AKAP-4: a novel cancer testis antigen for multiple myeloma
Multiple myeloma (MM) is a B-cell malignancy where mature plasma cells (PC) accumulate in the bone marrow (BM) causing bone destruction and BM failure (Atanackovic et al, 2007). Despite the improvement of conventional anti-tumour therapies, MM still remains incurable (Kyle & Rajkumar, 2004) and immunotherapy promises to be more effective and less invasive (Gouttenfangeas & Rammensee, 2000). The identification of novel tumour antigens that are capable of stimulating immune responses in cancer patients is pivotal (Scanlan et al, 2002). Cancer testis antigens (CTA) are a class of tumour-associated antigens (TAA) that show a restricted expression in the human germ line and cancer, a strong immunogenicity and a weak expression or absence in normal tissues (Scanlan et al, 2004). AKAP proteins, a growing family of scaffolding proteins, control signal transduction (Pawson & Scott, 1997) by targeting cyclic adenosine monophosphate-dependent protein kinase-A, and directing its actions (Turner et al, 2001). The present study investigated AKAP-4 expression in MM and in normal tissues to verify whether it can be considered a novel TAA.
A mRNA human normal tissue panel (Applied Biosystems, Foster City, CA, USA) and a normal tissue panel array (Pantomics, San Francisco, CA, USA) were analysed. PC of 19 MM patients (purified by BB4 antibody) and 6 MM cell lines (8226, ARK-B, U266, OPM2, ARP-1 and KMS11) were investigated. Clinical materials were obtained with approval from the local ethics committee and the patients’ consents. AKAP-4 expression was evaluated by reverse transcription polymerase chain reaction (RT-PCR), immunohisto/cytochemistry (IHC/ICC), cytochemistry immunofluorescence (IF) and fluorescence-activated cell sorting (FACS). For protein detection, we used anti-AKAP-4 polyclonal antibodies raised in rabbits (developed in our laboratory).
Briefly, 1 μg of total RNA extracted from cells by Tri-reagent (Sigma, St Louis, MO, USA) was DNAse I digested (Ambion, Austin, TX, USA) and reverse-transcribed by random hexamers. The primer sequences were as follows: 5′-GCG TAC TCT GAT ACT ACA ATG ATG-3′ and 5′-GGG GTT TTG GGT AAA GTC A-3′ (35 cycles, annealing 55°C). RNA integrity in each sample was checked on ACTB expression. All results were confirmed in three independent RT-PCR.
The tissue array was baked at 60°C, incubated for 15 min in 3% H2O2, and treated at room temperature with AKAP-4 polyclonal primary antibodies [1:100 dilution in phosphate-buffered saline (PBS1×)/bovine serum albumin 0·1%] for 45 min. Sections were incubated for 30 min at room temperature with the DAKO Envision™ system (DAKO, Carpinteria, CA, USA), followed by 5-min dark incubation with diaminobenzidine DAB (DAKO). Slides were counter-stained with hematoxylin (Fisher Scientific, Pittsburg, PA, USA) and evaluated by light microscope (Leica DMLA, Albuquerque, NM, USA).
Multiple myeloma PC and MM cell lines were spun in a cytospin column (5 × 104 cells/slide), fixed with SlideRite (Fisher Scientific) and air-dried overnight. Each sample was permeabilized (P) in PBS1×/0·1% Triton X-100 for 15 min at 4°C or not permeabilized (NP). For ICC, cells were treated with anti-AKAP-4 primary antibodies (1:100 dilution), incubated for 30 min with the Envision System (DAKO) and 5 min with DAB (DAKO). The ICC reaction was observed by light microscope (Leica). For IF, cells were incubated overnight in a wet chamber at 4°C with anti-AKAP-4 primary antibodies (1:100 dilution), then with fluorescein isothiocyanate-conjugated rabbit IgG secondary antibodies (1:500; Abcam, Cambridge, MA, USA). Results were analysed using an Olympus IX71 inverted microscope equipped with a Fluoview 300 confocal laser system (Olympus America Inc., Center Valley, PA, USA.
The expression of AKAP-4 was demonstrated by FACS analysis as previously described with a double stain reaction (Lim et al, 2001). Briefly, MM PC were incubated with monoclonal anti-AKAP-4 primary antibodies, or PBS1× as a negative control. Analysis was performed using a fluorescence-activated cell scanner (B&D Bioscience-PharMingen, Franklin Lakes, NJ, USA).
An enzyme-linked immunosorbent assay (ELISA) was performed on the sera of 19 MM and 11 healthy patients with no known abnormalities. Polystyrene 96-well flat-bottom plates were coated with AKAP-4 recombinant protein and incubated overnight at 4°C. After washing and blocking with SuperBlock® buffer (Pierce, Rockford, IL, USA), plates were placed at 37°C for 4 h. Each sample and the negative controls (PBS/fetal bovine serum) were diluted 1:1000 in SuperBlock® buffer and incubated for 4 h at room temperature. After washing with PBS/0·05% Tween 20, horseradish peroxidase-conjugated goat anti-human IgG (Pierce), diluted 1:5000 in SuperBlock®, was added and incubated at room temperature for 2 h. SuperSignal® ELISA Pico Chemiluminescent Substrate (price) was then added to each well for colour development, the intensity of which was measured by a Victor 2 microplate multilabel counter (PerkinElmer, Waltham, MA, USA). All samples were run in triplicates.
None of the normal tissues displayed AKAP4 expression (Fig 1A(i)), which was also confirmed by IHC (data not shown). AKAP4 expression was detected in eight of 19 patients (42%) (Fig 1A(ii) and in 5/6 (83%) of the established MM cell lines (Fig 1A(iii). (Fig 1B) shows representative positive staining in both MM PC and the ARP-1 MM cell line, evaluated by ICC, IF and FACS.
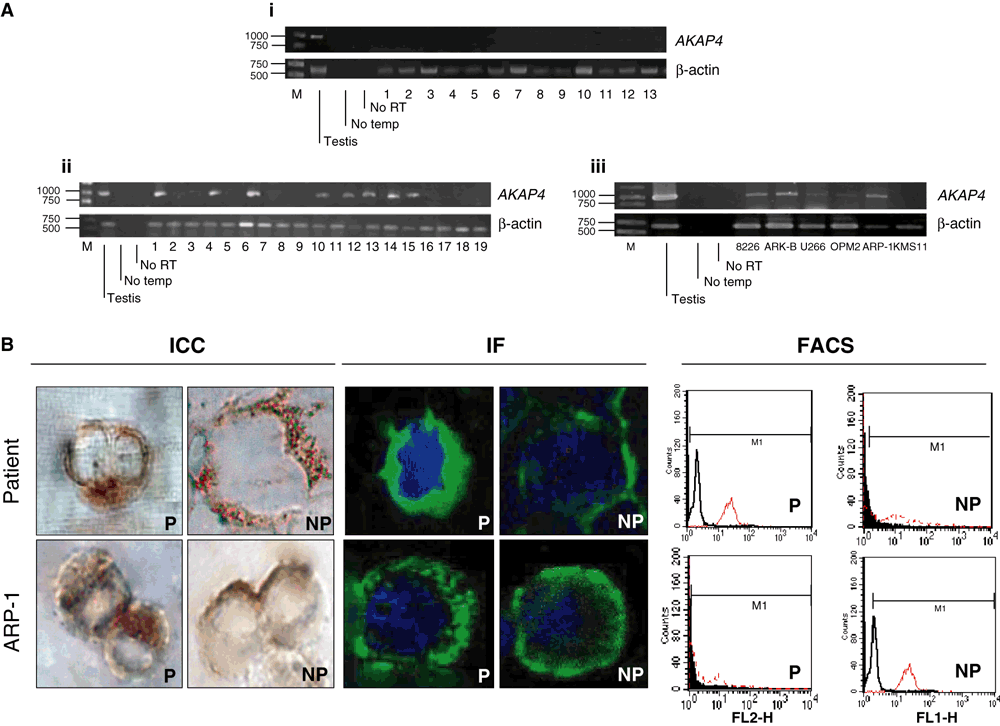
Polymerase chain reaction (PCR) analysis on human normal tissue panel and PCR, immunocytochemistry (ICC), immunofluorescence (IF) and fluorescence-activated cell sorting (FACS) on multiple myeloma (MM) patients and MM cell lines. (A). (i) PCR analysis did not show positive signal bands for any tissue, except for the positive control (testis). Investigated tissues: 1) brain, 2) breast, 3) colon, 4) heart, 5) kidney, 6) liver, 7) lung, 8) ovary, 9) pancreas, 10) skeletal muscle, 11) spleen, 12) stomach and 13) bone marrow; (ii) PCR analysis showed positive signal bands for eight of the 19 patients; and (iii). PCR performed for different MM cell lines showed positive band signals in the case of 8226, ARK-B, U266, Opm-2 and ARP-1 cell lines. We confirmed the purity of the results by means of two negative controls, which indicated that our primers were specific for AKAP4. No retro-verse transcriptase (no RT) indicates that there was no DNA contamination in the samples. (B). Plasma cells (PC) of a positive patient were evaluated. The panel for ICC and IF shows specific cytoplasmic (P) and surface (NP) staining. FACS analysis confirmed our observations for both, P and NP cases giving evidence of phycoerythrin signal shift (red graph) in the case of AKAP-4 stained PC. In the case of the ARP-1 MM cell line ICC and IF allow to visualize both the cytoplasmic (P) and surface (NP) presence of AKAP-4. Observations were confirmed by FACS analysis where PTTG-1 presence is evidenced in red. Pictures were taken at 100× objective magnifications.
To determine immunogenicity the presence of IgG antibodies against AKAP-4 was investigated in the serum of 19 MM patients by ELISA analysis (Fig 2). A positive signal was shown in 6/19 of the analysed cases (32%). The healthy controls, used to determine the cut-off point [mean + three times standard deviation (SD)], were significantly low (OD425 nm = 0·0491) (SD 0·0028), while negative controls showed a mean absorbance 19% less than the healthy controls (OD425 nm = 0·040).
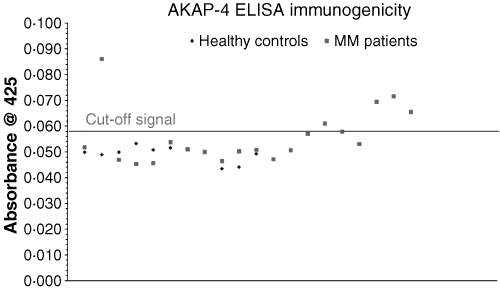
Enzyme-linked immunosorbent assay (ELISA) immunogenicity assay. Healthy donor and multiple myeloma (MM) patient’s serum samples, diluted 1:1000, were analysed using ELISA to determine the presence of IgG antibodies against AKAP-4. The cut-off signal, taken from the mean absorbance value at 425 nm + three times standard deviation, has a value of 0·058. Values above 0·058 were considered positive for the presence of IgG antibodies against AKAP-4.
Here we report, for the first time, the expression of AKAP-4 in MM at both the transcriptional and the protein level with no evidence of expression in human normal tissues other than in the testis (Turner RM et al, 2001). This suggests that AKAP-4 is a novel CTA in MM. As MM generates systemic immunosuppression (Goodyear et al, 2005) and, because antibodies against AKAP-4 have been found in patients’ sera, these findings strongly support the development of a polyvalent therapeutic vaccine that could be added to the standard treatments in MM (Chiriva-Internati et al, 2007).
Acknowledgements
This project was supported by the Institutional Research Program of the Texas Tech University Health Sciences Center and Southwest Cancer Treatment and Research Center Program. We thank Teri Fields for her assistance in editing this manuscript and Drs Janet Dertien and Jose-Louis Redondo for their assistance and support in using the confocal microscope. W. Martin Kast holds the Walter A. Richter Cancer Research Chair.




