Atypical presentation of acute promyelocytic leukaemia
We report an unusual case that illustrates the potential pitfalls in establishing a correct diagnosis for small round blue cell tumours (SRBCT) in paediatric patients while showing an exceedingly rare initial presentation of acute promyelocytic leukaemia (APL). A 14-year-old male was admitted to the Montreal Children's Hospital with paraparesis and otherwise unremarkable physical examination. For the previous 2-months he had back pain and numbness of his right leg. Difficulty to walk occurred two days prior to consultation. No urinary or stool incontinence or retention had been noted as well as no weight loss, night sweats or fever. His complete blood counts, blood smear, coagulation and biochemical profiles were normal. An emergency magnetic resonance imaging of the spine showed a pathological process in the bodies of L-2 to L5 and an extradural mass compressing the thecal sac at L3. Laminectomy of L-3 to L-5 and removal of the mass were performed, and patient had a good return of function. A gallium scan showed multiple sites of skeletal uptake. Computed tomography scanning of his lungs, abdomen and pelvis showed left pleural thickening. Pathology revealed an undifferentiated mitotically active SRBCT with slightly granular cytoplasm and oval nuclei (Fig 1A). Initial immunoperoxidase stains showed focal non-specific staining for CD45, diffuse staining for MIC-2 (CD99) and Neuron Specific Enolase and some rosette formation, potentially supporting a diagnosis of primitive neuro-ectodernal tumour (PNET)/Ewing's sarcoma (Fig 1A). However, ultrastructural studies (electron microscopy) performed in parallel, revealed the presence of Auer rods that are exclusively found in myeloid malignancies (Fig 1B). Additional staining for myeloperoxidase (MPO), lysozyme and Leder confirmed the diagnosis of an extra-medullary myeloid tumour (EMMT) (Fig 1B). Bone marrow (BM) aspirates performed on both posterior iliac crests showed only 6% myeloblasts with Auer rods. BM biopsy of the right posterior iliac crest showed replacement of the normal marrow by the same population of blasts (Fig 1C) while the biopsy from the left posterior iliac crest was normal and further confirmed the chloromatous behaviour of the leukaemic blasts in this child (Fig 1C). Based on cell phenotype, EMMT and absence of coagulation abnormalities, our initial diagnosis was an M2 subtype of acute myeloid leukaemia (AML) (French–American–British classification). This diagnosis was revised to APL upon the unexpected detection of t(15;17) by reverse transcription polymerase chain reaction on tumour cells from the extra-medullary mass. Of note, regular karyotyping was normal and fluorescent in situ hybridisation using the break-apart RARA probe (Mistry et al, 2003) was negative on marrow cell cultures, probably due to sampling and heterogeneous distribution of blasts. Treatment was subsequently modified to follow the Cancer and Leukemia Group B APL protocol that incorporates all-trans-retinoic acid (ATRA) (Gallagher et al, 1997). The patient achieved a complete remission and is now disease free 21 months after the end of treatment.
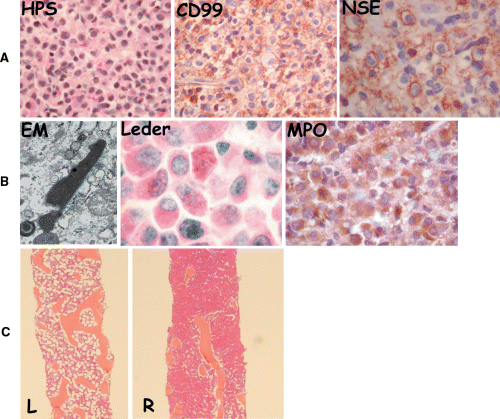
(A) Routine staining (haematoxylin–phloxine–saffron stain, HPS) of the extradural mass showing a proliferation of poorly differentiated cells. Tumour cells show diffuse staining for CD99 (MIC-2) and neuron specific enolase (NSE); positivity for these markers is highly evocative of primitive neuro-ectodermal tumour/Ewing's Sarcoma. (B) Ultrastructural studies (electron microscopy, EM) showing an elongated structure typical of an Auer rod in a tumour cell, suggesting myeloid differentiation. Myeloid origin was further confirmed through an additional Leder stain, which shows needle-shaped Auer rods in myeloblasts and by staining with myeloperoxidase (MPO). (C) Patchiness and chloromatous-like distribution of the leukaemic infiltrate in bone marrow (BM). Right panel represents left and right BM biopsies with near-complete infiltration of marrow space by neoplastic cells on the right side (R), while the left side (L) is normocellular (HPS).
In APL, EMMT is mostly observed at relapse (1–5%) and recent data suggest an increase in its incidence potentially linked to ATRA therapy (Wiernik et al, 1996; Specchia et al, 2001). The diagnosis of APL/AML could easily have been missed and the child treated as a sarcoma based on the localisation of the tumour, on the absence of frank marrow involvement (chloromatous presentation leading to sampling issues) and on initial pathological analysis (Menasce et al, 1999). Given the strong positivity for enolase, vimentin and CD99, a diagnosis of PNET/Ewing's would have been reasonable in our patient. However, as clearly shown in this report, SRBCT are misleading, underpinning the need for complete and multidimensional pathological assessment that includes a broad immunohistochemical panel, molecular biology and ultrastructural analysis despite understandable issues of cost management. Failure to use the correct tools to ensure diagnosis can tragically lead to inappropriate therapy, undue toxicity and treatment failure.
Acknowledgements
This work was supported by the Penny Cole foundation. N. Jabado is a recipient of a Chercheur Boursier Award from Fond de la Recherche pour la Sante au Quebec.




