A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction
Conflicts of interest: This study was supported by Immunex Corporation (Seattle, WA, U.S.A.), a wholly owned subsidiary of Amgen Inc. S.T. has received research support from Amgen; C.E.M.G. has been a paid consultant for Wyeth and Amgen; A.M.N and R.Z. are both full-time employees of Amgen.
Summary
Background In previous studies, etanercept significantly improved plaque psoriasis and was well tolerated.
Objectives To examine further the efficacy and safety of etanercept and to assess maintenance of treatment effect after dose reduction of etanercept.
Methods In this multicentre 24-week study in the U.S.A., Canada and Western Europe, patients were at least 18 years old; had active, clinically stable plaque psoriasis involving at least 10% of body surface area; had a minimum Psoriasis Area and Severity Index (PASI) of 10 at screening; and had received or were a candidate to receive systemic psoriasis therapy or phototherapy. During the first 12 weeks of the study, patients were randomly assigned to receive by subcutaneous injection etanercept twice weekly (BIW) at a dose of 50 mg or 25 mg, or placebo BIW in a double-blind fashion. During the second 12 weeks, all patients received etanercept 25 mg BIW. The primary endpoint was a 75% or greater improvement from baseline in PASI (PASI 75) at 12 weeks.
Results Five hundred and eighty-three subjects were randomized and received at least one dose of study drug. At week 12, a PASI 75 was achieved by 49% of patients in the etanercept 50 mg BIW group, 34% in the 25 mg BIW group, and 3% in the placebo group (P < 0·0001 for each etanercept group compared with placebo). At week 24 (after 12 weeks of open-label 25 mg etanercept BIW), a PASI 75 was achieved by 54% of patients whose dose was reduced from 50 mg BIW to 25 mg BIW, by 45% of patients in the continuous 25 mg BIW group, and by 28% in the group that received placebo followed by etanercept 25 mg BIW. Etanercept was well tolerated throughout the study.
Conclusions Etanercept provided clinically meaningful benefit to patients with chronic plaque psoriasis, with no apparent decrease in efficacy after dose reduction.
Psoriasis is associated with major morbidity, disability, economic loss, psychological impairment, and reduction in quality of life.1–3 Methotrexate and ciclosporin, traditionally used as systemic antipsoriatic agents, have been demonstrated to have excellent efficacy in short-term studies in psoriasis.4 However, cumulative, dose-dependent toxicities are known to occur with both (reviewed in Krueger et al.5).
The role of tumour necrosis factor (TNF) in psoriasis has been previously characterized.6 Etanercept is a soluble receptor TNF antagonist that competitively inhibits the interaction of TNF with cell-surface receptors, preventing TNF-mediated cellular responses and modulating the activity of other proinflammatory cytokines that are regulated by TNF. The safety and efficacy of etanercept have been demonstrated in patients with rheumatoid arthritis,7,8 psoriatic arthritis,9,10 ankylosing spondylitis11 and in two previous studies in patients with psoriasis.12,13
In this study, we further evaluated the safety and efficacy of etanercept in patients with chronic plaque psoriasis. The study had a two-part design: in the initial 12-week period, placebo was compared with two dosing regimens of etanercept, 25 mg twice weekly (BIW) and 50 mg BIW in a double-blind fashion; in the second 12-week period, all patients received etanercept 25 mg BIW. Of particular interest was whether a decreased dosage of etanercept would continue to provide benefit to patients originally randomized to the higher dose group.
Patients and methods
Patients
All patients gave written, informed consent before any study-related tests were performed. Patients were eligible for study enrolment if they were men or women aged at least 18 years with active, but clinically stable, plaque psoriasis involving ≥ 10% of total body surface area (BSA) at screening. Patients were to have a minimum Psoriasis Area and Severity Index (PASI)14 of 10 during screening and were to have received at least one previous phototherapy or systemic therapy for psoriasis (or to have been a candidate to do so in the opinion of the investigator). Patients were to have adequate haematological, renal and hepatic function.
Patients were excluded if they had received antibiotics within 1 week of study drug initiation or had an active severe infection within 4 weeks of study screening; had skin conditions other than psoriasis that would interfere with study evaluations; or had active guttate, erythrodermic or pustular psoriasis at the time of the screening visit. Patients were not to have received systemic psoriasis therapy or psoralen plus ultraviolet (UV) A phototherapy for 4 weeks before the study; topical corticosteroids, vitamin A or D analogue preparations, dithranol or UVB phototherapy for 2 weeks before the study; or etanercept or an anti-TNF antibody at any time. Patients were allowed to use topical corticosteroids of moderate strength on the scalp, axilla and groin, or tar compound or steroid-free topical emollients.
Treatment
Etanercept (Enbrel®; Immunex-Wyeth) was supplied to patients in syringes, each containing the contents of one reconstituted vial of etanercept or matching placebo. All study drug was self-administered by the patient by subcutaneous injection.
Study design
The study protocol was approved by the institutional review boards or independent ethics committees of the participating medical centres. The multicentre, randomized, parallel-group study consisted of two periods: during the initial 12-week period, patients were randomly assigned (using an Interactive Voice Response System) to receive placebo or etanercept at 25 mg BIW or 50 mg BIW in a double-blind manner; during the second 12-week period, all patients received etanercept 25 mg BIW (Fig. 1). Although all patients were treated with the same dose of etanercept during the second period, the patients, study site personnel, and all sponsor representatives remained blinded to the initial randomized treatment groups until the last patient had completed the week 24 efficacy and safety assessments, at which time the study was unblinded.
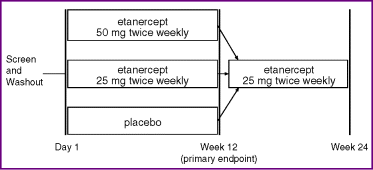
Study design.
Patients were stratified at randomization into two groups based on whether or not they had previously used systemic therapy or phototherapy, to maintain balance between treatment groups. To maintain the treatment blind, all patients received two injections per dose of study drug, with placebo making up the balance of injections for the 25-mg regimen. Safety and efficacy were evaluated at weeks 2, 4, 8, 12, 16, 20 and 24. Study drug was administered to the first patient in May 2002, and the last week 24 visit occurred in July 2003.
Efficacy endpoints
The primary efficacy endpoint was achievement of a 75% or greater improvement from baseline in the PASI (PASI 75) after 12 weeks of double-blind treatment. Secondary efficacy endpoints at week 12 included the PASI 50 and PASI 90 responses (achievement of at least 50% and 90% improvement, respectively) and percentage improvement from baseline in PASI; and the static physician's global assessment (sPGA) of clear or almost clear psoriasis (score of 0 or 1 on a six-point scale where 0 = clear psoriasis and 5 = severe disease). In addition, several patient-reported endpoints were assessed; the results of patient-reported outcomes will be summarized in a separate communication.
The above efficacy endpoints also were assessed during the second 12 weeks of the study. In addition, photographs of a subset of patients were taken at baseline and at weeks 12 and 24 to give visual confirmation of the effect of etanercept on psoriatic lesions. In some cases unscheduled photographs were taken in other weeks.
Safety endpoints
Safety was assessed throughout the study by summarizing reports of adverse events, infections and injection site reactions (ISRs); abnormalities in laboratory variables; and antibody formation to etanercept. Standard laboratory tests were done at screening, baseline and weeks 12 and 24, and included haematology, serum chemistry and urinalysis profiles. Adverse events and abnormalities in laboratory values were graded on a scale derived from the Common Toxicity Criteria of the National Cancer Institute. Serum samples obtained at baseline and at weeks 12 and 24, or at early termination from the study, were tested for antibody to etanercept using an enzyme-linked immunosorbent assay (ELISA).15 Samples that were positive by ELISA were tested for neutralizing antibodies with the use of a binding assay.
Statistical analysis
The planned sample size of 600 patients was based on efficacy results observed in an earlier phase II study12 and to expose an adequate number of patients to treatment for the assessment of safety. With 200 patients per treatment group, the study had greater than 99% power to detect a 25% absolute difference between either regimen of etanercept and placebo for the primary endpoint, using a two-sided Fisher's exact test at a significance level of α = 0·05.
The prespecified primary analysis for all efficacy endpoints included all randomized patients who received at least one dose of blinded study drug. In these analyses, missing postbaseline efficacy data were imputed using last observation carried forward (LOCF). In addition, a sensitivity analysis was performed on the binary efficacy endpoints (PASI 50, PASI 75 and PASI 90 responses, and clear/almost clear status on the sPGA) to evaluate the robustness of the primary analysis. This sensitivity analysis included all randomized patients, regardless of whether they received study drug. In addition, rather than using LOCF imputation, patients with missing data at a given visit were assumed to have not met the response criteria for that endpoint.
All analyses compared each of the two etanercept regimens vs. placebo. For binary endpoints, the analyses used the Cochran–Mantel–Haenszel test stratified at randomization by prior psoriasis therapy. Analyses for continuous endpoints used the van Elteren stratified rank test adjusting for prior psoriasis therapy.
For the primary endpoint, Hochberg's step-up procedure16 for multiple comparisons was used to control the overall significance level at 0·05 for the comparisons of the two etanercept regimens with placebo. For the secondary endpoints, the overall significance level of 0·05 was controlled by allocating 0·025 to a set of physician-reported endpoints (PASI 50, PASI 90, percentage improvement from baseline in PASI, and sPGA clear/almost clear) and to a set of patient-reported endpoints (not discussed in this study). Within the set of four physician-reported endpoints, Hochberg's step-up procedure was used to maintain an overall significance level of 0·025. Only those etanercept regimens found to be statistically significantly different from placebo for the primary efficacy endpoint were declared statistically significant for the secondary endpoints. For all other endpoints, the significance level was 0·05 unadjusted for multiplicity. All reported P-values are nominal.
All safety analyses were summarized by original treatment arm.
Results
Patients
Fifty investigative sites in the U.S.A., Canada and Western Europe screened 715 patients for the study, and 611 patients were randomly assigned to treatment. In total, 583 patients received at least one dose of study drug and are included in the primary analyses (Fig. 2). The groups were well matched across treatment groups for demography and disease history (Table 1). Most patients were men (66%), and most were white (91%). The median age was 45 years. Patients had a median duration of psoriasis of 19 years, and 89% had received previous systemic therapy or phototherapy for psoriasis; 26% had a prior diagnosis of psoriatic arthritis. Patients had a median BSA affected by psoriasis of 23% and a median baseline PASI of 16·4.
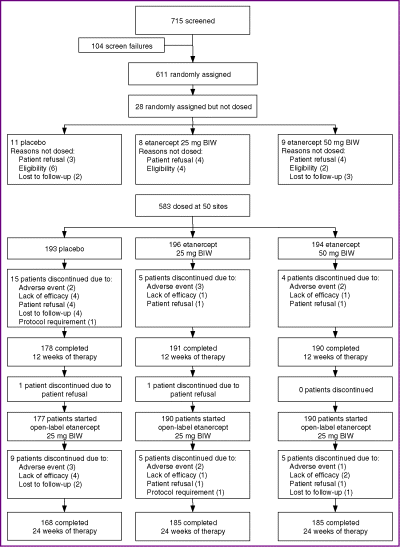
CONSORT diagram: patient disposition. BIW, twice weekly.
| Placebo (n = 193) | Etanercept | All (n = 583) | ||
|---|---|---|---|---|
| 25 mg BIW (n = 196) | 50 mg BIW (n = 194) | |||
| Men, n (%) | 124 (64) | 128 (65) | 130 (67) | 382 (66) |
| White, n (%) | 175 (91) | 180 (92) | 173 (89) | 528 (91) |
| Age (years), median (range) | 44·0 (18·0–80·0) | 46·0 (20·0–87·0) | 44·5 (21·0–80·0) | 45·0 (18·0–87·0) |
| Duration of psoriasis (years), median (range) | 17·5 (1·4–51·2) | 21·5 (0·8–64·6) | 18·1 (0·8–60·5) | 19·0 (0·8–64·6) |
| Body surface area affected (%), median (range) | 20·0 (10·0–95·0) | 23·0 (7·8–95·0) | 25·0 (10·0–80·0) | 23·0 (7·8–95·0) |
| PASI scores, median (range)a | 16·0 (7·0–62·4) | 16·9 (4·0–51·2) | 16·1 (7·0–57·3) | 16·4 (4·0–62·4) |
| History of psoriatic arthritis, n (%) | 50 (26) | 54 (28) | 50 (26) | 154 (26) |
| Any prior psoriasis therapy, n (%)b | 171 (89) | 174 (89) | 171 (88) | 516 (89) |
| Phototherapy, n (%)b | ||||
| PUVA | 65 (34) | 69 (35) | 63 (32) | 197 (34) |
| UVB | 118 (61) | 112 (57) | 111 (57) | 341 (58) |
| Systemic therapy, n (%)b | ||||
| Oral retinoids | 47 (24) | 51 (26) | 45 (23) | 143 (25) |
| Methotrexate | 76 (39) | 69 (35) | 74 (38) | 219 (38) |
| Ciclosporin | 31 (16) | 30 (15) | 34 (18) | 95 (16) |
| Topical therapy, n (%)b | ||||
| Steroids | 175 (91) | 181 (92) | 177 (91) | 533 (91) |
| Vitamin A analogue | 15 (8) | 18 (9) | 17 (9) | 50 (9) |
| Vitamin D analogue | 119 (62) | 122 (62) | 118 (61) | 359 (62) |
| Investigational product, n (%)b | 60 (31) | 51 (26) | 55 (28) | 166 (28) |
| Other psoriasis therapies, n (%)b | 57 (30) | 53 (27) | 49 (25) | 159 (27) |
- BIW, twice weekly; PASI, Psoriasis Area and Severity Index; PUVA, psoralen plus ultraviolet A phototherapy; UVB, ultraviolet B phototherapy. aPASI scores range from 0 (no psoriasis) to 72 (severe disease) and incorporate measures of erythema, desquamation, induration and affected body surface area. Inclusion criteria required a minimum PASI of 10 at screening. bPatients may have taken more than one prior psoriasis therapy.
Overall, 559 patients (96%) completed the initial 12 weeks of the study. Reasons for early termination included adverse event (1%), lack of efficacy (1%), patient refusal (1%), loss to follow-up (1%), and protocol requirement (< 1%). Of the 559 patients who completed the first 12 weeks, 557 patients continued to the second 12-week period and received etanercept 25 mg BIW, and 538 (97%) of these subjects completed 24 weeks of the study.
Efficacy
In the primary analysis of all patients who received at least one dose of study drug, the PASI 75 was achieved at week 12 by 96 patients (49%) in the etanercept 50 mg BIW group and 67 patients (34%) in the 25 mg BIW group, compared with six patients (3%) in the placebo group (P < 0·0001 for each etanercept group compared with placebo; Table 2). In the sensitivity analysis, which included all randomized patients regardless of whether they received study drug and in which patients with missing data were considered as not having achieved a PASI 75 response, results were almost identical to the primary analysis (Table 2): the PASI 75 at week 12 was achieved by 94 patients (46%) in the etanercept 50 mg BIW group and 66 patients (32%) in the 25 mg BIW group, compared with six patients (3%) in the placebo group (P < 0·0001 for each etanercept group compared with placebo).
| Placebo | Etanercept | ||
|---|---|---|---|
| 25 mg BIW | 50 mg BIW | ||
| Primary analyses | n = 193 | n = 196 | n = 194 |
| PASI 50 response, n (%) | 18 (9) | 126 (64)* | 150 (77)* |
| PASI 75 response, n (%) | 6 (3) | 67 (34)* | 96 (49)* |
| PASI 90 response, n (%) | 1 (1) | 21 (11) * | 40 (21) * |
| Static physician's global assessment, | 7 (4) | 77 (39) * | 111 (57) * |
| clear or almost clear, n (%) | |||
| Sensitivity analyses | n = 204 | n = 204 | n = 203 |
| PASI 50 response, n (%) | 18 (9) | 124 (61)* | 147 (72)* |
| PASI 75 response, n (%) | 6 (3) | 66 (32)* | 94 (46)* |
| PASI 90 response, n (%) | 1 (< 1) | 20 (10) * | 39 (19) * |
| Static physician's global assessment, | 7 (3) | 75 (37) * | 109 (54) * |
| clear or almost clear, n (%) | |||
- BIW, twice weekly; PASI 50, PASI 75 and PASI 90, improvement from baseline of at least 50%, 75% and 90%, respectively, in the Psoriasis Area and Severity Index. Results shown in bold indicate an evaluation of marked clearing of disease. *P < 0·0001, compared with placebo group.
Several subgroup analyses were performed to assess the PASI 75 response rates by baseline covariates (PASI, age, sex, race, prior systemic therapy or phototherapy, and BSA). Notably, in patients with BSA < 2·0 m2 (median baseline BSA), the PASI 75 was achieved at week 12 by 56% of patients in the etanercept 50 mg BIW group, 43% in the 25 mg BIW group and 4% in the placebo group; in patients with BSA ≥ 2·0 m2, the PASI 75 was achieved at week 12 by 41% of patients in the etanercept 50 mg BIW group, 23% in the 25 mg BIW group and 3% in the placebo group. In addition, logistic regressions were done to explore further the influence of baseline covariates on the primary endpoint. None of the treatment-by-covariate interaction terms was statistically significant, indicating that the treatment effect is not dependent on these covariates. Although the group of patients with larger BSA had slightly lower PASI response rates than those with smaller BSA, the treatment effect of etanercept was greater than the influence of BSA on the likelihood of achieving PASI 75 response at week 12.
Statistically significant differences also were seen between each active group and placebo at week 12 in the PASI 50 response, the PASI 90 response, and the achievement of a clear or almost clear assessment on the sPGA (score of 0 or 1 on a scale of 0–5; Table 2).
The response to etanercept was dose dependent: statistically significant differences in the PASI 75 response rates were observed as early as week 4 of the study between the etanercept 50 mg BIW group (10%) and placebo (2%), and as early as week 8 of the study between the etanercept 25 mg BIW group (20%) and placebo (3%; Fig. 3). Of note, at week 12, the difference between the etanercept 50 mg group and the 25 mg group was also statistically significant (P = 0·004). In addition, as early as week 2 of the study, the mean percentage improvement from baseline in the PASI was 18% in the etanercept 50 mg BIW group, 16% in the 25 mg BIW group and 4% in the placebo group (P < 0·0001 for each etanercept group compared with placebo). By week 12 of the study, the mean percentage improvement from baseline in the PASI was 68% in the etanercept 50 mg BIW group, 57% in the 25 mg BIW group and 0·2% in the placebo group (P < 0·0001 for each etanercept group compared with placebo). The median PASI scores at baseline (Table 1) improved to 4·5 in the etanercept 50 mg BIW group, 6·2 in the 25 mg BIW group and 15·6 in the placebo group at week 12.
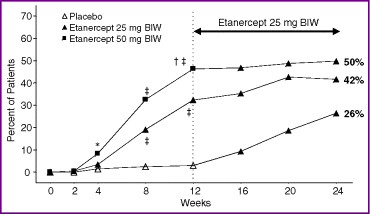
Percentage of patients achieving a PASI 75 response (improvement from baseline of at least 75% in the Psoriasis Area and Severity Index) over time: sensitivity analysis. This includes all randomized patients, regardless of whether they received study drug, and assumes that patients with missing data at a given visit did not meet the response criteria. BIW, twice weekly. *P = 0.0013 vs. placebo; ‡P < 0.001 vs. placebo; †P = 0.004 vs. etanercept 25 mg BIW.
PASI 75 response rates at week 24 of the study were sustained or improved compared with those at week 12. In the primary analysis at week 24, a PASI 75 was achieved by 54% of patients in the group following a dose reduction from etanercept 50 mg BIW to 25 mg BIW (n = 194), by 45% of patients after continuous treatment with etanercept 25 mg BIW (n = 196) and by 28% of patients in the group that began receiving etanercept 25 mg BIW after an initial 12 weeks of placebo (n = 193). Results of the sensitivity analysis were very similar: PASI 75 was achieved by 50% of patients in the group with a dose reduction from etanercept 50 mg BIW to 25 mg BIW (n = 203), by 42% of patients in the continuous etanercept 25 mg BIW group (n = 204) and by 26% of patients in the group that received etanercept 25 mg BIW after an initial 12 weeks of placebo (n = 204; Fig. 3).
An important clinical question was whether treatment success achieved with the 50 mg BIW dose could be maintained on an individual patient basis following dose reduction to 25 mg BIW. This could not be ascertained from the group analyses above, which average out the effect across patients within the group. To address this issue, the PASI 75 response status at week 12 and 24 of patients in this group was compared. Only those patients with available PASI assessments at both week 12 and week 24 (n = 179) were included: four of the patients receiving 50 mg BIW withdrew before the 24th week of the open-label period and an additional seven were missing either 12-week or 24-week data. One patient withdrew at week 24, but was included in the analysis. While this could represent a slight bias towards responders, only two of the patients who withdrew did so because of a lack of efficacy.
Of the 91 patients who were PASI 75 responders at week 12, the majority (70; 77%) maintained the PASI 75 response at week 24. Of the 21 patients who did not maintain a PASI 75 response at week 24, only three failed to maintain a PASI 50 response.
Notably, 32% of the 88 patients who had not achieved a PASI 75 response at week 12 did so by week 24, despite the decrease in etanercept dose from 50 mg BIW to 25 mg BIW (Table 3). Finally, photographs taken at baseline and throughout the study were consistent with the results of physician-reported assessments (4, 5).
| PASI 75 responder at week 24 | |||
|---|---|---|---|
| No | Yes | Total | |
| PASI 75 responder at week 12, n (%) | |||
| No | 60 (68) | 28 (32) | 88 (100) |
| Yes | 21 (23) | 70 (77) | 91 (100) |
| Total | 81 (45) | 98 (55) | 179 (100) |
- PASI 75, improvement from baseline of at least 75% in the Psoriasis Area and Severity Index.
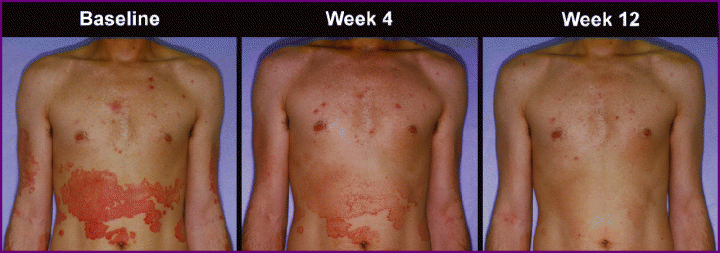
Etanercept provided improvement in psoriasis: photographs of a patient in the etanercept 50 mg twice weekly group at baseline, week 4 and week 12.
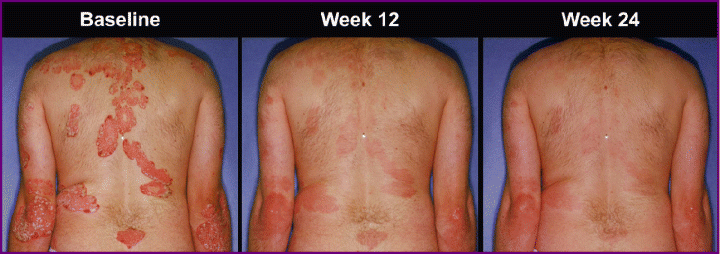
Psoriasis improvement was maintained after dose reduction: photographs of a patient in the etanercept 50 mg twice weekly (BIW) group at baseline and week 12, and at week 24 (after 12 weeks of etanercept 25 mg BIW).
Safety
Adverse events and infections occurred in similar proportions of patients in each group during the initial 12 weeks of the study (Table 4), and most events were of mild or moderate intensity. Twelve subjects experienced serious adverse events (including one serious infectious event, in a placebo patient) during the initial 12 weeks, leading to two discontinuations. ISR was the most commonly reported event, occurring in 18% of patients in the etanercept 50 mg BIW group, 13% of patients in the 25 mg BIW group and 6% of patients in the placebo group. All ISRs were mild or moderate; however, two patients discontinued from the study due to an ISR. Etanercept continued to be well tolerated during the second 12 weeks of the study. Adverse events and infections occurred in similar proportions of patients as observed in both the active and placebo groups in the initial 12-week period (Table 4). Throughout the 24 weeks, two patients withdrew because of infections, and nine patients discontinued due to other non-ISR adverse events. Of the nine discontinuations, five were considered to be drug related.
| Initial treatment group | Baseline to week 12 | Week 13 to week 24 | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 193) | Etanercept | Placebo (n = 177) | Etanercept | |||
| 25 mg BIW (n = 196) | 50 mg BIW (n = 194) | 25 mg BIW (n = 190) | 50 mg BIW (n = 190) | |||
| Injection site reaction | 11 (6) | 26 (13) | 35 (18) | 18 (10) | 9 (5) | 7 (4) |
| Upper respiratory infection | 25 (13) | 26 (13) | 25 (13) | 29 (16) | 30 (16) | 24 (13) |
| Headache | 15 (8) | 23 (12) | 21 (11) | 6 (3) | 9 (5) | 11 (6) |
| Injection site ecchymosis | 22 (11) | 24 (12) | 15 (8) | 4 (2) | 12 (6) | 5 (3) |
| Accidental injury | 12 (6) | 8 (4) | 13 (7) | 11 (6) | 7 (4) | 9 (5) |
| ‘Flu syndrome’ | 3 (2) | 9 (5) | 8 (4) | 3 (2) | 11 (6) | 5 (3) |
- All patients received open-label etanercept 25 mg twice weekly (BIW) during week 13 to week 24.
Most laboratory abnormalities observed throughout the study were mild or moderate (grade 1 or grade 2). During the initial 12 weeks, two patients had transient grade 3 changes in laboratory variables: one patient in the placebo group had a high haemoglobin level, and one in the etanercept 50 mg BIW group had a high alanine aminotransferase level. No additional grade 3 laboratory abnormalities occurred during the second 12 weeks. No grade 4 laboratory abnormalities occurred throughout the study.
Of 549 patients in the study who had baseline and postbaseline samples available for analysis, antietanercept antibodies were observed in six patients (1·1%) during the initial 12-week period. Samples from four of these six patients subsequently converted to negative by week 24. Longer-term exposure did not appear to increase the antigenicity of etanercept. During the second 12 weeks, nine additional patients (1·6%) developed antibodies; at a follow-up visit, samples from seven of these nine patients converted to negative. Of the nine patients who developed antibodies in the second 12 weeks, two had received placebo for the first 12 weeks. All patients had low levels of antibodies, and no patient developed neutralizing antibodies. No apparent differences in safety or efficacy profiles were observed between these patients and those who did not develop antibodies, although the small numbers of patients provide limited statistical power.
Discussion
In this study of patients with moderate to severe plaque psoriasis, etanercept treatment at 25 mg or 50 mg BIW resulted in statistically and clinically significant improvements in psoriasis that were dose dependent. The proportion of subjects achieving 75% or more improvement in PASI at week 12 compared with baseline was 49% in the etanercept 50 mg BIW group, 34% in the 25 mg BIW group and 3% in the placebo group (P < 0·0001 for each etanercept group compared with placebo). As early as week 2 of the study, the mean percentage improvement from baseline in the PASI was 18% in the etanercept 50 mg BIW group, 16% in the 25 mg BIW group and 4% in the placebo group (P < 0·0001 for each etanercept group compared with placebo).
Although it is problematic to compare efficacy across clinical studies, particularly trials that differ significantly in scope and design, some new and traditional psoriasis therapies have demonstrated similar or higher PASI response rates in short-term studies. In a recent nonplacebo-controlled comparison of ciclosporin and methotrexate, for example, 60% of patients on methotrexate and 71% of those on ciclosporin achieved a PASI 75 response after 16 weeks of therapy.4 However, more than a quarter (12 of 44) of patients discontinued methotrexate because of elevated liver enzyme levels.
While rare cases of demyelinating events, tuberculosis, lymphomas and lupus-like syndromes have been reported with etanercept and other TNF antagonists, etanercept was well tolerated in the initial 12-week period reported here, with most adverse events and infections occurring at similar frequency in both etanercept groups and in the placebo group. The overall percentage of etanercept patients who reported an ISR was 14%; however, this rate was markedly lower than that reported in clinical trials of patients with rheumatoid arthritis (37%). All reactions were mild, although two patients were discontinued because of an ISR. No increases in rates of adverse events or infections were observed during longer-term exposure to etanercept in the second 12-week period. No clinically significant laboratory abnormalities attributable to study drug were observed throughout the study. No neutralizing antibodies to etanercept were observed.
The results at 12 weeks in this study are highly consistent with another psoriasis study done at these doses.13 This is the first study, however, systematically to examine the maintenance of etanercept treatment effect following dose reduction. Treatment success, as measured by PASI 75 response, was measured in the group of patients who underwent a dose reduction from 50 mg BIW to half that dose. Importantly, with this group, most of the patients who achieved PASI 75 after treatment at the higher dose were able to maintain the response despite dose reduction. Of the patients who achieved a PASI 75 at week 12, 97% maintained at least a PASI 50 at week 24. In addition, almost a third of the patients who had not achieved a PASI 75 response at week 12 on the high dose did so by week 24 despite dose reduction.
At week 24, there was a clinically important, but not statistically significant, difference in the proportion of PASI 75 responders between those who underwent the dose reduction at week 12 (54% PASI 75 responders) and those who had received 25 mg BIW throughout (45% PASI 75 responders; P = 0·09). However, the power to detect this level of difference between these two treatment groups was low (38%). The ability to induce more rapid clearing in a large number of patients at the higher dose, and to maintain this response in a majority of these patients at a lower dose, makes this an attractive regimen. In addition, while the short-term safety of the 50 mg BIW regimen has been established in this and other studies,13 a reduction in exposure to any therapeutic agent is always a desirable goal, if satisfactory treatment response can be maintained.
Etanercept was approved for use in adults with rheumatoid arthritis in 1998, for children with juvenile rheumatoid arthritis in 1999, and in more recent years, for adults with psoriatic arthritis or ankylosing spondylitis. Etanercept has been used as chronic, continuous therapy in rheumatological diseases and has an excellent benefit–risk profile, both in clinical studies and in postmarketing use in over 200 000 patients.
In this study, the two dosing regimens of etanercept provided clinically meaningful benefit in a dose-dependent fashion to patients with chronic plaque psoriasis. Using a regimen of a starting dose of 50 mg BIW for 12 weeks followed by a maintenance dose of 25 mg BIW, etanercept provided significant improvement in psoriasis and continued maintenance of response, thus offering dermatologists a more flexible treatment paradigm for their patients with psoriasis.
Acknowledgments
Yuni Kim PhD assisted with the study conduct; Andrea Wang MA and Naree Sukumoljan BS assisted with the statistical programming and analysis; and Linda Melvin BA assisted with the writing of the paper. The Etanercept Psoriasis Study Group includes the following additional members: Peter Altmeyer, Ruhr-Universität Bochum, Bochum, Germany; John Berth-Jones, George Eliot Hospital, Nuneaton, U.K.; Karl Beutner, Solano Clinical Research, Vallejo, CA, U.S.A.; Isaac Bodokh, Cannes Hospital, Cannes, France; Erin Boh, Tulane University School of Medicine, New Orleans, LA, U.S.A.; Bernd Bonnekoh, Otto-von-Guericke Universität Magdeberg, Magdeberg, Germany; Marc Bourcier, Dermatology Clinic, Moncton, NB, Canada; Michelle Chambers, Radiant Research-Columbus, Columbus, OH, U.S.A.; Louis Dubertret, Hôpital Saint-Louis, Paris, France; Carin Endzweig-Gribetz, Mount Sinai School of Medicine, New York, NY, U.S.A.; John Ervin, The Center for Pharmaceutical Research, Kansas City, MO, U.S.A.; Richard Fitzpatrick, Dermatology Associates of San Diego County, Encinitas, CA, U.S.A.; W.F.M. Goldschmidt, Academic Medical Centre, University of Amsterdam, Amsterdam, the Netherlands; Alice Gottlieb, UMDNJ-Robert Wood Johnson Medical School, New Brunswick, NJ, U.S.A.; David Gratton, International Dermatology Research Inc., Montreal, QC, Canada; Gérard Guillet, CHU de Poitiers, Poitiers, France; Wayne Gulliver, NewLab Clinical Research, St John's, NF, Canada; Bruno Halioua, Cabinet Médical, Paris, France; Tiffani Hamilton, Atlanta Dermatology, Vein & Research Center, Alpharetta, GA, U.S.A.; Dan Henderson, Rockwood Clinic, PS, Spokane, WA, U.S.A.; David Horowitz, Dermatology Research Associates, Nashville, TN, U.S.A.; Michael Jarratt, Derm Research Inc., Austin, TX, U.S.A.; Roland Kaufman, Universitätsklinikum der Johann-Wolfgang-Goethe Universität Frankfurt, Frankfurt, Germany; Gerald Krueger, University of Utah Health Sciences Center, Salt Lake City, UT, U.S.A.; Richard Langley, Eastern Canada Cutaneous Research Associates Inc., Halifax, NS, Canada; Patricia Lee, UTMB Center for Clinical Studies, Houston, TX, U.S.A.; Craig Leonardi, Central Dermatology, St Louis, MO, U.S.A.; Anna Magee, Charlottesville Medical Research, Charlottesville, VA, U.S.A.; Gustav Mahrle, Universität zu Köln, Cologne, Germany; Michael Meurer, Universitätsklinikum Carl Gustav Carus der Technischen Universität Dresden, Dresden, Germany; Ulrich Mrowietz, Universitätsklinikum Schleswig Holstein Campus Kiel, Kiel, Germany; H.A.M. Neumann, Erasmus Medical Centre, Rotterdam, the Netherlands; Jean-Paul Ortonne, Hôpital de l'Archet II, Nice, France; Ray Parker Jr, Bressinck, Gibson, Parker, Dinehart, Sangster Dermatology, Little Rock, AR, U.S.A.; Gerd Plewig, Ludwig-Maximilians-Universität München, Munich, Germany; Yves Poulin, Centre de Recherche Dermatologique du Québec Metropolitain, Sainte-Foy, QC, Canada; E.P. Prens, Ziekenhuis Walcheren, Vlissingen, the Netherlands; Nathalie Provost, Innovaderm Research Inc., Montreal, QC, Canada; Elyse Rafal, DermResearch Center of New York Inc., Stony Brook, NY, U.S.A.; Michael Reardon, MSHJ Research Associates, Halifax, NS, Canada; Les Rosoph, North Bay Dermatology Center, North Bay, ON, Canada; Gilles Rostain, Cabinet Médical, Nice, France; Malcolm Rustin, Royal Free Hospital, London, U.K.; Patricia Speelman, Dermatology Specialists Inc., Vista, CA, U.S.A.; Wolfram Sterry, Charité, Berlin, Germany; Michael Sticherling, Universitätsklinikum Leipzig, Leipzig, Germany; Darryl Toth, Probity Medical Research, Windsor, ON, Canada.




