Enhancement of gilthead seabream (Sparus aurata) larval growth by dietary vitamin E in relation to two different levels of essential fatty acids
Abstract
The objective of this study was to determine the effect of dietary vitamin E on gilthead seabream (Sparus aurata) growth and survival, at two different highly unsaturated fatty acids (HUFAs) levels. Eighteen days old gilthead seabream larvae were fed four formulated experimental diets combining two different dietary levels of HUFAs (M: medium 2.5 + 1.5, DHA + EPA, H: high 5 + 2.5 DHA + EPA g per 100 g) with two different levels of vitamin E (M: medium 540 mg kg−1, H: high 2900 mg kg−1): MM, MH, HM, HH (HUFA/vitamin E). After 2-week feeding trial, the average survival rate was 52.6% and there were no significant differences found among treatments. Increase in vitamin E up to high level markedly improved larval growth, particularly when dietary HUFA levels were lower, suggesting a higher protection value when these fatty acids are more limiting. At medium dietary HUFA levels, increase in vitamin E from medium to high level enhanced larval growth performance in terms of total length. Moreover, increase in vit E enhanced HUFAs content in the larval polar lipids denoting the anti-oxidative effect of vitamin E.
Introduction
Fish feeds, and particularly those for the larval stages, are rich in highly unsaturated fatty acids (HUFAs fatty acids with 20 or more carbon atoms and 3 or more double bonds), such as eicosapentaenoic (20:5n-3; EPA) and docosahexaenoic (22:6n-3; DHA) acids, which are molecules with a great susceptibility to oxidative damage (Halliwell & Gutteridge 1996). When HUFAs are exposed to either oxygen or reactive oxygen species, they form lipid radicals, lipid peroxy radicals and other derivatives, which are toxic and damage various biomolecules, such as protein and DNA. Peroxidation risk is even higher in larval than in juvenile diets as they are micro-particles with a large surface area to volume ratio that frequently remain in the water for a long period of time (Izquierdo 2005). Thus, a functional defence against oxidized fat is particularly important in fish larvae. Furthermore, fish larvae tissue lipids are also very high in HUFA, what implies a higher risk of peroxidation (Sargent, Bell, McEvoy, Tocher & Estevez 1999) and, hence, cellular damage (Sakai, Murata, Endo, Yamauchi, Tabata & Fukudome 1989; Kanazawa 1991, 1993).
Endogenous enzymes play an important role in physiological antioxidant protection (Blazer 1982) including radical scavenging enzymes, such as catalase and superoxide dismutase, acting on hydrogen peroxide (H2O2) and superoxide (O−2), respectively, and glutathione peroxidase, which scavanges H2O2 and lipid hydroperoxides (Halliwell & Gutteridge 1996). Also, non-enzymatic low-molecular-weight molecules such as α-tocopherol (vitamin E, vit E) acts together with selenium and ascorbic acid preventing the chain reactions of HUFAs peroxidation. Thus, vit E functions as a chain breaking antioxidant, reacting with the lipid peroxide radicals and preventing the further reaction with a new HUFA. In addition, vit E plays an important role in the fish immune response (Montero, Tort, Izquierdo, Robaina & Vergara 1998; Wang, Mai, Liufu, Ma, Xu, Ai, Zhang, Tan & Wang 2006), reproduction (Izquierdo, Fernández-Palacios & Tacon 2001), stress resistance (Montero, Tort, Robaina, Vergara & Izquierdo 2001), larval growth (González, Izquierdo, Salhi, Hernández- Cruz & Fernández-Palacios 1995a). Vit E also improves growth in juveniles fed oxidized oil, reducing the lipid peroxidation products in gilthead sea bream (Sparus aurata) and turbot (Psetta maxima) (Tocher, Mourente, Van der Eecken, Evjemo, Diaz, Wille, Bell & Olsen 2003).
In gilthead seabream, vit E requirements were found to be close to 250 mg α-tocopherol per kg in broodstock diets (Fernández-Palacios, Izquierdo, Gonzalez, Robaina & Valencia 1998) and at least 136 mg kg−1 in larval diets (González et al. 1995a). However, the same authors found in a later study that the elevation of vit E up to 1500 mg kg−1 in larval diets did not negatively affected larval growth and lead to a much higher survival than the previous studies (Gonzalez 1997). Moreover, vit E requirements can be strikingly affected by the dietary levels of other nutrients (Hamre, Hjeltnes, Kryvi, Sandberg, Lorentzen & Lie 1994; Hamre & Lie 1995a; Hamre & Lie 1995b), such as selenium, vitamin C which protects fish against vit E deficiency (Hamre, Waagbo, Berge & Lie 1997) and HUFAs peroxidation (Blazer 1982). In fact, vit E oxidation is accelerated by HUFAs presence, as the nature of these fatty acids makes them prone to oxidation and, thus, they have the potential to increase the need for vit E (Watanabe 1982). For instance, increasing the polyunsaturated fatty acids (PUFA, fatty acids with 18 or more carbon atoms and 2 or more double bond) level in diets for Atlantic salmon (Salmo salar) reduced the liver vit E concentration (Waagbø, Sandnes, Torrissen, Sandvin & Lie 1993). Therefore, increasing dietary PUFAs seem to require increased vit E to avoid lipid peroxidation (Watanabe, Takeuchi, Wada & Uehara 1981; Watanabe, Takeuchi & Wade 1981; Sargent, McEvoy & Bell 1997; Izquierdo et al. 2001), although this topic has not yet been studied in gilthead seabream. Hence, the objective of the present study was to determine the effect of dietary vit E levels on gilthead seabream growth and survival in relation to two different HUFAs levels.
Materials and methods
Gilthead sea bream (S. aurata) larvae were obtained from natural spawning from broodstocks at the GIA facilities (Grupo de Investigación en Acuicultura, Las Palmas de Gran Canaria, Spain) where the experiment was carried out. A trial was conducted to test four microdiets in triplicates. Larvae were previously fed enriched rotifers (DHA Protein Selco; INVE, Dendermonde, Belgium) until they reached 18 days posthatch. Gilthead seabream larvae (total length 5.20 ± 0.077 mm, dry body weight 144 ± 0.08 μg) were randomly distributed into the experimental tanks at a density of 1800 larvae per tank and were fed one of the experimental diets tested in triplicates for 14 days, at a water temperature ranged from 18.8 to 20.3°C. All tanks (170 L light grey colour cylinder fibreglass tanks) were supplied with filtered sea water (37 ppm salinity) at an increasing rate of 0.4–1.0 L min−1 during the feeding trial. Water entered the tank from the bottom and get out from the top and was continuously aerated (125 mL min−1), attaining 5–8 ppm dissolved O2 and saturation ranged between 60% and 80% in all tanks. Therefore, water quality in terms of temperature, dissolved O2 and pH were appropriate for this species. Photoperiod was kept at 12 h light:12 h dark by fluorescent lights. Fish larvae were manually fed 14 times per day each 45 min from 9:00 to 19:00 hours. Daily feed supplied was 2 and 2.5 g per tank during the first and second week of feeding respectively.
Four isonitrogenous and isolipidic (70.22 ± 0.21/16.95 ± 0.19; mean ± SD analysed protein/lipid dietary contents) experimental microdiets (pellet size < 250 μm) were formulated using DHA50 and EPA50 (CRODA, East Yorkshire, UK) as sources of DHA and EPA respectively (Table 1). The desired lipid content was completed with a non-essential fatty acid source, oleic acid (Oleic acid vegetable; Merck, Darmstadt, Germany). The protein source used (squid meal) was defatted (three consecutive times with a chloroform:squid meal ratio of 3:1) to allow a better control of the fatty acid profile of the microdiet, only a 1.67 g per 100 g lipids remaining in the defatted squid meal. Two different dietary levels of HUFAs were formulated: 2.5 + 1.5 (medium, M) and 5 + 2.5 (high, H) (DHA + EPA) combined with two vit E (DL-α-Tocopherol acetate; Sigma-Aldrich, Madrid, Spain) levels: 500 (medium, M) and 2900 (high, H) mg kg−1 (Table 2). Therefore four experimental diets (MM, MH, HM and HH) were tested according to HUFA and vit E levels respectively. The microdiet was prepared in the following manner: the squid powder was carefully mixed with the other hidrosoluble ingredients (attractants, minerals and hidrosoluble vitamins) in a mortar. In a separated mixture, oils and fat-soluble vitamins were combined to obtain a homogeneous mix, which blended together with the powder mixture. Then, gelatin was dissolved in warm water and, when its temperature was lower than 35°C, added to the rest of the previously mixed ingredients. The paste was compressed pelleted (Severin, Suderm, Germany) and dried in an oven (Ako, Barcelona, Spain) at 38°C for 24 h. Pellets were ground (Braun, Kronberg, Germany) and sieved (Filtra, Barcelona, Spain) to obtain the desired particle size. Diets were analysed for proximate composition, α-tocopherol and fatty acids levels (Tables 2 and 3). Diets were kept at −20°C until each feeding.
| Diet b | EPA 50c | DHA 50d | Oleic acide | Soy lecithinf | Astaxa-nthing | Vitamin C h | Defatted squid Powder i |
|---|---|---|---|---|---|---|---|
| MM | 2 | 3.4 | 7.6 | 2 | 5 | 180 | 69.0 |
| MH | 2 | 3.4 | 7.6 | 2 | 5 | 180 | 69.0 |
| HM | 4.2 | 7.4 | 1.4 | 2 | 5 | 180 | 69.0 |
| HH | 4.2 | 7.4 | 1.4 | 2 | 5 | 180 | 69.0 |
- a Lipid, protein sources (g per 100 g) and antioxidant ingredients (mg per 100 g).
- b Diet: MM, MH, HM, HH (HUFA/vitamin E).
- c CRODA, East Yorkshire, UK (containing 12.7 g per 100 g DHA and 46.5 g per 100 g EPA).
- d CRODA (containing 53.8 g per 100 g DHA and 17.7 g per 100 g EPA).
- e Vegetable oleic acid; Merck, Darmstadt, Germany.
- f Acofarma, Barcelona, Spain.
- g Roche, Madrid, Spain.
- h ROVIMIX Stay-C-35; Roche, Paris, France.
- i Squid meal (Riber & Son, Bergen, Norway).
| Diet b | Protein | Total lipid | Moisture | α-Tocopherolc |
|---|---|---|---|---|
| MM | 70.49 ± 0.29 | 17.18 ± 0.24 | 7.99 ± 0.21 | 542 |
| MH | 70.22 ± 0.1 | 16.86 ± 0.16 | 7.73 ± 0.25 | 2956 |
| HM | 69.9 ± 0.22 | 16.86 ± 0.17 | 8.11 ± 0.12 | 541 |
| HH | 70.26 ± 0.22 | 16.9 ± 0.19 | 7.62 ± 0.13 | 2900 |
- a Protein, total lipid, moisture (g per 100 g) and α-tocopherol contents (mg kg−1).
- b Diet: MM, MH, HM, HH (HUFA/vitamin E), values are mean ± SD, n = 3.
- c DL-α-Tocopherol acetate; Sigma-Aldrich, Madrid, Spain.
| Fatty acid | Experimental dietsb | |||
|---|---|---|---|---|
| MM | MH | HM | HH | |
| 12:0 | 0.15 ± 0.02a | 0.15 ± 0.02a | 0.01 ± 0.00b | 0.02 ± 0.00b |
| 14:0 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.02 | 0.09 ± 0.01 |
| 14:1n-5 | n.d. | n.d. | 0.1 | 0.01 |
| 15:1n-5 | 0.02 | 0.01 | 0.03 ± 0.02 | 0.03 ± 0.01 |
| 16:0 iso | 0.01 | 0.01 | 0.01 | 0.01 |
| 16:0 | 1.03 ± 0.12 | 0.98 ± 0.02 | 0.84 ± 0.06 | 0.85 ± 0.05 |
| 16:1n-7 | 0.04 ± 0.01b | 0.04 ± 0.00b | 0.16 ± 0.03a | 0.16 ± 0.02a |
| 16:2n-6 | n.d. | n.d. | 0.02 | 0.02 |
| 16:2n-4 | 0.01 | 0.1 | 0.02 | 0.02 |
| 17:0 | 0.02 | 0.02 | 0.04 ± 0.02 | 0.04 ± 0.01 |
| 16:3n-4 | 0.02 ± 0.00b | 0.01 ± 0.00b | 0.05 ± 0.01a | 0.05 ± 0.01ab |
| 16:3n-3 | 0.01 | 0.01 | 0.02 | 0.02 |
| 16:3n-1 | 0.01 | 0.45 ± 0.36 | 0.01 | 0.01 |
| 16:4n-3 | n.d. | n.d. | 0.01 | 0.01 |
| 18:0 | 0.31 ± 0.02 | 0.3 ± 0.02 | 0.37 ± 0.05 | 0.37 ± 0.03 |
| 18:1n-9 | 7.97 ± 0.11a | 7.71 ± 0.33a | 2.3 ± 0.24b | 2.17 ± 0.09b |
| 18:1n-7 | 0.14 ± 0.02b | 0.15 ± 0.05b | 0.28 ± 0.02a | 0.28 ± 0.02a |
| 18:1n-5 | 0.01 | 0.01 | 0.02 | 0.02 |
| 18:2n-9 | 0.01 | n.d. | 0.01 | 0.01 |
| 18:2n-6 | 2.24 ± 0.16a | 2.1 ± 0.14a | 1.19 ± 0.07b | 1.19 ± 0.08b |
| 18:2n-4 | 0.02 | 0.02 | 0.05 | 0.05 |
| 18:3n-6 | 0.02 | 0.02 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| 18:3n-4 | 0.01 | n.d. | 0.04 ± 0.01 | 0.04 ± 0.01 |
| 18:3n-3 | 0.15 ± 0.03 | 0.14 ± 0.02 | 0.18 ± 0.01 | 0.18 ± 0.04 |
| 18:4n-3 | 0.1 ± 0.01b | 0.1 ± 0.02b | 0.24 ± 0.04a | 0.24 ± 0.02a |
| 18:4n-1 | 0.01 | 0.01 | 0.03 | 0.03 |
| 20:0 | 0.04 ± 0.01 | 0.4 ± 0.1 | 0.06 ± 0.02 | 0.06 ± 0.02 |
| 20:1n-9 | 0.18 ± 0.04b | 0.18 ± 0.02b | 0.3 ± 0.07a | 0.29 ± 0.03a |
| 20:2n-9 | 0.05 ± 0.06 | 0.1 | 0.03 | 0.03 |
| 20:3n-9 | 0.01 | 0.01 | 0.02 | 0.02 |
| 20:3n-6 | 0.02 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| 20:3n-3 | 0.02 | 0.02 | 0.05 | 0.05 |
| 20:4n-6 | 0.16 ± 0.01b | 0.16 ± 0.03b | 0.37 ± 0.02a | 0.36 ± 0.04a |
| 20:4n-3 | 0.06 ± 0.03b | 0.06 ± 0.03b | 0.15 ± 0.02a | 0.15 ± 0.01a |
| 20:5n-3 | 1.67 ± 0.21b | 1.67 ± 0.24b | 3.77 ± 0.66a | 3.81 ± 0.16a |
| 22:1n-11 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.08 ± 0.01 | 0.08 |
| 22:4n-6 | 0.11 ± 0.02b | 0.11 ± 0.01b | 0.26 ± 0.03a | 0.25 ± 0.04a |
| 22:5n-6 | n.d. | n.d. | 0.1 | 0.1 |
| 22:5n-3 | 0.14 ± 0.02b | 0.14 ± 0.02b | 0.34 ± 0.04a | 0.34 ± 0.04a |
| 22:6n-3 | 2.34 ± 0.17b | 2.47 ± 0.19b | 5.33 ± 0.34a | 5.27 ± 0.52a |
| Saturated | 1.64 ± 0.08a | 1.57 ± 0.09ab | 1.42 ± 0.08b | 1.43 ± 0.09ab |
| Monosaturated | 8.39 ± 0.22a | 8.17 ± 0.62a | 3.17 ± 0.45b | 3.03 ± 0.37b |
| n-3 | 4.49 ± 0.21b | 4.6 ± 0.43b | 10.08 ± 0.82a | 10.05 ± 0.6a |
| n-6 | 2.55 ± 0.18a | 2.47 ± 0.12a | 1.96 ± 0.11b | 1.95 ± 0.1b |
| n-9 | 8.32 ± 0.18a | 7.9 ± 0.29a | 2.6 ± 0.14b | 2.53 ± 0.21b |
| n-3HUFA | 4.22 ± 0.3b | 4.42 ± 0.13b | 9.65 ± 0.46a | 9.6 ± 0.16a |
| ARA/EPA | 0.1 ± 0.02 | 0.09 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| EPA/DHA | 0.71 ± 0.08 | 0.68 ± 0.06 | 0.71 ± 0.06 | 0.73 ± 0.05 |
| n-3/n-6 | 1.76 ± 0.18b | 1.87 ± 0.05b | 5.19 ± 0.16a | 5.17 ± 0.12a |
- n.d., not detected; DHA, docosahexaenoic acid: 22:6n-3; EPA, eicosapentaenoic acid: 20:5n-3; ARA, arachidonic acid: 20:4n-6.
- a Fatty acid (g per 100 g diet dry weight).
- b Diet: MM, MH, HM, HH (HUFA/vitamin E), values are mean ± SD, n = 3. Different letters for a given data denote significant differences among experimental groups (P < 0.05).
After feeding, larvae were observed under the binocular microscope to determine feed acceptance. Final survival was calculated by individually counting all the larvae alive at the beginning and at the end of the experiment. Growth was determined by measuring dry body weight and total length (Profile Projector; Nikon V-12A, Tokyo, Japan) of 30 fish per tank at the beginning, in the middle and at the end of the trial. The specific growth rate (SGR) was determined according to the equation: SGR = [(ln final body weight)−(ln initial body weight)] × 100/t, where t is days of the feeding period. On the last day of the experiment an activity test was conducted by lifting 20 larvae per tank out of the water in a scoop net for 1 min and subsequently allocating them in another tank supplied with clean seawater and aeration to determine survival after 24 h. Another 20 larvae per tank were transferred to 15°C sea water, to determine survival after 24 h. At the end of the trial, the remaining larvae in each tank were starved for 12 h then washed with distilled water, sampled and kept at −80°C for biochemical composition.
Moisture (A.O.A.C. 1995), crude protein (A.O.A.C. 1995) and crude lipid (Folch et al. 1957) contents of larvae and diets were analysed. Dietary vit E content was determined using high performance liquid chromatography (HPLC: column: C18, mobile phase:mixture of water-acetonitrile-trifluoroacetic acid, Software: Waters Empower, Waters Alliance). Neutral and polar fractions of the larval total lipid were separated using adsorption chromatography on silica cartridges (Sep-pak; Waters S.A., Milford, MA, USA) using 30 mL chloroform and 20 mL chloroform/methanol (49:1, v/v) as solvent for neutral lipid, then 30 mL methanol for polar fractions according to Juaneda and Rocquelin (1985). Fatty acid methyl esters were obtained by transmethylation of polar lipids as described by Christie (1982) separated using gas liquid chromatography (GLC), quantified using flame ionization detection (GC-14A; Shimadzu, Tokyo, Japan) under the conditions described in Izquierdo, Watanabe, Takeuchi, Arakawa and Kitajima (1990) and identified by comparison to previously characterized standards and gas-liquid chromatography-mass spectrometry.

Results
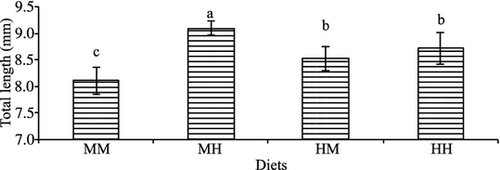
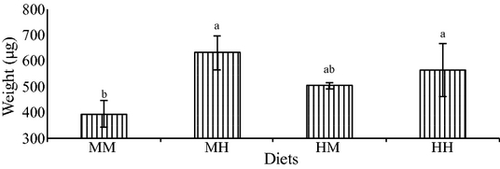
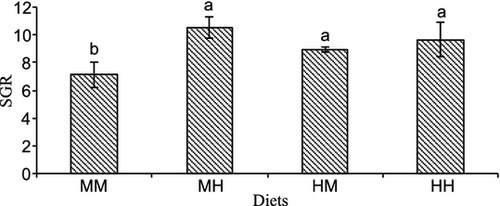
All experimental microdiets were well accepted by larvae. At the end of the trial, the average survival rate was 52.6% and there were no significant differences among treatments. After only 1 week of feeding, there was no significant difference among treatments in any of the growth-related parameters. However, at the end of the experiment, growth, in terms of total length, was significantly higher in fish larvae fed on the diet with medium HUFA and high vit E levels (MH) compared with the other treatments (Fig. 1). Increase in HUFA elevated larval length at medium vit E levels (HM versus MM) (Fig. 1). The two-way anova analysis showed that dietary vit E significantly affected larval total length (P = 0.04) and there was a significant interaction between vit E and HUFA dietary levels (P = 0.015) (Table 4). Similarly, whole body weight increased significantly in fish larvae fed on diet with medium HUFA and high vit E levels (MH) (Fig. 2). The two-way anova showed that vit E significantly affected whole body weight (P = 0.01), and that there was a significant interaction between vit E and HUFA dietary levels (P = 0.044). Specific growth rate followed a similar trend, with larvae fed diet MM showing significantly lower SGR than larvae from the other treatments (Fig. 3) and the dietary increase in vit E or HUFA with respect to this diet significantly improved SGR. The two-way anova analysis showed that vit E significantly affected SGR (P = 0.07), and that there was a significant interaction between vit E and HUFA dietary levels (P = 0.024).
| Probability of contrasts | |||
|---|---|---|---|
| Vit E | DHA/EPA | Vit E × DHA/EPA | |
| Total length (mm) | 0.04 | NS | 0.015 |
| Weight (μg) | 0.01 | NS | 0.044 |
| SGR (% body weight gain day−1) | 0.07 | NS | 0.024 |
- NS, not significant.
At the end of the experiment, the survival after the two activity tests (air or low water temperature exposure) were not showed significant differences among treatments (average values 75.5% and 85% respectively).
The diets were similar in their levels of saturated fatty acids, whereas they differed in their monounsaturated and polyunsaturated fatty acids contents (Table 3). Diets containing medium HUFA showed higher monoenoic and n-6 fatty acids than diets containing high HUFA, particularly due to higher oleic (18:1n-9, 254.8% increase) and linoleic acids (18:2n-6, 84.5% increase), respectively, and n-3 docosapentaenoic acid (22:5n-3: DPA) (23.88% increase), together with lower levels of PUFA, particularly (52.9% reduction), EPA (20:5n-3, 49.6% reduction) and DHA (22:6n-3, 55.37% reduction).
In comparison with the initial larvae and independently of experimental diet, fatty acid composition of polar lipids from larvae at the end of the experiment showed a reduction in saturated fatty acids, particularly myristic (14:0) and palmitic (16:0) fatty acids, PUFAs, such as ARA (arachidonic acid; 20:4n-6) and HUFAs such as EPA and DPA, displaying higher values in palmitoleic fatty acid (16:1n-7) and other minor fatty acids (Table 5). In comparison with larvae fed diets containing high HUFA, larvae fed diets medium HUFA, showed a very similar fatty acid composition between them, displaying higher levels of monounsaturated and n-6 fatty acids, particularly oleic and linoleic acid. Besides, larvae fed medium HUFA diets were lower in 17:0 (19.1% reduction), 18:1n-7 (13.2% reduction), 18:4n-1 (11.4% reduction), ARA (8.8% reduction), 20:4n-3 (4.8% reduction), 22:4n-6 (20.9% reduction) and DHA (9.9% reduction), than larvae fed high HUFA diets, but only slightly lower in EPA (0.7% reduction). Regarding fatty acid composition of polar lipids from larvae fed high HUFA diets, elevation of vit E from medium to high levels slightly increased polyunsaturated fatty acids, manifested in larval ARA (3.5% increase), 20:4n-3 (9.5% increase), 20:3n-9 (90% increase), EPA (5.4% increase), DPA (2.8% increase), n-3 HUFA (2.8% increase) and, particularly, DHA (2.3% increase). In medium HUFA treatments (MM, MH) the increase in dietary vit E from medium to high was accompanied by a slight elevation in dietary EPA and DHA (1.5% and 4.99% respectively), this increase in dietary vit E did not affect the EPA and DHA larval contents (1.5%, 0.23% increases respectively). In high HUFAs diets (HM and HH), the increase in vit E from medium to high was accompanied by an elevation of dietary EPA (0.42%) and a reduction in dietary DHA (1.05%), this increase in dietary vit E produced a slightly increase in the larval contents of EPA and DHA (5.7%, 2.3% respectively).
| Total lipid – fatty acid | Initial | Final | |||
|---|---|---|---|---|---|
| MM | MH | HM | HH | ||
| Total lipid | 2.52 ± 0.09 | 2.15 ± 0.17 | 2.17 ± 0.14 | 2.24 ± 0.16 | 2.23 ± 0.18 |
| Fatty acid | |||||
| 12:0 | 0.12 ± 0.00 | 0.15 ± 0.05 | 0.13 ± 0.08 | 0.15 ± 0.02 | 0.14 ± 0.04 |
| 14:0 | 1.12 ± 0.09 | 0.6 ± 0.05 | 0.6 ± 0.04 | 0.69 ± 0.11 | 0.69 ± 0.08 |
| 14:1n-7 | 0.05 ± 0.00 | 0.04 ± 0.0ab | 0.03 ± 0.01b | 0.05 ± 0.01a | 0.05 ± 0.01ab |
| 15:0 | 0.42 ± 0.02 | 0.24 ± 0.01 | 0.26 ± 0.04 | 0.27 ± 0.03 | 0.26 ± 0.05 |
| 15:1n-5 | 0.77 ± 0.05 | 0.51 ± 0.04 | 0.51 ± 0.04 | 0.6 ± 0.08 | 0.61 ± 0.08 |
| 16:0 iso | 0.23 ± 0.00 | 0.13 ± 0.02 | 0.13 ± 0.02 | 0.16 ± 0.02 | 0.15 ± 0.03 |
| Me-16:0 | 0.64 ± 0.03 | 0.4 ± 0.09 | 0.39 ± 0.08 | 0.41 ± 0.05 | 0.46 ± 0.12 |
| 16:0 | 25.41 ± 2.23 | 21.02 ± 1.91 | 19.5 ± 1.68 | 20.9 ± 2.87 | 20.81 ± 2.44 |
| 16:1n-9 | 0.95 ± 0.05 | 0.58 ± 0.4 | 0.79 ± 0.06 | 0.7 ± 0.09 | 0.72 ± 0.06 |
| 16:1n-7 | 6.03 ± 0.53 | 2.34 ± 0.26 | 2.53 ± 0.27 | 2.77 ± 0.23 | 2.86 ± 0.38 |
| 16:1n-5 | 1 ± 0.11 | 0.69 ± 0.06 | 0.75 ± 0.15 | 0.78 ± 0.09 | 0.77 ± 0.13 |
| 16:2n-6 | 1.27 ± 0.12 | 0.63 ± 0.42 | 0.38 ± 0.06 | 0.41 ± 0.05 | 0.76 ± 0.61 |
| 16:2n-4 | n.d. | 1.2 ± 0.05 | 1.18 ± 0.12 | 1.3 ± 0.14 | 1.19 ± 0.03 |
| 17:0 | 0.95 ± 0.07 | 0.68 ± 0.08b | 0.76 ± 0.07ab | 0.87 ± 0.08a | 0.91 ± 0.11a |
| 16:3n-3 | 1.91 ± 0.10 | 1.34 ± 0.3 | 1.38 ± 0.31 | 1.42 ± 0.17 | 1.57 ± 0.4 |
| 16:3n-1 | 0.43 ± 0.02 | 0.98 ± 0.18 | 0.96 ± 0.14 | 0.71 ± 0.05 | 0.78 ± 0.16 |
| 16:3n-4 | 0.3 ± 0.04 | 0.29 ± 0.06 | 0.3 ± 0.06 | 0.34 ± 0.03 | 0.39 ± 0.1 |
| 18:0 | 10.5 ± 1.3 | 10.39 ± 1.06 | 9.8 ± 0.98 | 10.63 ± 0.65 | 10.25 ± 1.14 |
| 18:1n-9 | 14.01 ± 1.23 | 19.62 ± 1.58a | 19.87 ± 1.22a | 13.73 ± 1.82b | 13.23 ± 1.29b |
| 18:1n-7 | 5.85 ± 0.43 | 3.55 ± 0.35 | 3.7 ± 0.38 | 4.16 ± 0.41 | 4.19 ± 0.52 |
| 18:1n-5 | 0.35 ± 0.02 | 0.2 ± 0.02b | 0.22 ± 0.03ab | 0.26 ± 0.01a | 0.26 ± 0.02a |
| 18:2n-9 | 1.31 ± 0.10 | 0.55 ± 0.32 | 0.57 ± 0.27 | 0.67 ± 0.02 | 0.7 ± 0.08 |
| 18:2n-6 | 3.96 ± 0.24 | 5.23 ± 0.26a | 5.31 ± 0.32a | 3.22 ± 0.22b | 3.14 ± 0.24b |
| 18:2n-4 | 0.06 ± 0.00 | 0.06 ± 0b | 0.06 ± 0b | 0.13 ± 0.01a | 0.13 ± 0.01a |
| 18:3n-6 | 0.3 ± 0.01 | 0.34 ± 0.02a | 0.34 ± 0.03a | 0.41 ± 0.03b | 0.41 ± 0.03b |
| 18:3n-4 | 0.06 ± 0.00 | 0.04 ± 0 | 0.07 ± 0.03 | 0.09 ± 0.03 | 0.07 ± 0.04 |
| 18:3n-3 | 0.23 ± 0.02 | 0.27 ± 0.25 | 0.46 ± 0.43 | 0.46 ± 0.44 | 0.44 ± 0.47 |
| 18:3n-1 | n.d. | 0.07 ± 0.02 | 0.08 ± 0.03 | 0.13 ± 0.03 | 0.14 ± 0.04 |
| 18:4n-3 | n.d. | 0.06 ± 0 | 0.08 ± 0 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| 18:4n-1 | 0.38 ± 0.02 | 0.19 ± 0.03 | 0.2 ± 0.04 | 0.2 ± 0.02 | 0.24 ± 0.05 |
| 20:0 | 0.17 ± 0.00 | 0.26 ± 0.04bc | 0.26 ± 0.03c | 0.33 ± 0.03a | 0.32 ± 0.03ab |
| 20:1n-9 | 0.9 ± 0.05 | 0.91 ± 0.05 | 0.99 ± 0.08 | 0.97 ± 0.11 | 0.92 ± 0.09 |
| 20:1n-7 | 0.45 ± 0.02 | 0.29 ± 0.04 | 0.34 ± 0.04 | 0.36 ± 0.02 | 0.33 ± 0.05 |
| 20:2n-9 | 0.16 ± 0.01 | 0.16 ± 0.04 | 0.18 ± 0.07 | 0.14 ± 0.05 | 0.12 ± 0.01 |
| 20:2n-6 | 0.37 ± 0.02 | 0.32 ± 0.02 | 0.35 ± 0.04 | 0.31 ± 0.02 | 0.31 ± 0.02 |
| 20:3n-9 | 0.07 ± 0.00 | 0.047 | 0.08 ± 0.05 | 0.1 ± 0.07 | 0.192 |
| 20:3n-6 | 0.38 ± 0.02 | 0.23 ± 0.03 | 0.26 ± 0.03 | 0.23 ± 0.02 | 0.22 ± 0.05 |
| 20:4n-6 | 2.44 ± 0.19 | 1.85 ± 0.22 | 1.87 ± 0.2 | 1.98 ± 0.15 | 2.05 ± 0.16 |
| 20:4n-3 | 0.28 ± 0.02 | 0.17 ± 0.04 | 0.18 ± 0.01 | 0.19 ± 0.04 | 0.21 ± 0.03 |
| 20:5n-3 | 5.38 ± 0.33 | 3.98 ± 0.81 | 4.04 ± 0.68 | 4.36 ± 0.8 | 4.61 ± 0.83 |
| 22:1n-11 | 0.24 ± 0.01 | 0.11 ± 0.02 | 0.13 ± 0.01 | 0.15 ± 0.03 | 0.12 ± 0.01 |
| 22:4n-6 | 0.43 ± 0.03 | 0.73 ± 0.1 | 0.75 ± 0.11 | 0.93 ± 0.1 | 0.94 ± 0.11 |
| 22:5n-6 | 0.11 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.02 |
| 22:5n-3 | 2.01 ± 0.17 | 1.52 ± 0.36 | 1.58 ± 0.27 | 1.38 ± 0.36 | 1.42 ± 0.23 |
| 22:6n-3 | 8.01 ± 0.30 | 17.7 ± 4.28 | 17.66 ± 4.57 | 21.93 ± 5.33 | 22.43 ± 5.39 |
| Saturated | 39.13 ± 3.01 | 33.52 ± 3.35 | 31.69 ± 2.82 | 34.25 ± 3.76 | 33.84 ± 3.83 |
| Monosaturated | 30.6 ± 2.54 | 28.84 ± 2.36ab | 29.87 ± 2.11a | 24.54 ± 2.83b | 24.05 ± 2.57b |
| n-3 | 17.81 ± 1.43 | 25.02 ± 5.24 | 25.37 ± 5.28 | 29.82 ± 6.56 | 30.75 ± 6.22 |
| n-6 | 9.26 ± 0.66 | 9.38 ± 0.51a | 9.29 ± 0.16a | 7.43 ± 0.25b | 7.82 ± 0.36b |
| n-9 | 17.42 ± 1.32 | 21.85 ± 2.08a | 22.46 ± 1.18a | 16.32 ± 2.07b | 15.75 ± 1.62b |
| n-3HUFA | 15.67 ± 1.21 | 23.37 ± 5.46 | 23.46 ± 5.5 | 27.87 ± 6.49 | 28.66 ± 6.4 |
| ARA/EPA | 0.45 ± 0.03 | 0.47 ± 0.05 | 0.47 ± 0.04 | 0.46 ± 0.06 | 0.45 ± 0.06 |
| EPA/DHA | 0.67 ± 0.05 | 0.23 ± 0.01 | 0.23 ± 0.03 | 0.20 ± 0.02 | 0.21 ± 0.02 |
| n-3/n-6 | 1.92 ± 0.15 | 2.66 ± 0.52 | 2.74 ± 0.61 | 4 ± 0.81 | 3.96 ± 0.94 |
- n.d., not detected; DHA, docosahexaenoic acid: 22:6n-3; EPA, eicosapentaenoic acid: 20:5n-3; ARA, arachidonic acid: 20:4n-6.
- a Total lipid (g per 100 g) and fatty acid (% total identified fatty acids), values are mean ± SD, n = 3. Different letters for a given data denote significant differences among experimental groups (P < 0.05).
Discussion
Despite the particular importance of vit E as a defense against oxidized fat in fish, studies aimed to determine its significance along larval development and its relation with dietary polyunsaturated fatty acid levels are very scarce. This study pointed out the importance of dietary vit E for gilthead seabream larval growth and its interaction with dietary HUFA levels. In juveniles of the same species (1.1 g), dietary supplementation with vit E up to 1000 mg kg−1 increased fish growth (Tocher, Mourente, Van der Eecken, Evjemo, Diaz, Bell, Geurden, Lavens & Olsen 2002), particularly when fish were reared at high densities (Montero et al. 2001). In adult sea bream (150 g), the dietary supplementation with 1200 mg kg−1 vit E did not produced a statistically significant difference in SGR compared with 100, 600 and 1800 mg kg−1 vit E supplemented diet (Ortuño, Esteban & Meseguer 2000). The comparison of all these results suggested a reduction in the vit E requirements for this species in relation to increased age, being therefore much higher in very young larvae than in juveniles or adult fish.
Increased up to optimum dietary vit E levels during larval development also has been reported in different species. For instance, in larvae of striped trumpeter (Latris lineate) fed rotifers enriched with emulsions contained 40 000 mg kg−1 vit E, increased fish length significantly in comparison to those fed 0 or 5000 mg kg−1 vit E enriched rotifers (Brown, Dunstan, Nichols, Battaglene, Morehead, Anna & Overweter 2005). Indeed, analysed α-tocopherol fish body contents along larval development showed a steady increase from the first days of feeding until the end of metamorphosis in seabream (González, Izquierdo, Salhi, Hernández-Cruz & Fernández- Palacios 1995b) and sea bass (Guerriero, Ferroa, Russob & Ciarcia 2004), suggesting the high requirement of vit E during the larval stage.
In juvenile grouper (Epinephelus malabaricus) increasing of dietary vit E over 100 mg kg−1 improved the growth in two dietary lipid levels either 4 or 9 g per 100 g (Lin & Shiau 2005). In this study, increase in vit E levels up to high level markedly improved larval growth, particularly when dietary HUFA levels were lower, suggesting a higher protection value when these fatty acids are more limiting. In other fish species such as in rohu (Labeo rohita,) increased dietary vit E levels over 100 mg kg−1 improved weight gain, feed efficiency and other nutritional indices (Sau, Paul, Mohanta & Mohanty 2004). In mrigal (Cirrhinus mrigala) fry the increase from 19 to 210 mg kg−1 improved weight gain and SGR (Paul, Sarkar & Mohanty 2004).
In this study, when gilthead seabream larvae were fed higher dietary HUFA levels, increase in vit E from medium to high affected overall larval performance in terms of growth parameters and survival, and increased the HUFAs content in the larval polar lipids, despite there was the same percentage of these fatty acids in both diets. In agreement with the growth and survival results, the elevation of vit E up to high level in presence of medium HUFA levels significantly improved final larval biomass. These results denote the anti-oxidative effect of vit E, which is positioned in the membrane and competes with PUFA located at the phospholipids in donating a hydrogen atom to the lipid peroxyl radical, breaking the chain of reactions involved in lipid auto-oxidation. For instance, in rainbow trout (Oncorhynchus mykiss), increased dietary vit E contents from 300 to 1500 mg kg−1 reduced the rate of lipid oxidation in fish fillets and the formation of off-flavours (Chaiyapechara, Casten, Hardy & Dong 2003). Nevertheless, the positive effect of vit E on fish growth is not only related to its antioxidant properties, but also to other functions of this molecule in cellular metabolism, signal transduction (Traber & Packer 1995) or modulation of eicosanoid synthesis (Cornwell & Panganamala 1993), thus in the present study, at medium HUFA levels, the effects of vit E on growth were manifested, suggesting that a proportion of this vitamin is assigned to prevent HUFA oxidation, while the other proportion or proportions of this vitamin was used for the other beneficial functions.
However, with the same high HUFA levels diets (HM, HH) increasing a too high level of vit E (5909 mg kg−1, data not showed) not only did not further improved larval performance (final total length 8.40 ± 0.22 mm and 57 ± 16% survival), but also reduced PUFAs contents in larval polar lipid (26.65% total fatty acid), suggesting the pro-oxidant effect of excess vit E. Indeed, at high concentrations and under conditions where vit C and E radicals are allowed to accumulate, both antioxidants have been shown to act as pro-oxidants (Bowry, Ingold & Stocker 1992; Ingold, Bowry, Stocker & Walling 1993; Porter, Caldwell & Mills 1995). Thus, under certain conditions vit E may be present as tocopheroxyl radicals, which capture hydrogen atoms from PUFA at low rates and initiates lipid oxidation. Previous studies in our laboratory have shown that the elevation of dietary vit E up to 1500 mg kg−1 in larval seabream diets containing free ascorbic acid significantly reduced larval survival, whereas the same increase in α-tocopherol when vit C was supplemented as ascorbic acid polyphosphate caused a significant improvement in larval growth without affecting survival (Gonzalez 1997). In this study, all experimental diets were supplemented with ascorbic acid polyphosphate, several studies (Cort 1974; Packer, Slater & Wilson 1979; Niki 1987a; Niki 1987b) demonstrated the ability of vit C to reduce vit E radicals and thereby regenerate them to vit E (‘recycling’ of vit E). The beneficial combination of vit E and vit C on growth and related indices of fish has been reported in juveniles of certain species (Chavez De Martinez 1990; Roem, Kohler & Stickney 1990; Thorarinsson, Landolt, Elliott, Pascho & Hardy 1994; Chien, Hwang & Jeng 1999). In other species such as mrigal (C. mrigala) increase in dietary vit E over 210 mg kg−1 also reduced growth markedly (Paul et al. 2004).
In the present experiment, neither dietary vit E nor HUFAs contents affected significantly larval survival, confirming that neither of these nutrients was present in markedly deficient levels. In addition, the histological study of the muscle in larvae from the present experiment (data not showed) did not showed any anatomical alteration as it has been described in vit E deficient fish (Betancor et al. in press). However complete lack of vit E supplementation may cause low survival as found in other species such as juvenile golden shiner (Notemigonus crysoleucas) (Chen, Lochmann, Goodwin, Praveen, Dabrowski & Lee 2004).
Moreover, in the present experiment, comparison of results from larvae fed medium vit E at the two HUFA levels assayed showed even a better performance of the higher HUFA content fed larvae, particularly in SGR, which showed a higher DHA content but similar ARA and DPA. This fact, besides pointing out the growth promoting effect of these essential fatty acids (Izquierdo 1996), could be also related to the anti-oxidative effects of DHA reported in animal systems. DHA effectively decreases the levels of cellular lipid peroxide (Nishida, Orikasa, Ito, Yu, Yamada, Watanabe & Okuyama 2006) contradicting the classical concept that an increase in DHA levels in biological systems has deleterious effects by enhancing lipid peroxidation.
Vitamin E is well known to play an important role in stress resistance in fish (Montero et al. 2001). In this study, there was not a significant effect of vit E levels in seabream larvae resistance to air exposure or low temperature. The magnitude of both stressors has been shown to be enough to cause different response in larvae fed different levels of fatty acids (Izquierdo, Socorro, Arantzamendi & Hernandez-Cruz 2000; Liu, Caballero, El-Sayed, Izquierdo, Hernández Cruz, Valencia & Fernández-Palacios 2002). In juveniles of the same species, reduction in vit E levels from 167.5 to 18.5 mg kg−1 markedly reduced fish resistance to a chronic and acute stress (Montero et al. 2001). Despite juvenile requirements are probably lower than those for larvae, the minimum vit E levels in this study were 10 times higher than those assayed by Montero and co-workers and could be enough to prevent the damage of the type of stress assayed.
In summary, the results of the present study suggest that increasing dietary vit E level associated with medium HUFA enhanced larval growth performance in terms of total length. This indicates the importance of dietary vit E for larval growth and its interrelation to dietary HUFA.
Acknowledgments
The authors acknowledge the grant from Spanish International Cooperation Agency (AECI) to Eyad Atalah.




