Debaryomyces hansenii L2-enriched diet enhances the immunity status, gene expression and intestine functionality in gilthead seabream (Sparus aurata L.)
Abstract
This study evaluates the effects of the dietary administration of the live yeast Debaryomyces hansenii strain L2 on the immune responses of gilthead seabream for 4 weeks. Cellular immune parameters were measured from serum and head-kidney leucocytes respectively. The expression levels of immune-associated genes were quantified by real-time polymerase chain reaction. In addition, the profile of intestinal microbiota was studied by denaturing gradient gel electrophoresis. The results showed that seabream fed a diet containing D. hansenii had significantly increased cellular immune parameters. The yeast-supplemented diet up-regulated the expression of most seabream genes at week 2 and down-regulated all of them at week 4, except in the head-kidney. Finally, a reduction in the diversity of the intestinal microbiota was detected in those specimens receiving the yeast-supplemented diet. These results support the idea that the live yeast D. hansenii strain L2 stimulates the immune system of gilthead seabream.
Introduction
Debaryomyces is normally a non-pathogenic yeast classified within the Ascomycetes (Warren & Hazen 1998). Debaryomyces hansenii occurs in many habitats with low water activity: hypersaline sites, such as seawater, from which it was initially isolated, cheese, meat, wine, beer, soil and fruit (Barnett, Payne & Yarrow 2000). In agriculture, the antagonistic activity of D. hansenii isolated from the surface of fruit has been tested against Penicillum digitatum, the causative agent of green mould, in which it produced a significant reduction in the lesion area (Ahrendts & Carrillo 2004; Hernández-Lauzardo, Bautista & Velazquez 2007). To our knowledge, this strain isolated from citrus fruit has not been evaluated in animal husbandry, while in the fish industry, this is not the case; there is a broad range of data on yeasts as immunostimulants in fish (Gopalakannan & Arul 2010; Refstie, Baeverfjord, Ripman & Elvebø 2010). Yeasts contain various compounds, among them β-glucans and polyamines, which stimulate the immune system of fish (Reyes, Salinas, Cuesta, Meseguer, Tovar, Ascencio & Esteban 2008; Reyes, Tovar, Ascencio, Civera, Gracia & Barbosa 2008; Gopalakannan & Arul 2010). Recent studies showed that dietary D. hansenii stimulates both the immune and the antioxidant responses in juveniles of gilthead seabream and in leopard grouper Mycteroperca rosacea (Reyes, Salinas et al. 2008; Reyes, Tovar et al. 2008; Reyes, Tovar, Ascencio, Civera, Gracia & Barbosa 2010) after exposure to pathogens. In order to properly use this type of microorganism, it is important to evaluate its effect on bio-control activities. The genus Debaryomyces has only been studied superficially and further investigation is needed to exploit its immense potential as a tool for tailoring its biotechnological production and its use could be cheaper and easier than other commercially isolated compounds. The intake of microorganisms with probiotic potential has been demonstrated to modify the composition of intestinal microbiota or some of their groups (Gómez & Balcázar 2008; Tapia-Paniagua, Chabrillón, Díaz-Rosales, García de la Banda, Lobo, Balebona & Moriñigo 2010), but few studies have focused on the functional relationships between fish and their intestinal microbiota. The aims of the present work were (i) to determine whether D. hansenii strain L2 isolated from Mexican citrus fruit is able to tolerate low pH and different concentrations of NaCl, (ii) to evaluate the humoral and cellular immune responses occurring in vivo after including yeast cell in the diet, (iii) to evaluate the effects of this administration on the expression of immune-related genes by real-time polymerase chain reaction (PCR) and (iv) to evaluate the effects of the addition of D. hansenii L2 on the intestinal microbiota of gilthead seabream. These data will contribute to our knowledge of the efficacy of using D. hansenii L2 as a probiotic in fish farms.
Materials and methods
Live yeast
The live yeast D. hansenii strain L2 was provided by Centro de Investigaciones Biológicas del Noroeste (CIBNOR, México). Briefly, the yeast was isolated from the surface of lemon fruit and cultured by cross-streaking on YPD-agar (containing 2.0% glucose, 2.0% peptone, 1.0% yeast extract and 1.5% agar prepared with distilled water and supplemented with 0.05% chloramphenicol) at 30 °C. The yeast was removed with a bacteriological loop and suspended in YPD-medium (100 mL or 1000 mL in Erlenmeyer flasks), followed by a new incubation on a rotary shaker at 30 °C for 48 h with constant aeration until the early stationary phase was reached. Yeast cells were then removed from the growth medium by centrifugation (5 min, 1000 g, 4 °C) and the pellet was recovered, adjusting the final cell concentration.
Microbiological assays
Growth of D. hansenii L2 at different concentrations of sodium chloride
D. hansenii was grown in the presence of an NaCl YPD broth supplemented with different concentrations of salt (256, 427, 769 or 1110 mM NaCl). Six millilitres of sterile YPD containing different concentrations of NaCl was inoculated with 60 μL of a cell suspension (D. hansenii) in a 24-h YPD primary culture. Tubes were incubated on a rotary shaker at 30 °C and OD600 was determined in a microplate reader after 24, 48 and 72 h of incubation (in triplicate). Negative growth represented an OD600 comparable with the non-inoculated YPD medium containing an equivalent concentration of salt.
Resistance and growth of D. hansenii L2 at different pH
To test the pH resistance of D. hansenii L2, a viable cell population was determined by dilution and plate counting on YPD agar as described (Park, Hwang, Kim, Kim, Song, Lee, Jeong, Rhee, Kim & Kim 2006). The yeast (1.1%) was inoculated into 8 mL of YPD broth (pH 5.3), adjusted to pH 2.5, 3.5 or 4.5 with 0.1 N HCl and the flasks were placed in a thermostat adjusted to 30 °C under aerobic conditions for 30 min to simulate the gastric juices. Positive growth was represented by an inoculated YPD broth without HCl. Serial dilutions to 10−5 were prepared using YPD agar. Plates were incubated at 30 °C overnight. Each culture was performed in triplicate.
Fish and experimental design
Sixty specimens (80 ± 5 g mean body weight) of the hermaphroditic protandrous seawater teleost gilthead seabream (Sparus aurata L.) obtained from Culmarex S.A. (Murcia, Spain) were randomly placed in six running seawater tanks (10 fish per tank) (flow rate 1500 L h−1) at 20 °C with a 12 h dark/12 h light photoperiod. Fish in three aquaria were fed a non-supplemented commercial diet (ProAqua, Spain) (control group), while the fish of the other three tanks were fed the same diet supplemented with 1.1% D. hansenii strain L2 (corresponding to 106 CFU g−1, according to Tovar, Zambonino, Cahu, Gatesoupe, Vázquez & Lésel 2002) for 4 weeks. Briefly, the commercial pelleted diet was crushed, mixed with tap water (to which the yeast suspensions were added) and made again into pellets. The re-made pellets were allowed to dry and stored at 4 °C until use. The control diet underwent the same process as the experimental diet but without yeast. The viability of the yeast after incorporation into the feed was determined by colony counts on YPD agar. Briefly, 1 g aliquots of food were homogenized in 9 mL of saline, and serial dilutions were prepared to 10−5 (determining the colony counts after incubation for 24 h at 30 °C).
The control diet was administered to all fish for a 2-week conditioning period. The biomass in each aquarium was measured before the experiment and the daily ration was adjusted accordingly after each sampling. The fish were fed twice daily at 2% of their biomass. At the end of the feeding trial, the entire fish intestine was dissected to investigate the adhesion of live yeast according to the method of Sherr, Sherr and Fallon (1987).
Fish sample collection
The studies presented in this manuscript were approved by the Bioethical Committee of the University of Murcia. Five fish from each aquarium were randomly sampled at week 2 or 4 of the feeding assay. Before sampling, the fish were starved for 24 h. Head-kidney (HK) was dissected out sterilely by a ventral incision, cut into small fragments and transferred to 8 mL of an sRPM (RPMI-1640, Gibco, Grand Island, NY, USA; culture medium containing P/S, 2% FCS and 0.35% NaCl). Head-kidney leucocytes (HKLs) were isolated from each fish under sterile conditions (Esteban, Mulero, Muñoz & Meseguer 1998). Briefly, leucocytes were washed, counted in a Z2 Coulter Particle Counter (Beckman Coulter Spain S.A., Madrid, Spain) and adjusted to 107 cells mL−1 to determine the cellular immune parameters (leucocyte peroxidase, phagocytosis and respiratory burst activity).
The whole intestines from sampled fish were aseptically dissected to investigate the adhesion of live yeast according to the method of Sherr et al. (1987) or removed and stored at −80 °C for intestinal microbiota analysis. Tissue fragments from the skin, intestine, liver and HK were obtained and immediately stored at −80 °C in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) for RNA extraction.
Immunological assays
Leucocyte peroxidase activity
The peroxidase activity inside leucocytes was measured according to Quade and Roth (1997). To determine the leucocyte peroxidase content, 106 HKLs in sRPMI were lysed with 0.002% cetyltrimethylammonium bromide (Sigma, St Louis, MO, USA) and, after centrifugation (10 min, 400 g), 150 μL of the supernatants were transferred to a fresh 96-well plate containing 25 μL of 10 mM 3,3′,5,5′-tetramethylbenzidine hydrochloride (Sigma) and 5 mM H2O2. The colour-change reaction was stopped after 2 min by adding 50 mL of 2 M sulphuric acid and the optical density was read at 450 nm in a plate reader. Standard samples without leucocytes, respectively, were used as blanks. The peroxidase activity was determined defining as one unit the peroxidase that produces an absorbance change of 1 OD.
Phagocytic activity
The phagocytosis was studied by flow cytometry (Rodriguez, Esteban & Meseguer 2003). Heat-killed yeast cells were labelled with fluorescein isothiocyanate (FITC, Sigma), washed and adjusted to 5 × 107 cells mL−1 of sRPMI. Phagocytosis samples consisted of labelled yeast cells and leucocytes. Samples were mixed, centrifuged (5 min, 400 g, 22 °C), resuspended in sRPMI and incubated (22 °C, 30 min). At the end of the incubation period, the samples were placed on ice and 400 μL ice-cold PBS was added to each sample to terminate phagocytosis. The fluorescence of the extracellular yeasts was quenched by adding 40 μL ice-cold trypan blue (0.4% in PBS). Standard samples of FITC-labelled S. cerevisiae or leucocytes were included in each phagocytosis assay. All samples were analysed in a flow cytometer set to analyse the phagocytic cells. Phagocytic ability was defined as the percentage of cells with ingested yeast cells (green-FITC fluorescent cells, FL1+) within the phagocyte cell population. The relative number of ingested yeasts per cell (phagocytic capacity) was assessed in arbitrary units from the mean fluorescence intensity of the phagocytic cells.
Respiratory burst activity
The respiratory burst activity of gilthead seabream HKLs, measured as the production of reactive oxygen intermediates, was studied by chemiluminescence (Bayne & Levy 1991). Briefly, samples of 106 leucocytes in sRPMI were placed in the wells of a flat-bottomed 96-well microtitre plate, to which was added 100 μL of HBSS containing 1 μg mL−1 phorbol myristate acetate (PMA, Sigma) and 10−4 M luminol (Sigma). The plate was shaken and immediately read in a plate reader over a period of 1 h at 2-min intervals. The reaction kinetic was analysed and the maximum slope of each curve was calculated. Backgrounds of luminescence were calculated using reagent solutions containing luminol but not PMA.
Molecular analysis
RNA purification
Total RNA was extracted from 0.5 g of seabream skin, intestine, liver and HK tissue, using TRIzol RNA isolation reagent (Invitrogen). It was then quantified and the purity was assessed by spectrophotometry; 260:280 ratios were 1.8–2.0, and treated with DNase (Promega, Madison, WI, USA) to remove DNA contamination. Complementary DNA (cDNA) was synthesized from total RNA using the SuperScript™ III reverse transcriptase (Invitrogen) and using 1 μg of total RNA with the oligo-dT18 primer.
Real-time PCR
The expression of 12 selected genes (IgM, MHCIα, MHCIIα, C3, IL-1β, TLR, TNFα, CSF-1R, NCCRP-1, Hep, TCRβ and CD8) was analysed by real-time PCR using the SYBR Green PCR Core Reagents dye detection (Applied Biosystems, Foster City, CA, USA) according to Reyes, Salinas et al. (2008). Amplification was performed in a 96-well plate in a 20 μL reaction volume containing 10 μL of 2 × SYBR Green supermix, 5 μL of concentration primers and 5 μL of cDNA template. The thermal profile for SYBR Green real-time PCR was 95 °C for 10 min, followed by 40 cycles of denaturation (95 °C for 15 s), annealing (60 °C for 60 s) and extension (95 °C for 15 s). For each mRNA, gene expression was normalized to the ribosomal protein S18 content in each sample using the comparative Ct method (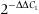 ) (fold), where the ΔCt value is determined by subtracting the average 18S Ct value from the average of each gen Ct. The results are expressed as the fold change in the yeast group compared with the control group. In all cases, each PCR was performed with triplicate samples and repeated at least twice. The primers used are detailed in Table 1.
) (fold), where the ΔCt value is determined by subtracting the average 18S Ct value from the average of each gen Ct. The results are expressed as the fold change in the yeast group compared with the control group. In all cases, each PCR was performed with triplicate samples and repeated at least twice. The primers used are detailed in Table 1.
| Gene | GenBank number | Primer sequence (5′–3′) |
|---|---|---|
| 18S | AY587263 | CGAAAGCATTTGCCAAGAAT |
| AGTTGGCACCGTTTATGGTC | ||
| IgM | AY619988 | TTACTACTGTCAGAGTTTTC |
| ATCCCAGTGAGGGGAAGC | ||
| MHCIα | DQ211540 | ATGAGGTTCTTGGTGTTTCTGG |
| TGGAGCGATCCATGTCTCTGC | ||
| MHCIIα | DQ019401 | ATGATGAAGATGATGAAGATGATG |
| TCACGCTCTGTCCGTTCTTGG | ||
| C3 | CX734936 | ATAGACAAAGCGGTGGCCTA |
| GTGGGACCTCTCTGTGGAAA | ||
| IL-1b | SAU277166 | GCTCAACATCTTGCTGGAGAGC |
| GGCACATATCCACTGGCTTG | ||
| TLR9A | AY751797 | ATTCCCGGAGTCCCAACAAG |
| GCTGCTGAACCATGAAGAAAGC | ||
| TNFα | AJ413189 | CTTCTCATCGCAGCACTTTG |
| CTCTGACAACTGGTTGGTTTCC | ||
| CSF-1R | AM050293 | ACGTCTGGTCCTATGGCATC |
| AGTCTGGTTGGGACATCTGG | ||
| NCCRP-1 | AY651258 | ACTTCCTGCACCGACTCAAG |
| TAGGAGCTGGTTTTGGTTGG | ||
| Hep | EF625900 | GCCATCGTGCTCACCTTTAT |
| CTGTTGCCATACCCCATCTT | ||
| TCRα | AY751745 | GGAAGTGGAACCAGACTGAACG |
| GATCACTCTGAGGCACAGGACG | ||
| CD8 | AJ878605 | AGGTCAAGGCCGTAACGGAG |
| CACGCTGGTGATGATGAGGAG |
Intestinal microbiota analysis
Total DNA was extracted from samples as described by Martínez, Shaw, Carrillo and Zanuy (1998). Agarose gel [1% (wt/vol)] electrophoresis in the presence of ethidium bromide was used to visually check for DNA quality and yield. In order to compare the denaturing gradient gel electrophoresis (DGGE) patterns of the intestinal microbiota of seabream receiving the different diets, the DNA was amplified using the 16S rDNA bacterial domain-specific primers 677R-GC (5′-CGGGGCGGGGGCACGGGGGGATMTCTACGCATTTCACCGCTAC-3′) and 309-F (5′-ACTCCTACGGGAGGCWGCAG-3′). The PCR mixtures (50 μL) contained 1 U Robust Taq polymerase (KapaBiosystem, Spain), 20 mM Tris-HCl (pH 8.5), 50 mM KCl, 3 mM MgCl2, 200 μM of each deoxynucleoside triphosphate, 5 pmol of the primers, 1 μL of DNA template and UV-sterilized water. The PCR was performed in a T1 thermocycler (Whatman Biometra, Göttingen, Germany) using one cycle of 94 °C for 2 min, 35 cycles of 94 °C for 30 s, 56 °C for 30 s and 68 °C for 30 min, followed by one cycle of 68 °C for 5 min. Aliquots (5 μL) were analysed by electrophoresis on 1% (wt/vol) agarose gels containing ethidium bromide to check for product size and quantity.
The amplicons obtained from the intestinal lumen-extracted DNA and the probiotic strain were separated by DGGE according to the specifications of Muyzer, De Waal and Uitterlinden (1993) using a Dcode TM system (Bio-Rad Laboratories, Hercules, CA, USA). Electrophoresis was performed in an 8% polyacrylamide gel (37.5:1 acrylamide-bisacrylamide). The gels contained a 30–55% gradient of urea and formamide increasing in the direction of the electrophoresis. A 100% denaturing solution contained 7 M urea and 40% (vol/vol) deionized formamide. The PCR samples were applied to gels in aliquots of 13 μL per lane. The gels were electrophoresed for 16 h at 85 V in 0.5 × TAE [20 mM Tris acetate (pH 7.4), 10 mM sodium acetate, 0.5 mM Na2-EDTA] buffer at a constant temperature of 60 °C (Sambrook, Fritsch & Maniatis 1989) and subsequently stained with AgNO3 (Sanguinetti, Dias-Neto & Simpson 1994). The number of DGGE bands and similarity indices were calculated from the densitometric curves of the scanned DGGE profiles using the software fpquest 4.5 (Applied Maths BVBA, Sint-Martens-Latem, Belgium). The similarity between DGGE profiles was determined by calculating a band similarity coefficient Pearson. Clustering of DGGE patterns was achieved by construction of dendrograms using the unweighted pair groups method using arithmetic averages.
In order to determine the structural diversity of the microbial community corresponding to the DGGE banding pattern, two indices were calculated: (1) the Shannon index (H′) was calculated following the function: H′=−∑Pi log Pi, where Pi is defined as (ni/N), ni is the peak surface of each band and N is the sum of all the peak surfaces of all bands, and (2) the specific richness (R) was calculated based on the total number of bands.
Statistical analysis
All bioassays were prepared in triplicate and all measurements were performed on three replicates; the mean ± SE for each immune parameter was calculated. One-way anova was performed to determine the effects of dietary live yeast on different parameters using spss v.15.0 software. Statistical differences in gene expression between control and dietary yeast samples were assessed using two-way anova to determine the effects of dietary live yeast on tissue, gen and tissue vs gen using statistica v.8.0 software. Differences were considered to be significant at P≤0.05. The intestinal microbiota analysis data were processed by analysis of variance using the program statgrafics Plus 5.0 (Statgraphics Corporation, Rockville, MD, USA).
Results
Microbiological assays
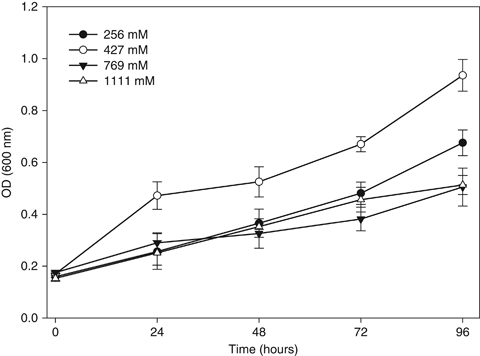
D. hansenii isolated from citrus fruit resisted high concentrations of NaCl showed an ability to grow at different concentration (256, 427, 769 and 1111 mM). More specifically, viable cell numbers increased at 427 mM of NaCl, corresponding to 2.5% of salinity (Fig. 1). To determine the selection criteria for probiotic development, the pH resistance of the D. hansenii L2 was compared at pH 2.5, 3.5 and 4.5, which yielded viability values of 88.2%, 98% and 94% respectively (Fig. 2).
|Growth ability of Debaryomyces hansenii L2 exposed to different concentrations of NaCl 256 mM (▪), 427 mM (♦), 769 mM (•) and 1111 mM (▴). Data represent the mean ± SD. The experiment was conducted in triplicate.
Resistance of Debaryomyces hansenii strain L2 isolates in YPD medium at various pH levels. The YPD was used as a control (pH 5.2). Data represent the mean ± SD. The experiment was conducted in triplicate.
Immunological assays
Peroxidase content
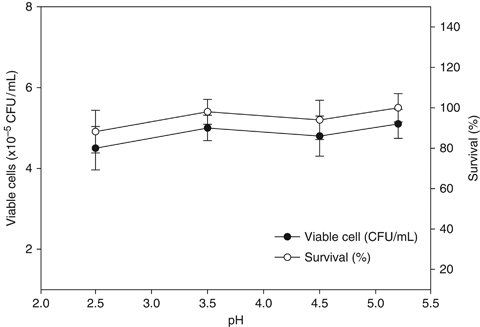
The peroxidase content of the HKL of gilthead seabream diet with D. hansenii strain L2 was significantly higher at week 4 compared with the control group (P<0.05) (Fig. 3).
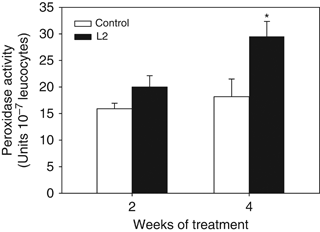
Leucocyte peroxidase activity from gilthead seabream fed non-supplemented (control) and Debaryomyces hansenii-supplemented diets (containing 106 CFU g−1). Data represent the mean+SD (n=10). Asterisk denotes statistically significant differences between the control and the yeast-fed groups (P≤0.05).
Phagocytic activity
The phagocytic ability of HKL was increased at any time of the experiment (2 or 4 weeks) in fish fed the D. hansenii L2-supplemented diet, although the increases were not statistically significant (Fig. 4). On the other hand, the phagocytic capacity of leucocytes was only significantly increased after 4 weeks of treatment (Fig. 5).
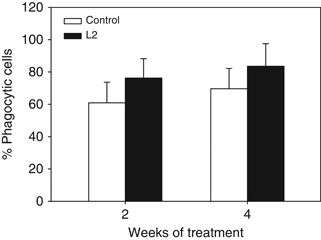
Phagocytic activity (percentage of cells with ingested yeast cells (green-FITC fluorescent cells, FL1+) within the phagocyte cell population) of head-kidney leucocytes from gilthead seabream fed the non-supplemented (control) and the Debaryomyces hansenii-supplemented diets (containing 106 CFU g−1). Data represent the mean+SD (n=10). Asterisk denotes statistically significant differences between the control and the yeast-fed groups (P≤0.05).
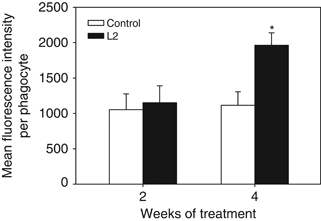
Phagocytic capacity (mean fluorescence intensity per phagocyte) of head-kidney leucocytes gilthead seabream specimens fed the non-supplemented (control) and the Debaryomyces hansenii-supplemented diets (containing 106 CFU g−1). Data represent the mean+SD (n=10). Asterisk denotes statistically significant differences between the control and the yeast-fed groups (P≤0.05).
Respiratory burst activity
The production of reactive oxygen intermediates of isolated HKL was significantly higher at week 2 but not at week 4 in fish fed the yeast-supplemented diet compared with the control group (Fig. 6).
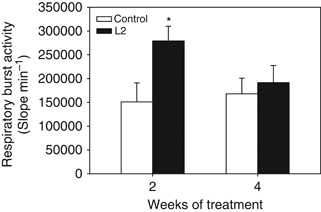
Respiratory burst activity of head-kidney leucocytes from gilthead seabream fed the non-supplemented (control) and the Debaryomyces hansenii-supplemented diets (containing 106 CFU g−1). Data represent the mean+SD (n=10). Asterisk denotes statistically significant differences between the control and the yeast-fed groups (P≤0.05).
Real-time PCR
A quantitative RT-PCR was used to estimate the regulation of immune gene expression in seabream fed with D. hansenii L2. Interestingly, the expression of most genes studied was significantly up-regulated at week 2 and down-regulated at weeks 4 (Fig. 7a and b). In this study, the number of mRNA transcripts of 12 selected immune-related genes was generally higher at week 2 in the skin, intestine and HK from fish fed the yeast-supplemented diet; however, in the liver, only IgM, TNF-α and CD8 expression was significantly affected by the experimental diet (at week 2). At the same time, the maximum transcript levels were found in the intestine for the major histocompatibility complex (MHC) genes, MHCI, MHCII, which were up-regulated more than 587- and 459-fold respectively. On the other hand, after 4 weeks, a relatively lower expression of genes was recorded in the skin, intestine and liver but not in the HK, where they were up-regulated, except for TNFα and CD8. Interestingly, the C3 transcript was up-regulated in the skin, liver and HK, although this high expression was increased more than 365-fold in the intestine of the yeast-fed group compared with the control group after 4 weeks of treatment.
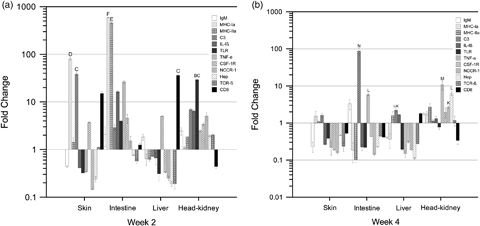
(a) Expression of immune-relevant genes determined by real-time polymerase chain reaction (PCR) in seabream following a diet with Debaryomyces hansenii L2 at week 2. Fold change in gene expression compared with the control fish is expressed. All the genes were considered to be up-regulated if the relative value was above 1 and down-regulated with a relative value below 1. Each bar represents the mean ± SD of triplicate samples. The groups marked with letters show statistically significant differences between tissue and gen. (b) Expression of immune-relevant genes determined by real-time PCR in seabream following a diet with D. hansenii L2 at week 4. Fold change in gene expression compared with the control fish is expressed. All the genes were considered to be up-regulated if the relative value was above 1 and down-regulated with a relative value below 1. Each bar represents the mean ± SD of triplicate samples. The groups marked with letters show statistically significant differences between tissue and gen.
Intestinal microbiota
A clustering analysis was applied to the PCR-DGGE patterns from the intestinal microbiota of the specimens to analyse their intravariability. In Fig. 8a, the dendrogram obtained at 2 weeks is shown, and it showed that fish fed with the control diet (non-supplemented) showed a high intragroup variability in their intestinal microbiota, with a similarity index lower than 10%. Specimens of gilthead seabream receiving the D. hansenii L2 for 2 weeks also showed a low similarity index (22%), although it was higher than that obtained for fish fed the control diet. When the same clustering analysis was carried out with fish sampled at 4 weeks, the dendrogram obtained (Fig. 8b) showed that fish receiving the D. hansenii L2 diet showed a drastic increase in their similarity index (nearly 100%), indicating that the intragroup variability of the intestinal microbiota of the specimens was very low. On the contrary, fish fed the control diet (non-supplemented diet) again showed a very high intragroup variability of the PCR-DGGE patterns derived from their intestinal microbiota.
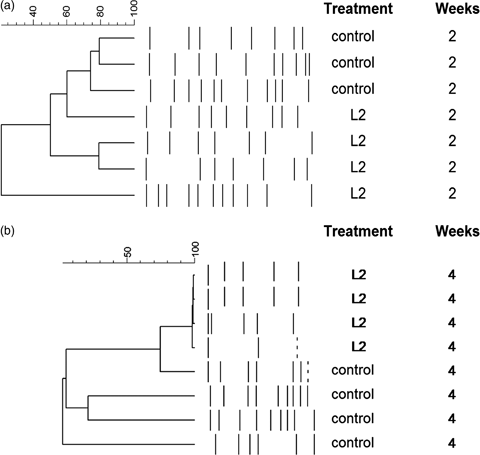
Similarity index of the DGGE patterns obtained from intestinal microbiota of Sparus aurata specimens fed a diet supplemented with Debaryomyces hansenii L2 for 2 (a) and 4 weeks (b).
Significant differences in the number of DGGE bands were detected between the diets assayed. The DGGE patterns from fish fed with the diet supplemented with D. hansenii L2 showed a lower number of bands in comparison with the DGGE profiles obtained from the specimens receiving the control diet, and this number reduced from weeks 2 to 4 (Table 2). The Shannon index (H′) calculated for fish from two treatments was significantly higher (P<0.05) in fish receiving the control diet compared with the values of the Shannon index obtained for fish fed the D. hansenii-supplemented diet. This significant difference increased with time; thus, while the H′ values for fish fed the control diet did not show significant differences at 2 or 4 weeks, the values of this index decreased significantly in fish receiving the supplemented diet for 2 or 4 weeks (1.96 and 0.66 respectively).
| Diet | Shannon index (H′) | Richness (R) |
|---|---|---|
| Control (non-supplemented) | ||
| 2 weeks | 2.004 ± 0.204 | 36 ± 1.581 |
| 4 weeks | 2.010 ± 0.213 | 36 ± 1.662 |
| D. hansenii (supplemented) | ||
| 2 weeks | 1.961 ± 0.217 | 10 ± 1.658 |
| 4 weeks | 0.661 ± 0.409 | 4 ± 0.707 |
Discussion
In the present study, the ability to grow in the presence of NaCl and the pH resistance of the D. hansenii strain L2 were tested as described above. This yeast strain could not only grow in saline environments but even showed increased cell numbers at 476 mM of NaCl (2.5% salt). D. hansenii is an important model organism for salt tolerance studies, and its mechanism of osmotolerance has been studied in detail (Hernandez-Saavedra, Ochoa & Vazquez-Dulhalt 1995; Butinar, Santos, Spencer-Martins, Oren & Gunde 2005). These investigations have shown that D. hansenii can tolerate salinity levels up to 25% and that it grows faster in the presence of 1 M NaCl than at lower concentrations. In marine aquaculture, these abilities are very important, taking into account that probiotic bacteria/yeast are exposed to the presence of salt seawater. On the other hand, when the pH of the gastrointestinal tract was simulated in the culture medium, D. hansenii L2 showed a higher per cent of survival at pH 3.5, followed by 4.5 and 2.5. The pH of the fish gastrointestinal tract ranges from 2 to 6, with an average close to pH 4 (Yúfera, Fernández-Díaz, Vidaurreta, Cara & Moyano 2004). It is reasoned that probiotic should be able to survive passage through the digestive tract and also have the capacity to multiply therein (Havenaar, Brink, Huis & Veld 1992). On the other hand, among the beneficial effects of probiotics, their capacity to enhance the fish immune system is considered to be very important (Salinas, Cuesta, Esteban & Meseguer 2005; Díaz-Rosales, Salinas, Rodríguez, Cuesta, Chabrillón, Balebona, Moriñigo, Esteban & Meseguer 2006). In the present study, cellular innate immune parameters were positively modulated by the D. hansenii-supplemented diet. Enhanced ROS production and peroxidase activity in HK leucocytes during oxidative burst had increased concomitantly at week 4, which suggests that an enhanced leucocyte microbe-killing capacity is a key factor in increased resistance to disease. Similar effects of yeast D. hansenii CBS 8339 isolated from fish have also been demonstrated in leopard grouper M. rosacea and gilthead seabream S. aurata (Reyes, Salinas et al. 2008; Reyes, Tovar et al. 2008). On the other hand, D. hansenii L2 showed a more sustained stimulatory effect in the phagocytic capacity of HK phagocytes in their phagocytic ability. As regards the respiratory burst, this activity was significantly increased in leucocytes from fish fed yeast-supplemented diets for 2 weeks. Respiratory burst activities are produced by phagocytes in order to attack invasive pathogens during phagocytosis, which suggests that an enhanced leucocyte microbe-killing capacity is a key factor in increased resistance to disease (Panigrahi, Kiron, Puangkaew, Kobayashi, Satoh, & Sugita 2005), indicating that respiratory burst was well correlated with the present results of phagocytic capacity. Real-time PCR was performed to analyse the expression of IgM, MHCIα, MHCIIα, C3, IL-1β, TLR, TNFα, CSF-1R, NCCRP-1, Hep, TCRβ and CD8 genes in different tissues, including the skin, intestine, liver and HK of seabream. The present work demonstrates that yeast-supplemented diets up-regulated the expression of these 12 genes after 2 weeks, especially in the skin, intestine and the HK. In contrast, yeast-supplemented diets down-regulated the expression of these genes after 4 weeks of feeding; however, these genes were only up-regulated in the HK, one of the main haemopoietic organs. D. hansenii diet produced a greater up-regulation of C3 at all sampling time points, this expression being 100-fold higher in the intestine and skin. Fish have a well-developed complement system that plays an important role in their innate immune response, including membrane attack complex (which lyses bacteria), opsonization and phagocytosis (Boshra, Li & Sunyer 2006). On the other hand, the mRNA transcript levels of MHC I, MHC II and CD8 showed a tendency to increase in the intestine at 2 weeks, suggesting the importance of the intestinal tract as a site of immune activity. Genes of the MHC encode two classes of structurally similar, but functionally distinct, glycoproteins, MHC class I and class II, both of which are involved in the immune response at various levels (Chen, Xu, Hu & Li 2004). CD8 is involved in the recognition of class I MHC molecules by developing thymocytes and mature cytotoxic T lymphocytes (Janeway 1992; Zamoyska 1994). NCCR-1 and TNF-α genes in the HK were considerably enhanced at week 4 in fish fed the yeast-supplemented diet. NCCR-1 genes encode leucocyte receptors that, in some instances, might be considered to be cell markers and several studies have concluded that they are very good candidates to this end (Cuesta, Esteban & Meseguer 2005). On the other hand, TNF-α is a key mediator in the response to microbial invasion and tissue injury and it can stimulate immune responses by activating lymphocytes, or by inducing the release of other cytokines capable of triggering macrophages, NK cells and lymphocytes (Panigrahi, Kiron, Satoh, Hirono, Kobayashi, Sugita, Puangkaew & Aoki 2007). Finally, the expression of genes for B and T lymphocytes in the HK was considerably enhanced at all sampling time points in fish fed a yeast-supplemented diet. However, the regulation mechanisms by which these genes are down-regulated in the liver and skin, but up-regulated in the HK and intestine, due to the presence of yeast in the microbiota, remain to be investigated. Bacterial diversity plays an important role in the function of microbial ecosystems (Bell, Newman, Silverman, Turner & Lilley 2005). The evidence indicates that the gut microbiota is an important component of the mucosal barrier has resulted in the promotion of the use of probiotics (Gómez & Balcázar 2008). In this study, a higher similarity of the PCR-DGGE patterns obtained from the intestinal microbiota of specimens fed the yeast-supplemented diet has been detected in comparison with fish receiving the control diet, especially after 28 days (4 weeks). These results suggest that the addition of the microorganism to the feed could exert an important influence on intestinal bacterial groups and yield a faster homogenization of the intestinal microbial community (Tapia-Paniagua et al. 2010). The intestinal microbiota of fish play an important role as a defensive barrier against pathogens (Ringø & Olsen 2008; Ringø, Løvmo, Kristiansen, Bakken, Salinas, Myklebust, Olsen & Mayhew 2010), and it can regulate the expression of a huge number of genes in the digestive tract involved in the epithelial proliferation, promotion of nutrient, metabolism and immune response (Rawls, Samuel & Gordon 2004; Rawls, Mahowald, Goodman, Trent & Gordon 2007). In this study, a stimulatory effect on the immunological system induced by the supplementation of feed with D.hansenii strain L2 has been detected. However, this immunostimulatory influence varied depending on the time that fish were receiving the diet supplemented with the microorganism. After 2 weeks of feeding the supplemented diet, fish showed an up-regulation of the expression of all genes studied in the skin, HK and intestine. At this time, the status of the intestinal microbiota corresponded to a high intragroup variability in both the experimental groups. On the contrary, 2 weeks later, fish receiving the yeast supplemented diet showed a down-regulation of the expression of these same genes in the intestine and skin, but an up-regulation in the HK. This different effect on the expression of genes in different organs corresponded to a very homogeneous status of the intestinal microbiota of fish fed the yeast-supplemented diet. It would very suggestive to think that the use of microorganisms such as this yeast could facilitate modification of the immunological status of fish, through the induction of changes in the intestinal microbiota. However, more research is necessary to understand the real role played by the microorganisms introduced through the diet on the changes in the ecology of the intestinal microbiota and the relationship with the changes in the immunological status of fish.
In summary, we report that D. hansenii L2, a non-indigenous yeast isolated from citrus fruit, can be considered to be a probiotic candidate to enhance the seabream immune system. Application for 1 month was sufficient to improve the immune status. However, further extensive testing is recommended, including microbial analyze, such as colonization assays, and challenge experiments in fish to observe their potential protective effectiveness against the opportunistic pathogens that cause economic losses in fish farming. This approach may well provide a safe novel treatment for use as a feed additive for fish farming. However, further extensive testing is recommended to evaluate the actual role played by the probiotic microorganisms on the host under changing environmental conditions and stressful situations.
Acknowledgments
We thank Rebeca Cerezuela and Francisco A. Guardiola for technical support. The first author received postdoctoral scholarships from Consejo Nacional de Ciencia y Tecnología of México (CONACYT grant 164653). This work was partially funded by the Ministerio de Ciencia e Innovación (AGL2008-05119-C02-01 and AGL2008-05119-C02-02, Spain) and Fundación Séneca de la Región de Murcia (06232/GERM/07).




