Immunomodulatory effect of recombinant RNA-dependent RNA polymerase protein of Macrobrachium rosenbergii nodavirus in giant freshwater prawn M. rosenbergii
Abstract
The present study evaluated the role of recombinant RNA-dependent RNA polymerase (RdRp) protein of Macrobrachium rosenbergii nodavirus (MrNV) in modulating the immune response and in reducing MrNV load in infected prawn. In the first experiment, prawns (25–30 g) were injected with recombinant RdRp protein (RP) at a concentration of 0, 1.0 and 10 μg, and immune parameters and expression of some immune-related genes were measured up to 14 days post injection (p.i.). In the second experiment, early juveniles were injected with a similar dose of RdRp and animals were challenged by immersion with MrNV. The infection status was detected in muscles by nested RT-PCR up to 21 days post challenge. Prawn injected with higher concentration of RP showed significantly higher total haemocyte count at different period post injection. Significant up-regulation of immune-related genes was observed within 24 h in prawn treated with lower dose of RP and after 7 days p.i. at higher level of RP injection compared with adult control. Most of the tested samples (63%) were found to be RT-PCR positive for MrNV at 48 h of post-immersion challenge. After 14 days, MrNV was detected only in control prawn, while both RP-injected groups were MrNV negative. This study elucidated the potential viral load reduction role played by RdRP in MrNV-infected prawn.
Introduction
The giant freshwater prawn Macrobrachium rosenbergii is one of the most important cultured crustacean species with high economic value in global scenario and undoubtedly a major contributor of aquaculture production. Increasing demand of M. rosenbergii in domestic as well as export market has led to a large-scale production through intensification of culture practices. The poor husbandry condition of the hatcheries as well as high stocking density and transport of brood stocks in adverse environmental conditions have led to the emergence of new infectious diseases. Since 2001, Macrobrachium hatcheries situated on the south-coast of India are facing a heavy loss because of an emerging disease called white tail disease or white muscle disease. Among the major viral diseases encountered by the giant freshwater prawn M. rosenbergii, white tail disease caused by Macrobrachium rosenbergii nodavirus (MrNV) is considered as the most devastating one. This virus-borne disease was first observed in the Island of Guadeloupe in 1995, followed by West Indies, Taiwan, China, Bangkok, India and most recently in Australia (Arcier, Herman, Lightner, Redman, Mari & Bonami 1999; Sahul Hameed, Yoganandhan, Sri Widada & Bonami 2004a; Shekhar, Azad & Jithendran 2006; Owens, Fauce, Jantunen, Hayakijkosol & Zeng 2009). The causative agent of WTD is identified as MrNV and is included in Nodaviridae family. The WTD-affected larvae and postlarvae show lethargy, milky whitish coloration of tail region and abdominal muscle, degeneration of telson and uropods, muscle necrosis and 100% mortality within few days. The discoloration starts at the abdominal segments and extends to anterior and posterior parts of the body. Mainly, the postlarvae and early larvae are more prone to this disease. It may be due to their poor immunity, whereas the adults are found to be the carrier of this virus (Bonami, Shi, Qian & Sri Widada 2005).
Macrobrachium rosenbergii nodavirus is an icosahedral, non-enveloped particle, 26–27 nm in diameter and has a genome with two pieces of single-stranded RNAs (ssRNA). RNA-1 encodes for an important viral enzyme RNA-dependent RNA polymerase (RdRp) of 2.9 kb and capsid protein translated from RNA-2 of 1.3 kb (Qian, Shi, Zhang, Cao, Liu, Li, Xie, Cambournac & Bonami 2003). During viral replication, a subgenomic RNA transcribed from RNA-1 was found to encode B2, a non-structural protein that is able to inhibit the cellular RNA interference (Pillai, Bonami & Sri Widada 2006). Genomic RNA1 of the virus is translated to RdRp that helps in the replication of virus and in the regulation of its development in the host cell (Bonami & Sri Widada 2011). RdRp was found to be the major target protein, which helps in the stability of the virus, containing the machinery for the production of capsid and other subunit RNAs (Wu, Lu & Chi 2010).
Although considerable progress has been made in MrNV and XSV molecular characterization (Pillai et al. 2006; Tripathy, Sahoo, Kumari, Mishra, Sarangi & Ayyappan 2006), no preventive or control measure has been worked out for this disease. It was mostly attributed to the poor understanding of MrNV/XSV replication mechanism as well as to molecular processes of the prawn immune responses being compromised during virus infections. On the other hand, considerable progress has been made on the use of using immunostimulants, rendering protection to various crustacean diseases (Balasubramanian, Sarathi, Venkatesan, Thomas & Sahul Hameed 2008) but these are not adequate to meet the demand in a fool-proof manner. They may control the disease from the hatcheries but the chances cannot be excluded from the larva and postlarvae from being infected in the grow-out stages. Hence alternatively, vaccine and immunization might offer an effective way for protection against the disease on a long-term basis, probably even in presence of short immune response and could be the most potential strategy to overcome the current situation. Recently, many authors have described about quasi-immune response in shrimp where the naturally virus-survived animals were protected against subsequent viral challenge (Venegas, Nonaka, Mushiake, Nishizawa & Muroga 2000). Various viral recombinant proteins have been studied so far as vaccines in different host systems with notable effects against pathogens (Heras, Prieto & Saint-Jean 2009). A high degree of protection was induced in Marsupenaeus japonicus against WSSV by intramuscular injection of WSSV-recombinant proteins, rVP26 and rVP28 (Namikoshi, Wu, Yamashita, Nishizawa, Nishioka, Arimoto & Muroga 2004; Caipang, Verjan, Ooi, Kondo, Hirono, Aoki, Kiyono & Yuki 2008). Thus, the development of subunit ‘vaccines’ based on MrNV replication-related proteins would be feasible and desirable as these proteins might interact with virus inside the host or triggering the host defence. RdRp plays crucial role in virus replication. Hence, recombinant RdRp protein (RP) may act as an immune modulator by producing immune factors against the protein, which will help in down regulation of MrNV progeny RNA synthesis (Wu et al. 2010). In order to find the immune-relevant factors in prawn modulated after exposure to RdRp, some of the differentially expressed genes were identified and their expression profiles in the hepatopancreas of the M. rosenbergii were studied. Prophenoloxiadase (PPO) enzyme is a well-known innate immunity-related enzyme of prophenol oxidase system, which leads to melanization and production of superoxide radicals, which are bactericidal in nature (Soderhall & Cerenius 1992). Pattern recognitions proteins like β-glucan binding proteins (BGBP) activates the PO system and are expressed abundantly in hepatopancreas (Wang, Chang & Chen 2007). α-2 macroglobulin (A2M) is a non-specific protease inhibitor, which plays a major role in inhibition and removal of harmful proteases as well as delivery of cytokines (Ho, Cheng & Cheng 2009). Cytochrome oxidase (cytox) gene is recognized as a part of respiratory pathway of host that helps in the production of superoxide radicals. During the process of phagocytosis, superoxide radicals are formed, which need to be eliminated promptly. Hence, antioxidant enzymes like superoxide dismutase (SOD) are used to convert toxic superoxide radicals to molecular oxygen.
In our previous study, we have cloned, expressed and characterized RP of MrNV (Shekhar, Sahoo, Dillikumar & Das 2011). The present study was carried out to measure the immunomodulatory role of two different dose levels of RP in juveniles of M. rosenbergii by looking into alterations in various haemolymph immune parameters and innate immune-related gene expression in hepatopancreas. The present study also measured the virus clearance efficiency of RP after challenging RP-exposed early juveniles of prawn with MrNV.
Material and methods
Prawn
The experimental animals M. rosenbergii (25–30 g, intermoult stage) were collected from the prawn complex of CIFA, Bhubaneswar, India, and acclimatized for 1 week at the wet laboratory before experimentation. During the experiment, the water temperature was maintained at 28±2 °C, pH 7.5–8.0, total hardness 80–100 mg L−1, dissolved oxygen content 6–7 mg L−1 and ammonia at <0.1 mg L−1. The prawns here were fed with a commercial pellet feed twice a day.
Preparation of recombinant RdRp
Plasmids [pET32a (+) vector, Novagen, Darmstadt, Germany with RdRp insert] isolated from the positive clones of DH5α cells were transformed into competent BL21 (DE3) pLysS cells for expression of recombinant protein as described previously (Shekhar et al. 2011). Briefly, MrNV RdRp was expressed as a recombinant fusion protein (44.5 kDa) in Escherichia coli and was analysed by SDS-PAGE. Purification of RP was carried out using an affinity column and purity checked in Western blot using anti-His mouse monoclonal antibodies.
Immunomodulation study
One hundred and twenty-six prawns were randomly distributed into three groups, Group A, B and C. In each group, 42 prawns were equally distributed in three tanks. The prawns were fed with standard commercial pellet feed during the whole experimental period at 4% of their body weight in two divided doses. In Group A, the prawns were individually injected with 100 μL of PBS intramuscularly into the second abdominal segment and were considered as the control group, whereas Groups B and C were injected individually with a concentration of 1 and 10 μg of RP dissolved in 100 μL of PBS, respectively, for immunomodulation study. After 0, 6, 12, 24, 72 h, and 7 and 14 days of injection, various immune parameters were studied to evaluate the immunomodulatory role of RP, and hepatopancreas was collected to study the expression of immune genes in immunized prawn.
Immune parameters
Total haemocyte count
Haemolymph (50 μL) was collected from the ventral sinus cavity of six prawns of each group for each time period using a 26-Gauge needle and 1 mL syringe containing 450 μL anticoagulant (sodium chloride 0.45 M, glucose 0.1 M, sodium citrate 30 mM, citric acid 26 mM, EDTA 20 mM, pH 4.5) and fixative solution (sodium cacodylate 0.1 M and 1.5% glutaraldehyde) in 1:1 ratio at 0, 6, 12, 24, 72 h, and 7 and 14 days p.i. for total and differential haemocyte counts. A drop of haemolymph was placed on the haemocytometer slide and different types of cells were identified according to Sierra, Guevara, Lasscurain, Perez, Agundis, Zenteno and Vazquez 2001. For haemolymph supernatant collection, haemolymph without anticoagulant was collected, allowed to clot and subsequently the clot was broken using a sterile needle and left at 4 °C for 2–3 h. The tube was then centrifuged for 10 min at 5000 × g, the supernatant was collected and kept at –30 °C for further use. Part of the supernatant was used to measure the total protein following the Bradford method (Bradford 1976) and remaining 50 μL was used for haemagglutination (HA) titre measurement using 1.5% rabbit RBC.
Preparation of rabbit RBC suspension
A rabbit maintained at the Institute animal house was bled and the blood was collected in Alsever's solution in a 1:1 ratio. Further, it was centrifuged at 4000 × g for 15 min and the supernatant was discarded. The packed RBCs was washed thrice with sterile PBS (containing Ca2+ and Mg2+, pH 7.3) and resuspended at 1.5% v/v in PBS for the measurement of HA titre (Sahoo, Pillai, Mohanty, Kumari, Mohanty & Mishra 2007). Briefly, double serial dilution of the haemolymph supernatant was made in PBS (with Ca2+ and Mg2+), and then 50 μL of 1.5% RaRBC was added to each well of the microtitre plate and incubated for 1 h at 37 °C. The HA titre was defined as the last dilution of serum showing minimal positive agglutination. Values were expressed as the reciprocal of HA titre.
Expression analysis of immune-related genes
Similarly for gene expression studies, hepatopancreas from four individual prawns were collected in RNAlater (Sigma, St. Louis, MO, USA) after bleeding for each time period of a group and RNA extraction was performed using TRI reagent (Sigma). To minimize the chances of genomic DNA contamination, the RNA was treated with DNAse I (Fermentas, Glen Burnie, MD, USA) and subsequently inactivated before reverse transcription following the instructions of the manufacturer. The purity and quantity of RNA were determined by measuring the ratio of optical density at 260/280 nm using a Nanodrop 1000 (NanoDrop Technologies Inc., Wilmington, DE, USA). Total RNA was reverse-transcribed taking 1 μg of RNA for the preparation of cDNA. Expression of five immune-related genes viz., BGBP, A2M, PPO, cytox and manganese superoxide dismutase (MnSOD) and a house-keeping gene β-actin was measured by semi-quantitative RT-PCR method. Each PCR reaction consisted of 40.70 μL of dH2O, 5 μL of 10 × PCR buffer, 1 μL of 10 mM dNTPs, 1 μL (10 pmol) of each forward and reverse primers, followed by 0.3 μL (1.5 U) of Taq DNA polymerase and 1 μL of cDNA. The amplification profile was 94 °C for 3 min followed by 39 cycles of denaturation for 45 s at 94 °C, at an appropriate annealing temperature for 45 s and extension at 72 °C for 1 min 30 s, followed by a final extension for 10 min at 72 °C. The primers used and the annealing temperature details are given in Table 1.
| Target gene | Primer name | Primer sequence (5′–3′) | Optimum annealing temperature (°C) | Size of PCR amplicon (bp) | Primer source |
|---|---|---|---|---|---|
| β-actin | MrBAF | TAG GTG GTC TCG TGG ATG CC | 59.0 | 438 | Liu, Tseng, Lai, Cheng & Kuo (2006) |
| MrBAR | GAG ACC TTC AAC ACC CCC GC | ||||
| α2-macroglobulin | A2M F | CGA GGT GCG AAC AGG AAG | 59.7 | 245 | FL657275 |
| A2M R | CCG GGC AGG TAC TGT GAC | ||||
| Prophenol oxidase | PPO F | GGA AGA GTT TTC TCC GT | 54.0 | 879 | Liu et al. (2006) |
| PPO R | GAA GTT GTG GAG GTC TC | ||||
| Cytochrome oxidase | Cyt ox F | CGG GCA GGT ACC CCT AAT A | 55.3 | 162 | FL657276 |
| Cyt ox R | GCC GCT AGT GGT GGA TAA AC | ||||
| Manganese superoxide dismutase | MnSOD F | TAC CTG CCA TCA AGT TCA A | 47.5 | 398 | EU077526.1 |
| MnSOD R | GTA CCG CTC GTT TAC ATT AG |
Agarose gel electrophoresis
The generated PCR products were analysed by electrophoresis on 1.0% agarose gel. The relative levels of expression of each gene were analysed by densitometry using alphaease®fc imaging software (Alpha Innotech, San Leandro, CA, USA). The ratios of immune-related genes/β-actin product were subsequently calculated after subtraction of the background pixel intensity for each gene of interest and mean values (±SE) were calculated.
Statistical analysis
Statistical differences in gene expression and immune parameters between experimental and control samples in each group were assessed using one-way anova. Means were compared with Duncan's multiple range test and the difference was considered significant when P<0.05.
Virus load reduction efficiency study
The prawn early juveniles utilized in this experiment were obtained from CIFA hatchery that had no previous history of MrNV infection. Further, the representative animal samples were screened for absence of MrNV using nested RT-PCR as detailed later. One hundred and twenty-six early juveniles (1–2 g) were distributed randomly into three groups A, B and C, having forty-two prawns in each group and the experiment was performed in triplicate. In Group A, the prawns were individually injected with 20 μL of PBS intramuscularly into the abdominal segment and were considered as control, whereas groups B and C were injected with a concentration of 1 and 10 μg of RP protein dissolved in 20 μL of PBS respectively. After 24 h of injection, an immersion challenge with MrNV suspension was performed. In the immersion challenge, 14 juveniles from each triplicate of a group were placed in a 2 L beaker containing freshwater with continuous aeration. The MrNV inoculum (obtained from OIE Reference Laboratory for WTD, Melvisharam, Vellore, India) was actually prepared from infected PLs collected from different hatcheries of Andhra Pradesh, India, and challenged at 0.1% of the total rearing medium (1 mL L−1), following the protocol of Ravi, Basha, Taju, Ram Kumar and Sahul Hameed (2010). After 3 h of exposure to the virus suspension, the animals were transferred to the glass jars containing freshwater and fed with artemia. The experimental animals were examined twice per day for clinical signs of WTD. The moribund/live animals were collected at 24, 48 h and 7, 14 and 21 days of post-challenge.
Virus detection using nested RT-PCR
In the challenge experiment, RNA from muscle samples were extracted using TRI reagent (Sigma) as per manufacturer's instruction and a nested RT-PCR was performed using published primers with CIFA-developed nested RT-PCR-based diagnostics using MrNV-specific primers (Anonymous 2004). Briefly, RNA was extracted from the tissues and RT was performed taking 5 μg of RNA as template for preparation of cDNA. PCR was carried out using two pairs of self-designed primers (designed from the sequence data of MrNV RNA-2, capsid protein) and 2.5 μL of cDNA. The steps of nested PCR are denaturation at 94 °C for 2 min followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 45 °C for 30 s and elongation at 72 °C for 1 min and further at 72oC for 10 min. The RT-PCR products were analysed by electrophoresis on 1% agarose gel to detect the presence of virus.
Results
Non-specific immune parameters
The effect of different concentrations of RdRp on total and differential haemocyte counts, HA titre and total protein concentration in haemolymph supernatant is determined and the results are presented in Table 2. Total haemocyte count was significantly (P<0.05) higher at 6, 24 h and 14 days p.i. of RdRp in comparison with control. A minor variation in different cell types was observed at 14 days of RdRp injection. There was no significant alteration in total protein concentration and HA titre in response to RdRp injection in M. rosenbergii.
| Time period | Group | THC (107 cells/mL) | Fusiform cells (%) | Large ovoid cells (%) | Undifferentiated cells (%) | Haemagglutination titre (log 2) | Total Protein(g/dL) |
|---|---|---|---|---|---|---|---|
| 0 h | A | 0.76±0.05 | 87.66±0.82 | 8.04±0.61 | 4.37±0.24 | 2.75±0.14 | 9.81±0.49 |
| B | 0.76±0.05 | 87.66±0.82 | 8.04±0.61 | 4.37±0.24 | 2.75±0.14 | 9.81±0.49 | |
| C | 0.76±0.05 | 87.66±0.82 | 8.04±0.61 | 4.37±0.24 | 2.75±0.14 | 9.81±0.49 | |
| 6 h | A | 0.31±0.04a | 78.83±4.16 | 14.65±3.21 | 6.63±0.79 | 4.5±1.04 | 8.41±1.21 |
| B | 0.55±0.06 b | 75.06±3.43 | 15.9±1.03 | 8.24±1.67 | 3.67±0.83 | 7.09±1.06 | |
| C | 0.51±0.03 b | 76.55±3.7 | 16.35±2.6 | 7.29±1.33 | 5.5±0.29 | 7.61±0.63 | |
| 12 h | A | 0.56±0.16 | 84.83±1.47 | 9.55±1.14 | 4.61±0.16 | 5.5±0.5 | 6.78±0.6 |
| B | 0.55±0.07 | 82.11±1.79 | 10.79±1.59 | 5.91±0.84 | 4± 0.76 | 6.59 ± 0.24 | |
| C | 0.7±0.11 | 82.72±0.71 | 11.06±0 | 4.86±0.06 | 4.75±0.43 | 7.43±0.9 | |
| 24 h | A | 0.59±0.06 a | 86.77±1.12 b | 8.98±0.7a | 4.14±0.44 | 3.17±0.6 | 6.63±0.6 |
| B | 0.63±0.05 a | 85.05±1.28ab | 10.2±0.83a | 4.73±0.44 | 4.17±1.36 | 7.92±0.81 | |
| C | 0.9±0.05b | 80.35±1.73 a | 13.84±1.13b | 5.44±0.34 | 6± 0 | 6.86 ± 0.43 | |
| 72 h | A | 0.85±0.07 | 81.66±1.8 | 12.41±1.67 | 5.69±0.17b | 6± 0 | 8.19 ± 0.95 |
| B | 0.69±0.08 | 86.96±1.14 | 8.52±1.36 | 4.85±0.18ab | 4± 0.87 | 7.47 ± 0.5 | |
| C | 0.89±0.13 | 85.77±3.48 | 8.03±0.94 | 3.89±0.52a | 2.67±1.45 | 9.85±1.34a | |
| 7 days | A | 0.67±0.04 | 86.87±1.41 | 8.55±0.73 | 4.56±0.69 | 4.67±0.88 | 7.28±1.01 |
| B | 0.81±0.06 | 86.09±1.99 | 7.67±0.61 | 5.84±1.57 | 5.75±0.14 | 9.18±0.97 | |
| C | 0.76±0.09 | 88.68±1.64 | 7.25±0.76 | 3.77±0.48 | 4.67±0.6 | 8.12±0.74 | |
| 14 days | A | 0.42±0a | 87.46±0b | 8.28±0b | 4.25±0b | 5.17±0.44 | 9.2±0.24 |
| B | 0.84±0b | 85.59±0.14a | 8.68±0c | 5.97±0c | 5± 0 | 9.26 ± 0.42 | |
| C | 0.91±0c | 89±0.58c | 7.06±0.01a | 3.91±0.05a | 4.83±0.73 | 8.23±0.47 |
- Means bearing different superscript alphabets at a particular time period in a column differ significantly (P<0.05). Group A: PBS control, Group B: injected with 1 μg of RdRp protein (RP) and Group C: injected with 10 μg of RP.
Expression of immune-related genes
A significant (P<0.05) up-regulation in BGBP gene was observed at 6 and 12 h p.i. in prawn injected with a lower concentration of RdRp, whereas the prawn treated with 10 μg of recombinant protein showed its peak at 7 and 14 days in comparison with control. A significant higher expression level of A2M was observed at 6 and 12 h in Group B prawn and 72 h and 7 days p.i. in Group C prawn and returned to control prawn level at 14 days p.i. The expression of PPO and MnSOD genes was significantly higher in Group B prawn at 6, 24 h p.i. and Group C animals at later time periods (14 and 7 days of injection respectively) compared with control. The transcript level of cytox was significantly higher at 6, 12 and 24 h p.i. in animals treated with lower dose of protein, whereas the peak levels of expression in higher dose animals were observed only at 7 days p.i. However, the expression level of the above genes returned back to control prawn levels at 14 days of post-RdRp injection, except for PPO and BGBP levels. There was marked up-regulation in most of the genes at lower dose of RdRp injection within 6–24 h in comparison with higher dose, whereas most of the genes were up-regulated after 7 days post-injection (p.i.) at higher level of RdRP administration (Fig. 1).
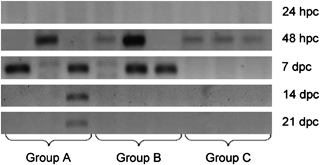
Bands showing the presence of viral transcripts at different interval of post challenge. Group A: injected with phosphate buffered saline only; Goup B: injected with 1 μg of recombinant RNA-dependent RNA polymerase (RdRp) and Group C: injected with 10 μg of recombinant RdRp.
Viral load reduction efficiency
In the second experiment, none of the groups showed the presence of the virus at 24 h of post challenge (p.c.). However, in all the three groups, most of the samples (63%) showed positive for MrNV at 48 h of post-immersion challenge. At 7 days of p.c., presence of virus was confirmed in almost all the samples of Groups A and B; however, virus was not detected in any of the samples of group C. At 14 and 21 days p.c., MrNV could be only detected in PBS-injected group, while all RdRp-injected groups showed negative for the presence of this virus (Fig. 2).
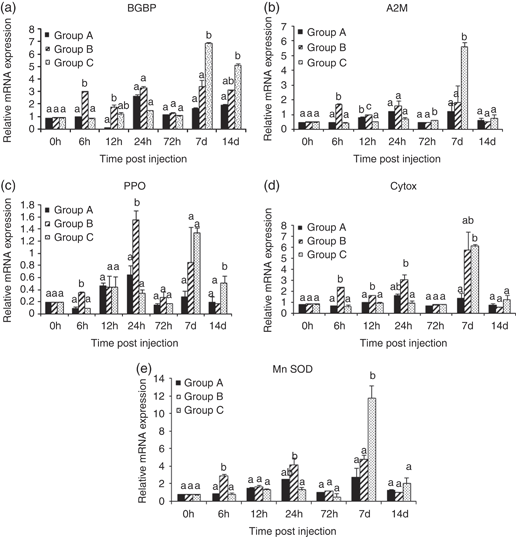
Effect of different doses of RNA-dependent RNA polymerase on expression of (a) β-glucan binding proteins (BGBP), (b) α-2-macroglobulin (A2M), (c) prophenoloxiadase (PPO), (d) cytochrome oxidase (ctyox) and (e) manganese superoxide dismutase (MnSOD) transcripts measured at different time periods post injection. Group A serves as phosphate buffered saline control, Group B injected with 1 μg of RdRp protein (RP) and Group C injected with 10 μg of RP. Bars showing different letter at a particular time differ significantly (P<0.05).
Discussion
The present study was carried out to find the efficacy of the recombinant RP of MrNV as an immunomodulator in modulating the immune status to inhibit the viral replication and propagation in infected prawn. In the first experiment, prawns were used to check the immunomodulatory effect of RP and in the second experiment, early juveniles were taken to study the virus load reduction. It is well understood that adult animals act as carriers of infection, whereas early stages (larvae, postlarvae and juveniles) are more prone to infection and mortality (Sahul Hameed, Yoganandhan, Sri Widada, Bonami 2004b).
Immune-relevant molecules from crustacean haemocytes play an essential role in defence against invasive pathogens (Johansson & Söderhäll 1989). THC reflects the immune status of a shrimp as it is involved in many cellular mechanisms such as phagocytosis, encapsulation, nodule formation and phenol oxidase activity. (Pipe & Coles 1995). Increase in THC is considered as a consequence of rapid proliferation of haematopoietic cells in host in order to respond to any stimulant or foreign pathogens (Liu, Soderhall & Jiravanichpaisal 2009). In this experiment, a significant increase in THC was observed at 6 and 24 h in M. rosenbergii treated with RP signifying proliferation of haemocytes in response to a viral protein. The increase in haemocyte count was also well correlated with an increase in the expression of PPO transcripts at the same time periods. This increase in THC along with PO activity indicates a crucial role against viral infection by host immune system. Ji, Yao and Wang (2009) observed a similar increase in THC at 6 h to 24 h in Litopenaeus vannamei after injection of laminarin and up to 12 h when the shrimp was immunized with poly I:C. However, Braak, Botterblom, Huisman, Rombout and van der Knaap (2002) suggested that an exposure to WSSV can cause loss of circulating haemocytes, which ultimately leads to death of animal. The MrNV affects the haemocyte proliferation in infected M. rosenbergii. Ravi et al. (2010) observed a significant reduction of THC in M. rosenbergii injected with MrNV and XSV on day 1 and 3 p.i. Therefore, the immediate regeneration of haemocytes may be a critical factor for the survival of animals after MrNV infection. Hence, the increase in THC by induction of RdRp as observed in this study indicates a possible beneficial role in protecting prawn from WTD infection.
Agglutinins in several crustaceans have been characterized as heat-labile and calcium-dependent immune-reactive molecules (Acharya, Mohanty & Sahoo 2004). The production of agglutinins, bactericidin and lysins following exposure to pathogens is related to the increase in resistance of the host. Apart from this, the total protein content of an animal is also an indicator of its good physical and nutritional status. However, in the present experiment no significant alteration was observed in agglutinin and in total protein levels of the host in response to RP injection.
The pattern recognition receptors (PRRs) like BGBP are shown to be a part of innate immune system and act as an acute phase protein by recognizing the carbohydrate structures in the microbial cell surface (Srityunlucksana, Lee & Soderhall 2002). Cellular mRNAs are capped, while viral RNAs have a 5′-phosphate that can be recognized by cellular PRRs (Pichlmair, Schulz, Tan, Naslund, Liljestrom, Weber & ReiseSouca 2006). Thus, ssRNA, dsRNA and viral glycoproteins constitute the basic structures, whereby PRRs recognize an invading virus and lead to production of cytokines (Workenhe, Hori, Rise, Kibenge & Kibenge 2009). BGBP expression has also been shown to be increased during WSSV infection in shrimp (Penaeus stylirostris) (Roux, Pain, Klimpel & Dhar 2002). BGBP is mainly expressed in the hepatopancreas (Wang, Chang & Chen 2008). We observed a significant up-regulation of BGBP transcript levels at 6 and 12 h p.i, in lower dose group animals and at 7 and 14 days in higher dose group animals, as compared with control. Probably, the higher dose of protein could be more than sufficient for early immune stimulation. The delayed response of BGBP level in higher dose protein-injected group is well correlated with an earlier study with β-1,3-glucan in L. vannamei. (Wang et al. 2008). In the present study, A2M was found to be significantly up-regulated at 6, 12, 72 h and 14 days in RdRp-treated groups. Lu, Sung, Liu and Sung (2007) also reported that elevated expression of A2M was observed after 1 h when prawn was treated with CpG ODN. A similar finding was observed when M. rosenbergii was challenged with LPS (Lu et al. 2007). Evidence of increased A2M at 24 and 12 h was also observed when the shrimps were injected with heat-killed Lactococcus garviae and Vibriyo alganolyticus respectively (Ho et al. 2009).
Animal cells generate energy in mitochondria through oxidative phosphorylation electron transport system (ETS). The ETS is a major source of reactive oxygen species (ROS), which are immunogenically active molecules that help in destruction of pathogens. Cytochrome oxidase is an electron transport enzyme that plays an important role in stimulating the immune response in invertebrates (Rensburg & Coyne 2009). In this study, cytox was up-regulated at 6 and 12 h post RdRp injection. Similarly, Lu, Sung, Liu and Sung (2009) found up-regulation of cytox at 6 and 12 h after the administration of LPS in M. rosenbergii (Lu et al. 2009).
As stated earlier, ROS are involved in destruction of pathogen, and to minimize the effect of ROS, SOD is used, which can catalyse the conversion of the highly toxic superoxide radicals to molecular oxygen (Cho, Lee, Bang, Kim & Nama 2009). SOD is an antioxidant enzyme, which has been widely used to evaluate the defence ability of shrimp against pathogens. Results of our present study showed the significant up-regulation of SOD activity in RP-treated groups at 6, 24 h and 7 days p.i. Campa-Córdova, Hernandez-Saavedra and Ascencio (2002) also observed an increased production of superoxide anion at 24 h in white shrimp L. vannamei challenged with Vibrio parahaemolyticus. Similarly, the up-regulated transcription level of MnSOD has also been marked in L. vannamei at 3–24 h after the shrimp were administered with laminarin. (Campa-Córdova et al. 2002). The level of SOD was also reported to increase significantly in Panaeus monodon when treated with DNA construct with VP28 gene of WSSV (encapsulated with chitosan nanoparticle) at 7 days p.i, which agrees to our observation in higher dose-treated animals (Rajesh Kumar, Venkatesan, Sarathi, Sarathbabu, Thomas, Basha & Sahul Hameed 2009). Similarly, when the Fenneropenaeus chinensis was fed with recombinant protein of WSSV (rVP28) orally, the SOD level was found to be up-regulated at third, 14th and 28th days of post-administration (Fu, Shuai, Xu, Li & Li 2010). It is interesting to mention that prawns experimentally infected with MrNV and XSV revealed a significant reduction in SOD activity and an increase in superoxide ion level up to 10 dpi (Ravi et al. 2010). This means the host defence might be highly reactive at the initial stage by producing free radicals to degrade virus, and in turn host antioxidant defence enzyme SOD might be exhausted during WTD infection. Thus, the increase in SOD activity observed in this study in RdRp-injected prawn might be helpful to combat MrNV/XSV load.
The PO system is the basis of non-specific immune system in crustacean and it catalyses the formation of quinine from phenolic compounds, which subsequently becomes melanin. Melanin is involved in wound healing and repair mechanism and quinine acts as an antifungal agent (Cerenius & Soderhall 2004). In the current study, PO expression was significantly increased when compared with control at 6 and 24 h in animals injected with 1 μg concentration of protein, whereas in Group C, the highest expression of PO was observed at day 14 only. Such a change in PO expression has been well documented in Chinese mitten crab Eriocheir sinensis, where PO is seen to be activated after 6 and 12 h of dsRNA infection (Dong, Zhao, Song, Wang, Qiu, Zheng, Li, Gai & Yang 2009). In general, most of the immune-related genes showed higher expression level at an early period after lower dose of RP injection, whereas at a later stage it occurred after exposure to higher dose of RP exposure. This could be probably due to the excess amount of protein that might be more than sufficient to stimulate the genes at higher dose because of feed-back mechanism. However, once the protein level might be reduced or degraded, then the level of expression gradually increased. In general, all these gene expressions are well correlated with the increase in THC level in prawns and probably act shortly to reduce the viral load as marked in the second experiment.
The challenge study was carried out here to evaluate the efficiency of RdRp in reducing the MrNV load in the injected prawns. The prawns were challenged after 1 day of RdRp treatment and nested RT-PCR was conducted to check the presence of virus. The virus was below detectable limit after 24 h of challenge. Similar to our finding, earlier studies have also observed detection of virus after 2–3 days of challenge (Sudhakaran, Syed Musthaq, Haribabu, Mukherjee, Gopal & Sahul Hameed 2006; Ravi et al. 2010). This is probably because the virus needs more than 24 h to establish and replicate inside the host or because there was insignificant amount of virus to be detected by nested RT-PCR method. After 48 h of immersion challenge, virus was detected in all the groups including control in this study. However at 7 days p.c., virus was detected only in control as well as in animals injected with lower dose of RdRp, whereas no viral particles could be detected in higher dose RdRp-injected animals. An earlier study suggested that the virus was cleared from the adult host after 25 days of infection naturally. However, the virus persisted in the system to a detectable level up to 20 days (Ravi et al. 2010). Hence, it is quite probable that the virus has been cleared from the system after 7 or 14 days of infection in RdRp-injected prawns because of the immunostimulatory action of RP exposure. RdRp might have played an important role in down regulating virus replication that needs further in-depth study. Besides, there might be modulation of the host immune response, as observed in this study, which could probably provide an additive effect in reducing viral load. However, further study in this regard is warranted to prove these concepts. Interestingly, at day 14 and 21, all samples of RdRp-treated group showed as nested RT-PCR negative, whereas the presence of this virus was detected only in PBS-injected control group. Although the mechanism is not clearly understood, it is confirmed that exposure to RP could be successful in protecting the host against MrNV.
Conclusion
The absence of viral particles in protein-injected prawn indicated the immunomodulatory effect of the recombinant RdRp. This result correlated with the bioassay tests indicating RdRp triggering the immune response with more production of haemocytes. The increase in haemocytes might be responsible for the release of inducible anti-viral substances and immune effector molecules in RdRp-injected prawn leading to virus reduction in infected prawns.
Acknowledgements
The authors wish to greatly acknowledge the Department of Biotechnology for sponsoring the project and thank Dr A. E. Ekanath, Director, Central Institute of Freshwater Aquaculture, Kausalyaganga, Bhubaneswar, India, for providing necessary facilities to carry out the study.




