Growth and survival of juvenile spider crabs, Maja brachydactyla (Balss, 1922), fed with fresh or frozen mussels
Abstract
The effects of diet freezing on the growth, survival and biochemical composition of the diets and juvenile spider crabs (Maja brachydactyla) were studied. Fresh and frozen (at −20 °C for 21 days) mussels, Mytilus edulis, were used as food. Two experiments were conducted and in each, spider crabs were placed in individual trays. During experiment I, 40 juvenile spider crabs (2 months old) were used. Twenty animals (9 ± 2 mg) were fed fresh mussels, and 20 animals (8 ± 2 mg) were fed frozen mussels. Spider crabs fed fresh mussels grew larger than the ones fed frozen mussels (304.0 ± 118.0 and 70.0 ± 40.1 mg respectively). During experiment II, 16 juvenile spider crabs (5 months old) were used. Eight animals (3.4 ± 0.8 g) were fed fresh mussel and eight animals (4.1 ± 1.3 g) were fed frozen mussel. Spider crabs fed with fresh mussels were larger than the ones fed with frozen mussels (92.5 ± 41.7 and 41.5 ± 17.7 g respectively). There were no significant differences in the protein, amino acids and fatty acid composition between fresh and frozen mussels. The freezing procedure makes mussels less adequate for the culture of 2-month-old early juveniles of M. brachydactyla up to 5 months, although they promoted acceptable growth and good survival in older animals (>5 months old).
Introduction
Maja brachydactyla (Balss 1922) is found along the East Atlantic Ocean, from the North Sea to North Africa, and in the Mediterranean Sea (Ingle 1980; Kergariou 1984; Le Foll 1993). Behaviour and requirements vary with size and age (González-Gurriarán & Freire 1994; González-Gurriarán, Freire & Bernárdez 2002). There is marked spatial segregation between juveniles and adults and between sexes and reproductive stage (González-Gurriarán & Freire 1994). Juveniles of Maja sp. live in low depths (<15 m), in sandy and rocky waters (Hines, Wolcott, González-Gurriarán, González-Escalante & Freire 1995). A wide variety of preys are part of the diet of both juveniles and adults, including echinoderms, molluscs and macro algae (Bernárdez, Freire & González-Gurriarán 2000).
Few studies on the growth for M. brachydactyla culture have been conducted, either in the laboratory (Corgos, Sampedro, González-Gurriarán & Freire 2007; Alaminos & Domingues 2008; Domingues & Alaminos 2008) or in nature (Le Foll 1993; Corgos et al. 2007). Similar Maja sp. have also been studied (Drach 1939; González-Gurriarán, Freire, Parapar, Sampedro & Urcera 1995; Iglesias, Sánchez, Moxica, Fuentes, Otero & Pérez 2002; Sanchez, Fuentes, Iglesias, Moxica & Otero 2007).
Iglesias et al. 2002 obtained adult spider crabs with a carapace length (CL) of 6.5 ± 0.8 cm, at 9.5 months of age, under semi-intensive culture conditions (outdoor 225 m3 tanks up to 50 days and then in indoor 500 L tanks), and survival rates of 66% at temperatures between 15 and 18 °C. For wild populations, Sampedro, González-Gurriarán and Freire (2003) reported increments of 27% in CL, while Corgos et al. (2007) reported intermoult increments in CL between 30% and 35%. González-Gurriarán et al. (1995); Drach (1939) and Le Foll (1993) also reported intermoult increments in CL between 30% and 35%, for both males and females and for laboratory-cultured or wild animals. Sanchez et al. (2007) have reported intermoult increments in CL close to 35%, for juvenile Maja squinado between the age of 2 and 6 months, cultured indoors, in 800 L tanks. The same author reported higher growth rates when spider crabs were fed either crabs or sea urchins.
The high growth rates make this species an excellent candidate for commercial aquaculture in template waters, a fact that is further supported by the high market value of M. brachydactyla (Sampedro 2001; Sampedro et al. 2003). The biochemical composition of this species has been reported by Marques, Teixeira, Barrento, Anacleto, Carvalho and Nunes (2010). They reported that the muscle and gonads have a low lipid content, while the hepatopancreas has a high lipid, unsaturated and monounsaturated, content. Also, Sirot, Oseredczuk, Bemrah-Aouachria, Volatier and Leblanc (2008) indicated that fatty acid (FA) compositions are among the highest in edible crustaceans, particularly eicosapentanoic acid (EPA, 20:5 n-3), docosahexanoic acid (DHA, 22:6 n-3), as well as n-3 and n-6 polyunsaturated fatty acid (PUFA).
Freezing and frozen storage allow a lower dependence on live or fresh prey, higher production and culturing of species inland. Nevertheless, this process considerably affects the technological properties of the muscle of marine species, particularly the myofibrillar protein composition (Matsumoto 1980; Shenouda 1980). Freezing produces huge structural and physiological modifications in intracellular components, with dehydration contributing to the grouping of muscle components. During storage, polar lipids (PL) and triacylglycerides (TAG) concentrations decrease, as they are hydrolysed, producing increasing concentrations of free fatty acids (FFA) and diacylglycerides (Mackie 1993). The increase in the concentration of FFA and the decrease in PL during the first 2 months of the freezing storage time have been reported for several marine species (Beltran & Moral 1991).
The objective of the present research was to determine the effects of freezing, during 21 days, of a usual fresh prey previously used to culture M. brachydactyla in our laboratory with good growth and survival of juvenile spider crabs, in order to reduce the high costs derived from maintenance and mortality of this prey, and allow us to store food for long periods of time.
Material and methods
Origin of the animals and acclimatization conditions
Juvenile spider crabs used were born in the research facility of the Centro IFAPA Agua del Pino (Andalucía, Spain), from fecunded females captured from the wild with net traps in coastal waters of Huelva (South Spain), in front of the research station. Fecunded females can spawn in the laboratory at least three times without the necessity of a male (Iglesias et al. 2002). Two experiments were conducted. Before their use in the experiments, which were conducted simultaneously, all spider crabs during the larvae and megalopae stages (up to 18–20 days) were cultured with live, newly hatched Artemia nauplii enriched with SELCO® (INVE Group, Deudermonde, Belgium). After settlement, spider crabs were fed with fresh mussels.
Experimental design and sampling protocol
Juvenile spider crabs used in experiment I (n=40) were 2 months on average, with an initial weight between 9 ± 2 mg (20 animals fed fresh mussels) and 8 ± 2 mg (20 animals fed frozen mussels). All of them were from the same spawn and female. Similarly, spider crabs used in experiment II, much older (5 months old on average), were from the same spawn and female, which was not the same as for experiment I. During experiment II, 16 juvenile spider crabs were used. Eight animals weighing 3.4 ± 0.8 g were fed fresh mussels, while the remaining eight animals, weighing 4.1 ± 1.3 g, were fed frozen mussels. There were no differences (P>0.05) in the initial weights of animals fed each diet in both experiments.
The experimental design (one individualized animal per compartment) was chosen after determining that cannibalism would be a major factor that could considerably affect the results, according to previous studies conducted by our research team. Also, individual data are stronger than those from grouped animals, which can provide inaccurate information, especially when mortality occurs during experimentation. Spider crabs were fed ad libitum, either fresh (alive) or frozen (at −20 °C for 21 days) mussels Mytilus edulis, in both experiments. The frozen mussels were from the same origin as the live ones. Before their use in the experiments, all mussels were fed microalgae (tetrasselmys sp.) for 1 week. Afterwards, half were frozen and used in the experiments after 21 days.
For experiments I and II, flow-through culture systems were used. For experiment I, a total of 40 individual, dark grey PVC compartments (6 × 8 cm, water depth of 2.5 cm, with a total volume of 0.14 L), and a water flow of 6.9 L h−1, allowing a total water renewal per compartment in just over 1 min, were used. For experiment II, a total of 16 individual trays (40 × 80 cm; water depth, 6 cm; total volume, 19.2 L), and a water flow of 5 L h−1, allowing total water renewal in <4 h, were used. Taking into consideration that culture density and available space can affect crustacean growth (Wilber & Wilber 1989), the dimensions of the individual containers or trays were chosen in order to allow spider crabs to have enough space to move around, minimizing stress. Water was previously passed through a sand and a biological filter, then a 25 μm filter and finally through UV lights, before entering the culture system. A natural light cycle (16:8 h) was used for both experiments, which were conducted simultaneously, between March and May. The temperature varied between 18 ± 2 °C and salinity varied between 35 ± 2 ppt.
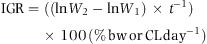
Experiments I and II lasted for 71 and 84 days respectively. Weight measurements were made using a precision scale (COBOS CB complet©, Barcelona, Spain), with three decimal cases, while only for experiment II, CL was measured using an electronic precision calliper (MITUTOYO Absolute coolant proof IP66©, Aurora, IL, USA), with two decimal cases. The decision of not measuring CL in experiment I was taken in order to avoid further handling and stressing, which causes mass mortality in very young animals (Domingues & Alaminos 2008).
After the end of experiment I, fresh and frozen mussels, and spider crabs fed each diet (six animals from each diet and each spider crab group) were sacrificed and homogenized to determine the biochemical composition. The spider crabs' carapace was separated and removed, and only the body composition was determined.
Proximal analysis
Moisture, total protein, total lipids (TL) and ash were determined following the methods established by AOAC (1990) (934.01, 976.05, 920.39 and 942.05 respectively).
Energy content
Mussel and spider crab energy contents were determined using a calorimetric pump (Parr®, Parr Instruments, Moline, IL, USA) calibrated with benzoic acid.
Amino acid (AA) analysis
Samples were freeze dried, before being processed, and brought to a particle size of 0.5 mm. Analysis of AA was performed using the HPLC (Waters, Mildford, MA, USA) method both in fresh and frozen mussel and in spider crabs fed with different diets.
Samples were hydrolysed for 4 h at 145 °C in 6 N HCl, using the technique described by Bidlingmeyer, Cohen and Tarvin (1984) and Cohen and Michaud (1993) after derivatization with 6-aminoquinolyl-N-hidroxisuccinimidilcarbamate. The concentration of essential amino acids (EAA) was determined in the different samples using an Acc-Q-Tag Nova Pak C18 column (particle size 4 mm) of 3.9 × 150 mm (Waters).
To determine the tryptophan (Trp) content, each sample was subjected to alkaline hydrolysis, using 4 N LiOH in a Tecator digester (Foss, Hillerod, Denmark) at 145 °C for 6 h (Lucas & Sotelo 1980). After drying in a rotovapor (Buchii, Mod. R, Büchi Larortechnik AG, Flawil, Switzerland), the hydrolysate obtained was filtered through Whatman paper No. 542, and the filtrate was brought to a 25 mL volume with distilled water. The Trp content was quantified colorimetrically using a spectrophotometer and absorbance was recorded at 590 nm. The obtained values were adjusted with a standard Trp curve [l-Trp, 98%, Batch 8940816, Sigma Chemical, St Louis, MO, USA).
Lipids and FAs
Total lipid was extracted with chloroform:methanol (2:1 v/v) containing 0.01% of butylated hydroxytoluene as an antioxidant (Christie 1982). The organic solvent was evaporated under a stream of nitrogen and the lipid content was determined gravimetrically. Lipid classes (LC) were separated by one-dimensional double development high-performance thin-layer chromatography using methyl acetate/isopropanol/chloroform/methanol/0.25% (w/v) KCl (25:25:25:10:9 by vol.) as the polar solvent system and hexane/diethyl ether/glacial acetic acid (80:20:2 by vol.) as the neutral solvent system. LC were quantified by charring with a copper acetate reagent, followed by calibrated scanning densitometry using a CAMAG TLC Scanner 3 dual-wavelength flying spot scanner (Muttenz, Switzerland) (Olsen & Henderson 1989). Total lipid extracts were subjected to acid-catalysed transmethylation for 16 h at 50 °C, using 1 mL of toluene and 2 mL of 1% sulphuric acid (v/v) in methanol. The resultant fatty acid methyl esters (FAME) were purified by thin-layer chromatography and visualized by spraying with 1% (w/v) iodine in CHCl3 (Christie 1982). Before transmethylation, non-adecanoid acid (19:0) was added to TL as an internal standard. FAME were separated and quantified using a Shimadzu GC-2010 gas chromatograph (Kyoto, Japan) equipped with a flame ionization detector (250 °C) and a fused silica capillary column RTX-WAXTM (10 m × 0.1 mm ID). Helium was used as a carrier gas and the oven initial temperature was 150 °C, followed by an increase at a rate of 90 °C min−1 to a final temperature of 250 °C for 2 min. Individual FAME were identified in reference to authentic standards and to a well-characterized fish oil (FAME Mix C4-C24 and Menhaden Oil, SUPELCO, Sigma-Aldrich, MO, USA).
Butylated hydroxytoluene, potassium chloride, potassium bicarbonate and iodine were supplied by Sigma Chemical. All organic solvents for gas chromatography used were of reagent grade and were purchased from Panreac (Barcelona, Spain).
Statistics
Statistical analysis (significance at P<0.05) was performed using the program statistica 6.0. For both experiments, student t-tests (Zar 1984) were conducted to determine differences in the growth of spider crabs fed the two diets, as well as differences in the biochemical composition. Before the statistical analysis, normality and homogeneity of variance from each sample were determined (Zar 1984).
Results
Growth parameters
Figure 1 shows the growth of juvenile spider crabs fed either fresh or frozen mussels during experiment I. Animals fed the fresh mussels grew larger (P<0.05), to a final average weight of 304.0 ± 118.0 mg, compared with the ones fed frozen mussels (70.0 ± 40.1 mg). The average growth rates were 5.0 ± 0.8 and 2.8 ± 0.9% bw day−1 for spider crabs fed the fresh or frozen mussels, respectively, and were higher (P<0.05) for the ones fed fresh mussels. Mortality was 10% and 25% for animals fed the fresh and frozen mussels respectively.
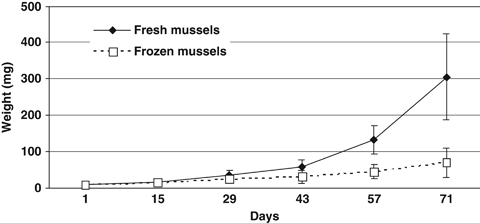
Growth of juvenile spider crabs (2 months old) cultured with fresh and frozen fresh mussels. Bars indicate standard deviations.
Figure 2 shows the growth in weight (g) of spider crabs fed the two diets tested during experiment II. Spider crabs fed the fresh mussel grew larger (P<0.05) than the ones fed frozen mussels (92.5 ± 41.7 and 41.5 ± 17.1 g respectively) at the end of the experiment. Similarly, the growth rates for animals fed the fresh mussel (3.3 ± 1.4% bw day−1) were higher (P<0.05) than the ones obtained with frozen mussel (2.4 ± 1.4% bw day−1). Animals in all groups were fed in excess, and some uneaten mussels were always present in every group and for both experiments. None of the 16 spider crabs used died during experiment II.
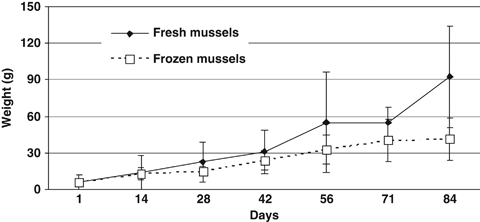
Growth of juvenile spider crabs (5 months old) cultured with fresh and frozen fresh mussels. Bars indicate standard deviations.
Biochemical composition: Mussels
There were no differences (P>0.05) between the chemical characteristics of fresh and frozen mussels. The protein content was 61%, TL 12% and ash 8% (Table 1). An energy content around 18500 kJ kg−1 DW was obtained on both types of diet: fresh and frozen mussel (Table 1). Carbohydrates calculated by differences showed values around 19% in both the diets (Table 1).
| Mean ± SE | ||||
|---|---|---|---|---|
| Fresh mussel | Frozen mussel | Crab feed fresh | Crab feed frozen | |
| Energy | 18530.0 ± 1111.8 | 18460.0 ± 923.0 | 19413.0 ± 970.6 | 17180.6 ± 1030.8* |
| Protein | 61.28 ± 3.06 | 60.43 ± 5.51 | 83.63 ± 8.57 | 89.53 ± 5.37 |
| Total lipid | 11.53 ± 0.92 | 12.87 ± 1.15 | 6.08 ± 0.75 | 2.94 ± 0.049* |
| Ash | 8.19 ± 0.57 | 7.95 ± 0.68 | 10.30 ± 0.5 | 8.81 ± 0.042* |
- * Significant differences (P<0.05) between spider crabs fed each diet.
- Values (n=3; mean ± standard error, SE) represented as percentage in dry weight (% DW) and as kilojoules per kilogram in dry weight (kJ kg−1 DW) for energy content.
The most abundant EAA were leucine (Leu) and lysine (Lys) (43.2 and 45.8 g kg−1 protein respectively), followed by threonine (26.4 g kg−1 protein), isoleucine (26.7 g kg−1 protein), phenylalanin (21.9 g kg−1 protein) and valine (26.8 g kg−1 protein) (Table 2). There were no statistical differences (P>0.05) in the EAA content between fresh and frozen mussel. The most abundant non-essential amino acid (NEAA) was glutamic acid (Glu) (83.3 g kg−1 protein), followed by aspartic acid (59.1 g kg−1 protein) and arginine (44.7 g kg−1 protein). The rest of the NEAA maintained values between 38.3 g kg−1 (glycine) and 8.0 g kg−1 [cystine (Cys)] protein (Table 2).
| Mean ± SE | ||||
|---|---|---|---|---|
| Fresh mussel | Frozen mussel | Crab feed fresh | Crab feed frozen | |
| EAA | ||||
| Tryptophan | 6.8 ± 0.3 | 6.9 ± 0.6 | 13.3 ± 1.2 | 12.5 ± 1.1 |
| Threonine | 26.4 ± 1.3 | 26.4 ± 1.6 | 38.6 ± 2.3 | 36.3 ± 3.3 |
| Isoleucine | 26.7 ± 1.3 | 26.7 ± 2.4 | 46.2 ± 2.8 | 43.4 ± 3.9 |
| Leucine | 43.2 ± 2.2 | 43.1 ± 2.6 | 75.6 ± 4.5 | 71.1 ± 6.4 |
| Lysine | 45.8 ± 2.3 | 45.8 ± 4.1 | 82.9 ± 5.0 | 77.9 ± 7.0 |
| Methionine | 13.8 ± 0.7 | 13.8 ± 0.8 | 26.8 ± 1.6 | 25.2 ± 2.3 |
| Phenylalanin | 21.9 ± 1.1 | 22.0 ± 1.3 | 40.2 ± 2.4 | 37.8 ± 3.4 |
| Valine | 26.8 ± 1.3 | 26.8 ± 1.6 | 44.8 ± 2.7 | 42.1 ± 3.8 |
| Histidine | 11.7 ± 0.6 | 11.8 ± 0.7 | 19.4 ± 1.2 | 18.2 ± 1.6 |
| NEAA | ||||
| Cystine | 8.0 ± 0.4 | 8.0 ± 0.7 | 10.7 ± 0.6 | 10.0 ± 0.9 |
| Tyrosine | 19.6 ± 1.0 | 19.6 ± 1.8 | 31.7 ± 1.9 | 29.8 ± 2.7 |
| Arginine | 44.7 ± 2.2 | 44.7 ± 4.0 | 83.2 ± 5.0 | 78.2 ± 7.0 |
| Alanine | 37.1 ± 1.9 | 37.1 ± 3.3 | 54.0 ± 3.2 | 50.7 ± 4.6 |
| Aspartic acid | 59.1 ± 3.0 | 59.1 ± 3.5 | 98.5 ± 5.9 | 92.6 ± 8.3 |
| Glutamic acid | 83.3 ± 4.2 | 83.3 ± 7.5 | 162.5 ± 9.7 | 152.7 ± 13.7 |
| Glycine | 38.3 ± 1.9 | 38.3 ± 2.3 | 57.5 ± 3.4 | 54.0 ± 4.9 |
| Proline | 25.0 ± 1.3 | 25.0 ± 2.2 | 31.4 ± 1.9 | 29.5 ± 2.7 |
| Serine | 27.4 ± 1.4 | 27.5 ± 1.6 | 37.5 ± 2.2 | 35.2 ± 3.2 |
- Values (n=3; mean ± SE) represented as grams per kilogram of protein (g kg−1 protein).
- EAA, essential amino acids; NEAA, non-essential amino acids.
The most abundant LC were TAG, followed by sterols, phosphatidylethanolamine and phosphatidylcholine (PC) in both fresh and frozen mussels (Table 3). There were little differences (P>0.05) between LC of fresh and frozen mussels, although PC and digalactosyl diacylglycerol from fresh mussels were significantly higher than those on frozen mussel (P<0.05; Table 3)
| Mean ± SE | ||||
|---|---|---|---|---|
| Fresh mussel | Frozen mussel | Crab feed fresh | Crab feed frozen | |
| Lysophosphatidylcholine | ND | ND | ND | ND |
| Sphingomyelin | ND | ND | ND | ND |
| Phosphatidylcholine | 12.33 ± 0.30 | 10.03 ± 0.74* | 11.13 ± 3.06 | 9.64 ± 2.46 |
| Lysophosphatidylserine | 3.06 ± 2.13 | 5.34 ± 1.52 | 1.41 ± 1.43 | tr |
| Phosphatidylserine | 9.96 ± 0.33 | 7.97 ± 1.29 | 2.04 ± 0.76 | 1.41 ± 1.46 |
| Phosphatidylinositol | 1.16 ± 1.03 | ND | 1.33 ± 1.22 | 2.38 ± 0.76 |
| Phosphatidylethanolamine | 13.34 ± 0.41 | 11.86 ± 1.06 | 7.45 ± 1.95 | 7.53 ± 2.43 |
| Digalactosyl diacylglycerol | 1.95 ± 1.15 | ND | ND | ND |
| Diacylglycerol | 0.74 ± 0.29 | 1.07 ± 0.99 | ND | ND |
| Sterols | 12.21 ± 0.66 | 13.04 ± 1.48 | 11.24 ± 2.92 | 12.85 ± 2.60 |
| Free fatty acid | 3.35 ± 0.90 | 5.88 ± 3.46 | ND | 2.86 ± 2.91 |
| Triacylglycerol | 28.44 ± 2.82 | 24.90 ± 3.81 | 20.89 ± 7.18 | 26.69 ± 9.86 |
| Sterol esters/wax esters | 9.76 ± 2.39 | 15.99 ± 4.93 | 41.09 ± 20.18 | 29.07 ± 24.75 |
| Unknown | 3.66 ± 0.86 | 3.89 ± 0.18 | 2.00 ± 3.46 | 6.19 ± 5.37 |
| Total polar lipid | 41.86 ± 0.98 | 35.30 ± 4.55 | 24.62 ± 7.91 | 22.34 ± 9.35 |
| Total neutral lipid | 57.85 ± 1.24 | 63.84 ± 5.29 | 75.38 ± 7.91 | 77.41 ± 9.24 |
- * Significant differences (P<0.05) between fresh and frozen mussels.
- Values (n=3, mean ± standard deviation, SD) represented as percentage of total lipid (%TL).
- TFA, total fatty acid; ND, not detected (≤0.3% TFA); tr, trace (0.3–0.5% TFA).
Table 4 shows the FA percentage of TL (% TFA) of fresh and frozen mussels (21 days at −20 °C). The most abundant FA were palmitic acid (16:0), EPA, 18:0 DMA and DHA. No significant differences (P>0.05) were found for any FA or FA groups, although it is interesting to note the higher percentage of n-3 highly unsaturated fatty acid (HUFA) detected in fresh mussels and the higher saturated fatty acids (SAT) in frozen mussel. The differences in n-3 HUFA were mainly caused by EPA and DHA. On the other hand, the differences in SAT were mainly caused by 16:0. In both cases, the high variability in the composition of frozen mussels prevented significant differences.
| Mean ± SE | ||||
|---|---|---|---|---|
| Fresh mussel | Frozen mussel | Crab feed fresh | Crab feed frozen | |
| 14:0 | 2.45 ± 0.48 | 1.95 ± 0.70 | 1.33 ± 0.16 | 1.72 ± 0.86 |
| 15:0 | tr | 0.64 ± 0.10 | 0.70 ± 0.08 | 0.88 ± 0.16 |
| 16:0 | 18.44 ± 0.52 | 21.30 ± 4.41 | 19.77 ± 0.81 | 18.26 ± 0.68 |
| 16:1 n-7 | 6.60 ± 0.87 | 6.09 ± 1.80 | 6.79 ± 0.97 | 5.98 ± 1.30 |
| 16:1 n-5 | 0.53 ± 0.38 | 0.57 ± 0.57 | 0.85 ± 0.06 | 0.73 ± 0.31 |
| 17:0 DMA | 0.90 ± 0.26 | 0.99 ± 0.78 | 1.11 ± 0.76 | 0.88 ± 0.61 |
| 16:2 n-4 | 0.61 ± 0.18 | 0.79 ± 0.27 | tr | tr |
| 17:0 | 0.74 ± 0.07 | 0.79 ± 0.17 | 0.79 ± 0.04 | 0.99 ± 0.08* |
| 18:0 DMA | 10.27 ± 2.52 | 10.96 ± 3.63 | 5.01 ± 0.91 | 5.21 ± 0.38 |
| 18:0 | 4.46 ± 0.22 | 4.48 ± 1.22 | 8.57 ± 0.53 | 9.66 ± 0.70 |
| 18:1 n-9 | 1.66 ± 0.36 | 1.75 ± 0.43 | 5.33 ± 0.64 | 6.13 ± 0.60 |
| 18:1 n-7 | 2.70 ± 0.37 | 2.88 ± 0.53 | 4.22 ± 0.24 | 5.64 ± 0.58* |
| 18:1 n-5 | ND | tr | tr | 0.53 ± 0.35 |
| 18:2 n-6 | 1.91 ± 0.11 | 1.72 ± 0.26 | 1.35 ± 0. 09 | 1.77 ± 0.15* |
| 18:3 n-3 | 0.78 ± 0.25 | 0.94 ± 0.28 | 0.60 ± 0.14 | tr |
| 18:4 n-3 | 1.23 ± 0.11 | 1.06 ± 0.27 | tr | ND |
| 20:0 | 1.09 ± 0.15 | 0.97 ± 0.41 | tr | 0.60 ± 0.08* |
| 20:1 n-9 | 3.43 ± 0.28 | 3.89 ± 0.95 | 2.59 ± 0.46 | 2.78 ± 0.16 |
| 20:1 n-7 | 1.46 ± 0.19 | 1.59 ± 0.15 | 1.74 ± 0.26 | 1.57 ± 0.21 |
| 20:2 n-6 | 0.53 ± 0.18 | 0.58 ± 0.13 | 0.63 ± 0.09 | 0.70 ± 0.08 |
| 20:4 n-6 | 2.48 ± 0.24 | 2.21 ± 0.53 | 3.05 ± 0.23 | 3.85 ± 0.50 |
| 20:5 n-3 | 17.98 ± 2.41 | 13.67 ± 5.62 | 16.17 ± 0.42 | 13.67 ± 2.40 |
| 22:2 NMID1 | 2.92 ± 0.41 | 3.53 ± 1.28 | 1.74 ± 0.22 | 1.97 ± 0.51 |
| 21:5 n-3 | 0.85 ± 0.22 | 1.05 ± 0.33 | 0.72 ± 0.09 | 0.59 ± 0.08 |
| 22:5 n-3 | 1.24 ± 0.16 | 1.19 ± 0.07 | 0.90 ± 0.05 | 0.66 ± 0.21 |
| 22:6 n-3 | 7.66 ± 0.37 | 6.70 ± 0.89 | 8.44 ± 0.62 | 7.62 ± 0.36 |
| UK | 4.09 ± 0.57 | 5.45 ± 1.49 | 3.98 ± 0.36 | 4.62 ± 1.00 |
| SAT | 27.95 ± 0.76 | 30.13 ± 3.63 | 31.57 ± 1.14 | 32.10 ± 0.57 |
| MON | 16.66 ± 1.44 | 17.23 ± 0.42 | 22.37 ± 0.89 | 23.65 ± 1.12 |
| PUFA | 40.14 ± 2.79 | 35.24 ± 4.15 | 35.97 ± 0.98 | 33.53 ± 1.04* |
| n-3 | 29.97 ± 3.33 | 24.80 ± 6.40 | 27.51 ± 0.96 | 23.44 ± 2.03* |
| n-6 | 5.60 ± 0.25 | 5.13 ± 0.83 | 5.72 ± 0.27 | 7.00 ± 0.59* |
| n-9 | 5.09 ± 0.24 | 5.64 ± 1.29 | 8.16 ± 0.47 | 9.16 ± 0.49 |
| n-3 HUFA | 27.92 ± 3.05 | 22.77 ± 6.28 | 26.24 ± 0.86 | 22.54 ± 2.09* |
| n-3/n-6 | 5.37 ± 0.74 | 5.02 ± 2.03 | 4.82 ± 0.35 | 3.38 ± 0.54* |
| EPA/DHA | 2.34 ± 0.24 | 2.00 ± 0.55 | 1.92 ± 0.11 | 1.80 ± 0.36 |
| ARA/EPA | 0.14 ± 0.03 | 0.19 ± 0.09 | 0.19 ± 0.02 | 0.29 ± 0.07 |
| ARA/DHA | 0.32 ± 0.04 | 0.34 ± 0.11 | 0.36 ± 0.03 | 0.50 ± 0.04* |
| MON/n-3 H | 0.60 ± 0.08 | 0.79 ± 0.18 | 0.85 ± 0.03 | 1.05 ± 0.05* |
| MON/PUFA | 0.42 ± 0.05 | 0.49 ± 0.04 | 0.62 ± 0.02 | 0.71 ± 0.01 |
| MON/SAT | 0.60 ± 0.06 | 0.58 ± 0.07 | 0.71 ± 0.04 | 0.74 ± 0.04 |
- * Significant differences (P<0.05) between spider crabs fed each diet.
- Values (n=3; mean ± SD) represented as percentage of total fatty acid (%TFA).
- PUFA, polyunsaturated fatty acid; HUFA, highly unsaturated fatty acid; n-3 H, n-3 highly unsaturated fatty acid; DHA, docosahexanoic acid (22:6 n-3); EPA, eicosapentanoic acid (20:5 n-3); ARA, arachidonic acid (20:4 n-6); MON, monounsaturated fatty acid; SAT, saturated fatty acid; NMID, non-methylene interrupted dienoic fatty acid; UK, undetermined fatty acid; ND, not detected (≤0.3% TFA); tr, trace (0.3–0.5% TFA).
Biochemical composition: spider crabs
Differences in the energy content of spider crabs fed experimental diets were observed, with higher values in animals fed fresh mussels than crabs fed frozen ones (P<0.05; Table 1). Also, differences were recorded in TLs and ash, with values 51% and 15% lower in animals fed frozen than fresh mussels (P<0.05; Table 1). There were no differences (P>0.05) in the protein content. For this reason, a mean value of 87% of protein can be proposed for M. brachydacyla used in the present study (Table 1).
There were no differences in the EAA content between spider crabs fed experimental diets (Table 2). In fact, EAA abundance followed the same tendency as that in the diets with high values for Lys (82.9 and 77.9 g kg−1 protein) and Leu (75.6 and 71.1 g kg−1 protein) and low values in histidine (His) (19.4 and 18.2 g kg−1 protein) and Trp (13.3 and 12.5 g kg−1 protein). A similar behaviour was observed of NEAA, with high values in Glu (162.5 and 152.7 g kg−1 protein) and low values in Cys (10.7 and 10.0 g kg−1 protein) (Table 2).
The most abundant LC were sterol esters/wax esters and TAG for animals fed both diets (Table 3). There were no differences (P>0.05) between LC of spider crabs fed fresh and frozen mussels (Table 3).
The results of the FA composition of spider crabs that had been fed either fresh or frozen mussels are shown in Table 4. The most abundant FA were 16:0, EPA, 18:0 and DHA. The sum of SAT and monounsaturated fatty acids in spider crabs was similar regardless of the diet. In contrast, PUFA and n-3 HUFA contents were higher for animals fed fresh mussels.
Discussion
Iglesias et al. (2002) obtained adult spider crabs with a survival of 66% and CL of 6.5 ± 0.8 cm, at 9.5 months of age under semi-intensive culture conditions, between 15 and 18 °C. This corresponds to an average growth rate of 0.5% bw day−1. These growth rates are much lower than the ones obtained during the present experiments (2.4–5% bw day−1). Animals are expected to grow faster during the early stages of the life cycle, as observed in the present experiments. The growth rates obtained here indicate that fresh mussel was a very good diet for these early stages. Nevertheless, frozen mussels were not appropriate to culture smaller animals (2 months old) due to much lower growth rates, although survival (75% in experiment I and 100% in experiment II) was similar.
Few growth results in the laboratory for juveniles or adults of M. brachydactyla presented in animal weight have been reported until now (Alaminos & Domingues 2008). Crustaceans in general, and Maja sp. in particular, usually grow by periodic replacement of the exoskeleton. During the ecdysis process (Chang 1989, 1995); the majority of the results reported are indicated in per cent of intermoult increments in CL. The results presented here are in weight increments during a time interval for both experiments. Because of this, direct comparisons with the majority of the results reported in the literature for the growth of Maja sp. are only possible for experiment I. Nevertheless, the results from experiment II can be directly compared with the ones reported by several authors such as Iglesias et al. (2002), and Alaminos and Domingues (2008), which provide growth in weight and CL for specific periods of time.
Wilber and Wilber (1989) and González-Gurriarán, Freire, Fariña and Fernández (1998) report that the holding time, isolation and tank size affect growth of spider crab and other crustacean species. In contrast, growth in isolation and relatively small chambers was high when fresh mussels were fed. Growth rates up to 5% bw day−1 in weight are very satisfactory, indicating that isolation might not be such a limiting factor for this particular species, compared with other crustaceans. Nevertheless, further studies have to be conducted in order to determine the actual effect of isolation on growth.
Higher water temperatures (18 °C) in the present experiments promoted higher growth compared with the ones from Iglesias et al. (2002) (15–18 °C).
The results obtained showed that fresh mussels are good food for the culture of the initial stages (after settling) of M. brachydactyla, while frozen mussels promote acceptable growth and good survival in older animals (>5 months old). This is a good base for the possible large-scale culture of this species, and allows researchers to focus on the development of a good, stable and storable artificial diet, which could reduce the production costs.
During frozen storage, marine organisms are highly susceptible to lipid oxidation due to it is high PUFA content (Nazemroaya, Sahari & Rezaei 2009), particularly during long storage periods (Ohman 1996). Previous works have reported remarkable rates of lipid hydrolysis mechanisms in different types of fish and oysters (Jeong, Ohshima & Koizumi 1991; Nazemroaya et al. 2009), with decreases in the PL contents and increases in the FFA fraction during the first 2 months of the freezing storage time (Beltran & Moral 1991). In these works, the percentage of PUFA decreased and SAT increased. The decrease in the PUFA was due to a decrease in 20:5 n-3 and 22:6 n-3 during storage. The increase in SAT was mainly due to the increase in 16:0. In this sense, it has been suggested that the EPA+DHA/C16 ratio is a good index to determine lipid oxidation (Jeong, Ohshima, Koizumi & Kanou 1990). In previous studies, this ratio decreased 50% during frozen storage in fish (Nazemroaya et al. 2009) and oysters (Jeong et al. 1991). The present results suggest that lipid mussel composition could also be affected by a short freezing process (at −20 °C for 21 days). PC decreased due to the freezing process. Moreover, TPL decreased and the FFA increased, although the differences were not significant due the high variability detected in mussels frozen samples. Higher frozen periods would possibly contribute to significant differences. Also, a decrease in PUFA and an increase in SAT percentages occurred with the freezing process in mussels, during the present experiment, although the differences were not significant. However, a significant increase in the EPA+DHA/C16 ratio was detected (data not shown).
In contrast, spider crab lipid composition was affected by diet. It is interesting to note that there were differences in the TL and ash contents of spider crab meat fed both diets. In fact, differences in the energy content could indicate differences in the nutrients that were used and stored. Moreover, differences in the FA content were detected in spider crab samples. The overall FA composition for spider crabs fed with frozen mussels had a higher percentage of n-6. In contrast, in spider crabs fed fresh mussels, the PUFA and n-3 were greater in importance, possibly due to the higher content of these two groups in fresh mussels (although not significant, due to the considerable variability in the mussel samples).
Differences in growth and spider crab composition obtained during this experiment may not have resulted from different ingestion rates, as all groups fed consistently on either fresh or frozen mussels. Nevertheless, as this was not quantified, due to the extremely small size of the experimental animals and amounts of food consumed, it is possible that differences could have occurred on feeding rates. These could have resulted from the different textures of fresh and frozen mussels. Although there were no statistical differences in protein, EAA and NEAA content between fresh and frozen mussels or between spider crabs fed experimental diets, this fact does not mean that freezing, even for a short time, cannot affect the structure of proteins and directly influence the texture of the mussels (which can be checked visually).
Further studies on other nutritional components of fresh and frozen mussels, such as myofibrillar protein should be conducted. Particularly, the effects of freezing on mussel and proteins structure and how it affects consumption rates should be conducted, in order to possibly explain the high differences in growth, as overall protein, AA and FA composition of fresh and frozen mussels were similar.
Acknowledgments
The authors wish to thank the ‘Plan Nacional de Centolla’ –JACUMAR– Project ‘Cría de centolla, Maja sp.’ 2007/2009, for the funding for this research. Sandra García-Garrido wishes to thank the ‘Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria’ (INIA) for the Pre-doctoral grant no 47 (BOE no 308 26/12/2006).




