Environmental quality standards for a deltamethrin sea louse treatment in marine finfish aquaculture based on survival time analyses and species sensitivity distributions
Abstract
This paper describes the use of time to event and Species Sensitivity Distribution (SSD) analyses to derive environmental quality standards (EQS) for the synthetic pyrethroid deltamethrin when used to treat lice in marine finfish aquaculture. Long-term EQS are of limited applicability for parasiticides used in coastal aquaculture because initially high concentrations are rapidly dissipated and diluted. Short-term EQS related to likely exposure duration are a more useful management tool. Accelerated Life Testing was used to analyse high-quality, time-specific survival data (LC10 values) for saltwater fish and crustacean species for which observations on survival over several time periods were available. These data were then plotted as SSDs, allowing the estimation of time-specific median HC5 values, protective of 95% of organisms in saltwater assemblages if the test data are representative of species in the field. These analyses show that after 3 h, the HC5 of LC10 values for deltamethrin in saltwater is 9.3 ng L−1, declining to 1.4 ng L−1 after 48 h of continuous exposure. Such values are consistent with data on effect concentrations from other lines of evidence, including mesocosm and field studies, and can be used as time-specific EQS when monitoring discharges from aquaculture facilities immediately after the treatment of fish with deltamethrin.
Introduction
Sea lice released from marine fish farms are suspected to be one of the contributory factors in the decline in wild salmonid stocks. To combat infections, maintain fish welfare, retain commercial viability and assist with initiatives to prevent adverse effects on wild stocks, fish farmers use a range of chemo-therapeutants licensed as veterinary medicines. However, due to their toxic properties, some of these compounds have the potential to pose an environmental risk to non-target organisms.
ALPHA MAX™ is a 10 mg mL−1 emulsifiable concentrate of the synthetic pyrethroid deltamethrin used as a bath treatment to control the infestation of farmed salmon by salmon lice (Lepeophtherius salmonis) and sea lice (Caligus elongatus). Deltamethrin is the (1R, cis; α S)-isomer of eight steroisomeric esters of the dibromo analogue of chrysanthemic acid, 2,2-dimethyl-3-(2,2-dibromovinyl) cyclopropanecarboxylic acid with α-cyano-3-phenoxybenzyl alcohol. This substance is a neurotoxin that acts by interfering with the sodium and potassium channels in the peripheral and central nervous systems of arthropods such as sea lice. Exposure to deltamethrin causes repetitive firings of the nerve endings as well as increased and sustained membrane action potentials that overwhelm the muscular neurotransmitters, resulting in paralysis and death (Pawlisz, Busnarda, Mclauchlin, Caux & Kent 1998).
When used in an aquaculture bath treatment, the fish within the net pen are surrounded with a tarpaulin and the deltamethrin treatment is added to the seawater. After a specified treatment period, the tarpaulin is removed and the spent solution is released into the environment. Following release, the deltamethrin is diluted and dispersed by tidal and wind-driven currents. Low water solubility (0.2 μg L−1) means that deltamethrin is expected to adsorb to suspended solids and sediment and has been shown to have a water column disappearance half-life of 2–4 h (Muir, Rawn & Grift 1985).
Before a fish medicine containing deltamethrin can be authorized for use in most jurisdictions, an environmental quality standard (EQS) must be derived, which represents a threshold concentration below which no significant environmental impact is expected (Crane, Barrett & Boxall 2008; SEPA 2009). In Europe, EQS are increasingly derived using procedures defined by the Water Framework Directive (Crane & Babut 2007). However, exposure of non-target organisms to deltamethrin used as a fish medicine is likely to be only brief and the effects will be overestimated if based on tests with exposures that last for much longer periods. If the maximum toxic response occurs primarily in a period shorter than the duration of a typical ‘short-term’ toxicity study (i.e. 96 h for fish and 48 h for invertebrates), then time-to-event (TTE) analysis may be a useful approach. Exposure duration, a critical determinant of exposure consequences, is explicitly included in TTE, providing a more accurate prediction of effects for different exposure durations. Using conventional testing methods, TTE methods draw from an experimental design in which groups of individual organisms are monitored through time (Crane, Chapman, Fenlon & Newman 2002).
Construction of a Species Sensitivity Distribution (SSD) is another technique that is increasingly being used to analyse environmental toxicity data (Posthuma, Suter II & Traas 2002). An SSD makes use of the full distribution of available good-quality ecotoxicity data and allows the calculation of a low percentile, which is then used as the basis for deriving a predicted no effect concentration (PNEC) or EQS. The fifth percentile of the distribution is usually selected, and is referred to as the HC5, or the Hazardous Concentration for an assumed 5% of species. It is assumed that the toxicity data used in the distributions are representative of those that might be exposed in natural ecosystems.
Methods and results
Accelerated Life Testing, implemented in the ace software package (USEPA 2003), was used to analyse high-quality time-specific survival data for one saltwater fish species (Cyprinodon variegatus, two datasets) and five saltwater crustacean species (Penaeus duorarum, Uca pugilator (two datasets), Acartia tonsa, Crangon crangon, and Americamysis bahia) for which observations on survival over several time periods were available (Bell 1997a, b; Heitmuller 1978a, b, c; LeLievre 1991a, b; Sousa 1990). The lethal concentration to 10% of the test population (LC10) was estimated at 3, 6, 12, 24 and 48 h. When two datasets were available for the same species, the LC10 value was conservatively estimated as the geometric mean of the two individual LC10 values at each time period.
The estimated LC10 values for each time period were fitted to a lognormal SSD using etx 2.0 software (RIVM 2004) to estimate the hazardous concentration for 5% of organisms (HC5) plus 90% confidence intervals around the HC5. All model fits passed Anderson–Darling, Kolmogorov–Smirnov and Cramer von Mises tests for normality at P=0.05, except for the 12-h exposure data (P=0.025). Figure 1 shows the toxicity curves for all test data and Table 1 summarizes the HC5 values for LC10 SSDs at the different estimated time periods. These show that after 3 h of exposure, the estimated LC10 HC5 is 9.3 ng L−1, declining to 1.37 ng L−1 at 48 h. If PNECs were set at these HC5 values for each time period, there would be no LC10 values for any of the tested species that fall below the respective LC10 HC5 values.
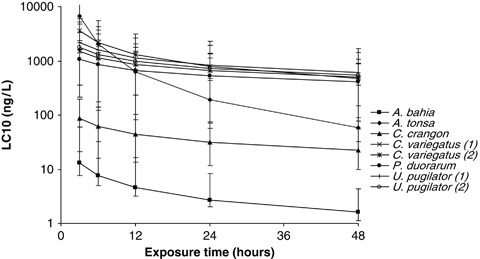
Toxicity curves for LC10 values of deltamethrin for six saltwater species calculated by Accelerated Life Testing (USEPA 2003). Error bars are 95% confidence intervals.
| Time (h) | HC5 (ng L−1) of LC10 values, plus90% confidence interval |
|---|---|
| 3 | 9.3 (0.09–73.5) |
| 6 | 6.62 (0.078–48.29) |
| 12 | 4.29 (0.054–30.3) |
| 24 | 2.53 (0.031–18.12) |
| 48 | 1.37 (0.015–10.36) |
Discussion
A large number of short-term, long-term and mesocosm studies have been performed with deltamethrin because of its widespread use as a plant protection product. The most sensitive results for freshwater and saltwater species are, unsurprisingly, for crustaceans and other arthropods, as these groups include the target pests. Sensitive results include 21-day growth and reproduction no observed effect concentrations (NOECs) of 4 and 9 ng L−1, respectively, for Daphnia magna (McNamara 1990). For saltwaters, the lowest reported 96-h LC50 from a reliable study is 3.7 ng L−1 for the mysid shrimp A. bahia (LeLievre 1991a), and the lowest reported long-term results are 6-day EC10 values for the survival and reproduction of the copepod Tisbe battagliai of 4.7 and 8.7 ng L−1 (Barata, Baird, Medina, Albalat & Soares 2002). Use of the standard assessment factor of 10 on the lowest of these data (EC 2003) would produce a PNEC as low as 0.37 ng L−1, based on exposure lasting 4 days.
The EU TGD (EC 2003) provides guidance on the use of SSDs in which it is recommended that at least 12 long-term datasets are used with at least eight different taxa when constructing SSDs for general risk assessment purposes. However, the TGD guidance is not relevant to the particular application of an SSD here because the mode of action of deltamethrin is well known and the most sensitive species (primarily crustaceans, but also fish) are represented in this SSD (Campbell, Arnold, Brock, Grandy, Heger, Heimbach, Maund & Streloke 1999).
The results of the TTE/SSD analyses suggest that short-term EQS of 9.3, 6.6, 4.3, 2.5 and 1.4 ng L−1 should be sufficiently protective of sensitive saltwater species after, respectively, 3, 6, 12, 24 and 48 h of exposure. These results are consistent with the most sensitive toxicity results summarized above. They are also consistent with analyses by Solomon, Giddings and Maund (2001), who fitted an SSD to 21 freshwater data for deltamethrin and calculated fifth and 10th percentile values for all acute LC/EC50 data of 3 and 9 ng L−1 respectively. However, uncertainty about no effect concentrations estimated using distributional approaches such as TTE and SSD may be high; hence, they should be used only as one line of evidence in an overall assessment of deltamethrin aquatic effects.
An important additional line of evidence comes from field studies, including those performed in mesocosms. Several studies on the effects of deltamethrin in freshwater communities are available. Hanson, Graham, Babin, Azam, Coutellec, Knapp, Lagadic and Caquet (2007) and Caquet, Hanson, Roucaute, Graham and Lagadic (2007) reported on the effects of deltamethrin in 9 m3 freshwater mesocosms in a 14-month study in which eight replicates were sprayed with a single dose of deltamethrin and compared with eight undosed controls. The main aim of the study was to mimic the effect of pond isolation on the recolonization of pesticide-impacted ponds; thus, the exposure concentration of 5 μg L−1 was deliberately selected to exert large effects on pond arthropods. Half of the replicates in each treatment group were then covered with a mesh cover to prevent external colonization, and half were left uncovered so that both internal and external recovery mechanisms could operate. Daphnidae were the crustacean zooplankton taxon most affected by exposure to deltamethrin, with calanoid copepods, another group of crustaceans, affected to a lesser extent. Recovery of daphnid populations occurred between 77 and 105 days. Most benthic insect groups recovered by 84 days after treatment. Hanson et al. (2007) cite a provisional NOEC for deltamethrin in water of 9.6 ng L−1, derived from previous mesocosm work. The ecologically acceptable concentration from a 6-month microcosm study has also been given as 3.2 ng L−1 based on invertebrates, including sediment-dwelling organisms (EC 2002).
Grøsvik and Andersen (1997) carried out a field trial in Norwegian coastal waters to determine the effect on non-target organisms of deltamethrin applied as ALPHA MAX™ in a marine fish farm. The farm chosen was considered to represent reasonable worst-case conditions. The trial was located in a secluded bay, with shallow waters, i.e. 15–20 m depth below the net pens, and with low water currents (0.01–0.03 m s−1 at 2 m depth). Caged marine shrimp, Palaemon elegans, was chosen as a relevant species for sentinel monitoring as this crustacean naturally occurs in shallow waters in Norway. A total of six net pens (11 × 11 × 2 m) were treated on a single day at a concentration of 5 μg a.i. L−1 (five pens) or 7.5 μg a.i. L−1 (one pen). Field-collected shrimps were divided into test replicates by placing 10 randomly chosen shrimps into a small net. The nets were placed at fixed positions 1–500 m from the farm, and at 2–3 m depths before initiation of the treatment, and acclimatized before the trial. The mortality rate during this period was 5.6%. Not all nets had the required acclimatization period due to variation in the current, which required repositioning. Each net pen was treated for 30 min, yielding six releases approximately 1 h apart. Salinity ranged from 32 to 33.5 ppt approximately and temperature 10 to 8 °C. A gradient in the mortality of shrimps was observed, dependent on their position and depth relative to the farm. Mortality at 48 h after application of ALPHA MAX™ is shown in Table 2.
| Depth | Distance from the farm | ||||
|---|---|---|---|---|---|
| 1 m | 5 m | 10–15 m | 30 m | 50–500 m | |
| 1 m | 96 | 70 | 40 | 5 | Mean 8.6%* |
| 3–5 m | 50 | 20 | 5 | 40 | |
| Near seabed | NR | 0 | 0 | 0 | |
- * A 350 m observation 74site was not included in the results as the net could not be located at 48 h. It was found 4 weeks after treatment and all the shrimps (30) were alive. NR, not reported.
The abundance of algae in the water, to which deltamethrin would normally be expected to adsorb, was considered to be unusually low for the time of year, which may have influenced the results in this study (Grøsvik & Andersen 1997). Also, definitive unexposed sites were compromised during the trial due to steadily changing current and wind directions, washing the outlet back and forth in the bay, exposing the ‘control’ sites close to the farm and possibly reaching the distant sites. However, the mean mortality rate (8.6%) at sites >50 m from the fish farm was not statistically significantly different from that of the acclimation period (5.6%), and is within the 10% mortality considered to be acceptable for laboratory controls in aquatic toxicity testing. This study therefore demonstrated that under realistic worse-case conditions, the spatial extent of adverse effects in a sensitive crustacean species was not significant at a distance >50 m from the fish pens.
Overall, the different lines of evidence from single species, mesocosm and field studies, and from TTE/SSD analyses suggest that short-term (i.e. 3 h) concentrations of deltamethrin below approximately 10 ng L−1 should not have substantial adverse effects on sensitive non-target arthropod species in the water column near to aquaculture facilities. The accuracy of this prediction should be monitored at representative fish farms, but is expected to be robust, given the different lines of evidence that support it.
Acknowledgments
This study was funded by PHARMAQ AS. We thank Asbjorn Bergheim and Rosa Richards for useful comments during the peer review of this manuscript.




