Live prey first feeding regimes for short-snouted seahorse Hippocampus hippocampus (Linnaeus, 1758) juveniles
Abstract
As with many species of seahorses, Hippocampus hippocampus wild populations are being subjected to uncontrolled exploitation in their natural environment. Thus, aquaculture could contribute to satisfy the commercial demand for animals while promoting the recovery of wild stocks. The present study was conducted to compare the effect of the substituting Artemia nauplii with rotifers for first feeding seahorse juveniles. Survival, growth and biochemical composition of prey organisms and fish were studied during the feeding trial. In addition, to help the biometric study, an anaesthetic test was also carried out using clove oil. The results showed excellent survival (average 60%) in juveniles exclusively fed with Artemia, with better values than those reported previously obtained by other authors for this species. By comparison, high mortality and poor growth were observed during first feeding with seahorses fed on rotifers. This could have been related to the lower energy intake and poorer nutritional value of the rotifers. Furthermore, clove oil concentrations of 25 ppm were found to work well as an anaesthetic for seahorse juveniles. Overall, first feeding Artemia alone was found to be an efficient and simplified method for feeding young H. hippocampus fry, building the principles for their culture for ornamental or re-stocking purposes.
Introduction
The state of seahorse populations shows the uncontrolled overexploitation of marine resources and the unsuitable management of wild areas (Vincent 1996). For this reason, all seahorse species have been included in appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES 2002) (González, Piloto, Chevalier & Rivero 2006). Reproduction in captivity is considered as one of the solutions to reduce the pressure on wild stocks by fishing and ornamental business operations. The species studied in this work, Hippocampus hippocampus, is present in the Canary Islands, and catalogued as data deficient by the red book of the IUCN (The World Conservation Union) and as ‘Vulnerable’ in the Catalogue of Threatened Species of the Canary Islands (Government of the Canary Islands, D. 151/2001, 23 July). For this reason, references about the life history of this species are scarce. In the wild, H. hippocampus populations appears to live close to seagrass areas related with artificial holdfast, living in low densities and little home ranges (Wilson & Vincent 1999; Curtis & Vincent 2005). In the natural environment, the short-snouted seahorse regime is composed basically on little crustaceans as mysis, amphipods and copepods species (Kitsos, Tzomos, Anagnostopoulou & Koukouras 2008). Like other seahorses spp., it is the male that becomes pregnant. After a period between 2 and 4 weeks (Boisseau 1967), depending on water temperature, the male releases his brood. The standard length of newborn seahorses averages 9 mm (Damerval, Détienne, Détienne & Vincent 2003; Lourie, Foster, Cooper & Vincent 2004). Early life steps of this fish are described with a first pelagic behaviour of 2–3 weeks (Vincent 2001; Damerval et al. 2003). After this, juveniles use the prehensile tail to settle. They reach sexual maturity around 16–18 weeks for males and 18 for females (Damerval et al. 2003).
Concerning breeding in captivity of H. hippocampus, although there are more than 100 successful references in ‘Aquariology Forums’, most of them are not applicable for large-scale production operations with the aim of restocking or for commercial trade activities. Usually, live copepods or other zooplanktonic animals caught in the wild are used in seahorse culture with high survival rates (Gardner 2004; Bentivegna 2006; Jones & Lin 2007). However, copepods are not generally used for rearing marine ornamental fish as reliable large-scale production methods are usually difficult to attain (Payne & Rippingale 2000). By contrast, rotifers and Artemia are commonly used as live prey food organisms in aquaculture, and have been also used in seahorse breeding with different degrees of success, depending upon animal size and species. Thus, the reported survival rates obtained in captivity for H. hippocampus (Linnaeus, 1758), have ranged between 30% and 45% (Damerval et al. 2003), animals being first fed with rotifers until day 4 and then fed with Artemia. Sometimes rotifers have been used in seahorse breeding, combined with other live prey such as Artemia, copepods or both. This is due to their adequate size and better digestibility for the first 5 days of culture, which is believed to be the critical bottleneck in seahorse culture (Damerval et al. 2003; Jones & Lin 2007). Other authors used exclusively Artemia, copepods, or both, with other seahorse species born in aquaria with varied results (Reyes-Bustamante & Ortega-Salas 1999; Wilson & Vincent 1999; Payne & Rippingale 2000; Woods 2000a, b; Choo & Liew 2006; González et al. 2006; Jones & Lin 2007; Martinez-Cardenas & Purser 2007; Sheng, Lin, Chen, Shen & Lu 2007; Truong Si Ky & Hoang Duc Lu 2007).
Sometimes, high survival rates in breeding experiments have been related with the use of relatively simple larval rearing techniques (Wilson & Vincent 1999) such as the decapsulation of Artemia cysts before hatching so as to eliminate pathogenic bacteria sources, the nutritional enrichment of live prey organisms before feeding, the use of proper acclimation procedures using overlapping feeding protocols, or the maintenance of scrupulous hygienic controls within seahorse rearing tanks. Moreover, the success of larval rearing is greatly influenced by first feeding regimes and the nutritional quality of starter diets (Izquierdo, Tandler, Salhi & Kolkovsky 2001). In this sense, the dietary lipids are recognized as one of the most important nutritional factors that affect larval growth and survival (Watanabe, Kitajima & Fujita 1983) and in particular highly unsaturated fatty acids (HUFA). Numerous studies have shown that the larvae of marine fish have a dietary requirement for arachidonic (ARA; 20:4n-6), eicosapentaenoic (EPA; 20:5n-3) and docosahexaenoic acids (DHA; 22:6n-3) (Webster & Lovell 1990; Lemm & Lemarie 1991; Rimmer, Reed, Levitt & Lisle 1994; Sargent, Bell, McEvoy, Tocher & Estevez 1999) and these must be supplied in the diet to ensure optimal growth and survival (Chang & Southgate 2001).
In addition to the nutritional quality of first feeding regimes, it is important to understand the morphological development and allometric growth patterns of fish (Koumoundouros, Divanach & Kentouri 1999; Gisbert, Merino, Muguet, Bush, Piedrahita & Conklin 2002) for the optimization of production in aquaculture. Biometric data can also provide an insight into possible functional trends and environmental preferences of different developmental stages (Fukuhara 1988). In most biometric studies, marine organisms need to be anaesthetized in order to avoid sacrificing animals (Choo & Liew 2006). Anaesthetic methods have generally been used to reduce fish stress (Ross & Ross 1999). Optimal doses must be used in order to achieve a 100% recovery rate from anaesthetic protocols. Anaesthetic episodes in fish can be divided into different periods, beginning with equilibrium loss (phase I), numb stage with operculum movement (phase II) and without this movement (phase III) (García-Gómez, de la Gándara & Raja 2002). Concerning the use of anaesthetics with seahorses, some researchers have cited the use of MS-222 (Boisseau 1967; Bull 2002; Jones & Lin 2007) and AQUI-S (Woods 2000a, b), which has been commonly used with different species. However, authors seldom detail their protocol. Natural clove (Syzygium aromaticum; Merr. & Perry) essential oil was found to present a number of advantages over other commonly used anaesthetics: a high efficacy at low doses, cost-efficiency, non-toxicity, potential positive side effects and no need for a withdrawal time for selling the fish after its use (García-Gómez et al. 2002). These characteristics are very important for routine tasks in handling cultured marine fish (Munday & Wilson 1997; Waterstrat 1999; Woody, Nelson & Ramstad 2002).
This study is part of a coordinated research project (http://www.iim.csic.es/proyectohippocampus) devoted to evaluate the wild seahorse resources of the Spanish Atlantic coasts, from NW Iberian Peninsula to the Canary Islands. The aim of this work was to evaluate the effect of dietary replacement of live Artemia nauplii by rotifers during the first 5 days of feeding for newborn short-snouted seahorses H. hippocampus (Linnaeus, 1758), by quantifying the effects on survival rate and growth during their first weeks (34 days). In addition, the lipid composition and fatty acid content of the prey organisms and fish were analysed during the trial so as to see if there was a correlation between animal survival and growth with the different food organisms.
Material and methods
One pregnant male seahorse was collected by scuba diving among seaweed (Cymodocea nodosa) in Melenara bay (27°59′N, 15°22′W), Eastern coast of Gran Canaria island (Canary Islands, Spain), in April 2007 (approximate photoperiod of 10 L:14 D). The male standard length (Villares 2005) was 11.3 cm and the wet weight was 3.48 g. The pregnant animal was then transported in a 10 L acclimatization tank with seawater and oxygen to the laboratory research facilities at the Instituto Canario de Ciencias Marinas (ICCM) in Telde (Gran Canaria, Canary Islands, Spain).
The adult seahorse was initially held in an aerated 100 L quarantine square glass aquarium supplied with flow-through ambient seawater, purified previously using a prefiltration tank equipped with an under-gravel filter, 5 μm cartridge filters and finally UV light (Philips, 25W/G25T8UV-C, Holland, the Netherlands). The flow rate was 1 L min−1. The salinity was 37‰, the temperature was maintained by a thermostat (Jäguer, 300 W, Wuestenrot, Germany) at 22–23 °C and the oxygen level ranged from 6.5 to 7 mg L−1. The aquarium was illuminated 10 h day−1 (Woods 2000b) using broad-spectrum fluorescent tubes (Sera blue daylight, 36 W, 12 000° K, Heinsberg, Germany). Furthermore, a continual cleaning to prevent hydroids and anemones from invading the tank was performed every day. The fish was fed twice a day with live mysids (Leptomysis sp. and Paramysis sp.) caught in the wild. In addition, two seaweed-like plastic attachment substrata were placed in the aquarium to help the fish to acclimatize.
After 10 days, the male released 468 newborn seahorses. Immediately (day 0), 15 offspring were randomly selected and sacrificed for biometric studies (length and dry/wet weight). The remaining seahorses were divided equally into six 35-L square glass aquaria (one triplicate per treatment). The stocking density within tanks was 2.3 seahorses L−1. The rearing tanks were supplied with flow-through ambient seawater also filtered with 5 μm cartridge filters and UV light (Sera, 25 W) at a flow rate of 2 L min−1. This high flow rate was established to assure that untouched living foods (rotifers and Artemia) were removed after each feeding periods. Aeration to each tank was provided by an air pump located out of the aquaria (125 mL min−1). Measurements of physical parameters like temperature ( °C) and oxygen level (mg L−1) were carried out every day. Nitrogen compounds than can be lethal for fish, including ammonia and nitrites (Otero-Ferrer 2001), were measured twice a week (Red Sea Europe, Marine and Freshwater Test Lab, Verneuil Sur Avre, France). Light was provided by one fluorescent tube (Sera blue sky, 36 W, 12 000° K, Germany) for each triplicate and the photoperiod was 10 L:14 D. During the first 2 weeks, we applied lighting from the bottom and also darkened off the top and sides of tanks. Seahorses eat their food below the surface thanks to the live prey's positive phototactic behaviour (Wilson & Vincent 1999; Woods 2000b; Warland 2003; Choo & Liew 2006). This period concerned the seahorse planktonic phase (Damerval et al. 2003; Warland 2003; Choo & Liew 2006; Sheng et al. 2007). The light intensity just above the water surface ranged between 60 and 100 lx (HT Digital Light Meter, HT170N, HT Instruments, Barcelona, Spain). After this (days 15–34), the fish were progressively acclimated to normal lighting applied from the top of tanks. The light intensity just above the water surface then ranged between 800 and 1000 lx. From the 10th day, two white plastic mesh (12 cm × 5 cm) were added in each aquarium to provide holdfasts and habitats for the animals (Villares 2005).
In this study, two experimental protocols were tried with different food sequences for seahorse first feeding. In the first treatment (RA), newborn seahorses were exclusively fed until day 5 after birth (DAB) with 5 rotifers mL−1μm (Brachionus plicatilis), L-strain (240 μm). Rotifers had been grown out in a batch culture system fed with commercial yeast (Saccharomyces cerevisiae) and enriched with DHA protein-Selco (INVE Aquaculture, Dendermonde, Belgium) following the standard protocols of ICCM (Roo, Hernández-Cruz, Socorro, Fernández-Palacios, Montero & Izquierdo 2009a). After which, enriched (Easy – DHA, INVE Aquaculture) Artemia Instar II (EG type 850 μm, INVE Aquaculture) were progressively included for 3 days in the seahorse diet (0.25, 0.50 and 0.75 Artemia mL−1), until their concentration reached 1 Artemia mL−1. Meanwhile, rotifers concentrations were reduced to 0 during feeding episodes. In the second treatment (A), seahorses were fed only with enriched (Easy – DHA, INVE Aquaculture) Artemia (EG type, INVE Aquaculture) Instar II (1 mL−1). The seahorses were fed twice per day (at 9:00 and 14:00 hours) after removing the uneaten food and faeces from the bottom of the tanks. During feeding, the rearing tanks remained without an inflow of fresh seawater for 2 h. In this experiment, factors that traditionally had relevance in seahorse breeding, like type (Damerval et al. 2003) and position (Woods 2000b; Warland 2003) of lighting, aeration intensity (Molina, Socorro, Herrera, Otero-Ferrer, Villares, Fernández-Palacios & Izquierdo 2007) or special tank design (Woods 2000a; Warland 2003; Matsushige & Melechinsky 2004), were incorporated into the experimental design.
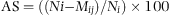
N i was the initial number of seahorses placed in each aquarium at day 1.
M ij was the total seahorses died since day 1 until the day j.
At 0, 5, 13, 22 and 34 DAB, three biometric measures were also taken: snout length (SnL), head length (HL) and trunk length (TrL) (Villares 2005), using a ‘profile projector’ (Mitutoyo PJ-A3000, Kawasaki, Japan). At the same time, an anaesthesia test was carried out, testing natural clove essential oil (Guinama®, Valencia, Spain), containing 87% eugenol anaesthetic. Thirty seahorses (five animals from each tank) of 22 were chosen randomly for the trial. Concentrations of 25 ppm of natural clove essential oil were prepared in 100 mL beakers with seawater, from a 0.5% (v/v) solution diluted in 96% ethanol (Panreac®, Barcelona, Spain). During the test, the seawater temperature was constant (20 °C). The five young seahorses from each aquarium (15 from RA and 15 from A treatment) were introduced into their respective beaker. Behaviour and possible side effects were observed and noted. When the animals showed symptoms of numbness with only operculum movement (phase II) (Iwama & Ackerman 1994), they were then placed into a Petri dish for the measurement with the profile projector. To test the efficiency of the anaesthesia, biometric measurements completed during the first 5 min (García-Gómez et al. 2002) after clove essential oil administration were considered as ‘positive’, and if they took >5 min to arrive to phase II anaesthetic, they were denominated ‘negative’. After that, the seahorses were immersed in a ‘recovery’ aerated tank in order to recover normal activity and regular breathing, before their return to the rearing aquaria.
Weight (dry and wet) was also evaluated at the end of the trial. For the dry weight, seahorses were placed in an oven (Jouan, EU 280 EL TS SN Inox, Saint-Herblain, France) at 105 °C until the weight became constant (AOAC 1995). The measurements were taken with a precision balance (Mettler Toledo, AG204, Greifensee, Switzerland). The crude lipids and fatty acid methyl esters content of seahorses (A and RA treatments) were determined at DAB 5 and DAB 34. In order to obtain prey nutritional quality, 3 g samples (wet weight) of rotifers (6 h enriched) and Artemia (24 h enriched) were collected throughout the trial. A commercial trademark protocol was followed to enrich live preys. The samples were taken at the same time that the seahorses were fed. Concerning seahorses, five random fish of each treatment replicate were taken at days 5 and 34. Samples were collected with an adequate-sized nylon mesh, measured with a precision balance and washed twice with distilled water and freeze-dried (−80 °C) for subsequent analysis. In order to obtain sufficient sample for lipid analysis in seahorses, three replicates from each treatment were combined before analysis (Chang & Southgate 2001).
Crude lipid (% wet weight) of seahorses and live prey (Artemia and rotifers) were extracted following the method of Folch, Lees & Stanley (1957). Fatty acid methyl esters from total lipids were prepared by transmethylation as described by Christie (1982) and separated and quantified by das liquid chromatography under the conditions described by Izquierdo, Watanabe, Takeuchi, Arakawa & Kitajima (1990).
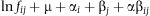
ln fij is the expected frequency in row i, column j of the two-way contingency table.
μ is the mean of the logarithms of the expected frequencies.
αi is the effects of category i of factor A (survival).
βj is the effects of category j of factor B (treatment).
αβij is the interaction term that expresses the dependence of category i of factor A on category j of factor B.
Variations between RA and A treatment concerning growth (length and weight) and seahorse lipid composition factors were tested using One-way anova (Zar 1996). All results were processed and analysed using spss statistical software system version 16.0.1. (SPSS, Chicago, IL, USA, 1999).
Results
General observations
Since the first day, visual inspection of seahorses during feeding periods showed hunting behaviour and a full orange (A treatment) (Fig. 1a) or white (RA treatment) gut. Until DAB 10, the fish of both treatments showed pelagic activity. The first seahorse showing ‘benthic’ behaviour was seen on DAB 15 for A treatment and on DAB 22 for RA treatment. At the end of the trial, all seahorses from both treatments had developed their prehensile tails and were fixed in the plastic nets. During the experiment, some animals presented bubble problems in the gut (Fig. 1b) or reduction in swimming activities in both treatments, with some remaining floating on the water surface and finally died. Others appeared to be able to expulse the gas bubble and survive. Finally, no sexual dimorphism was found.
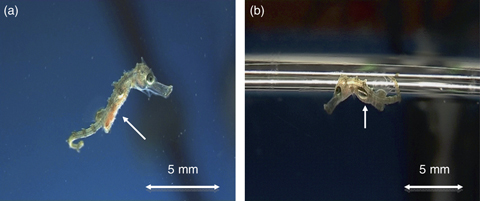
Day after birth five offspring's of Hippocampus hippocampus of treatment A with an orange gut after a feeding episode (a) and having a bubble gas problem, in RA treatment (b).
Survival
The average temperature during the experiment was 21.9±0.6 °C for all the aquariums. Oxygen levels were 6.8±0.2 and 6.7±0.2 ppm for RA and A treatment respectively. Concerning chemical parameters, ammonia and nitrites levels were always under 0.2 and 0.02 ppm during the trials.
Cumulative survival average at the end of experiment was significantly lower in RA treatment (25.93%±0.19) (ZRA,vivos=−6662; P<0.0001) compared with A treatment (59.90%±10.76) (ZA,vivos=+6662, P<0.0001) (Fig. 2). Beside, RA showed high mortality peaks between 5 and 9, whereas in A, low mortality was noted gradually (Fig. 2).
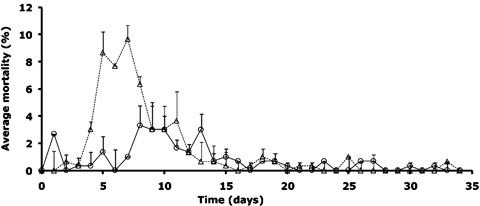
Daily average mortality observed in each treatment, RA (Δ) and A (O), during the experiment.
Growth
Biometric studies first showed significantly lower values with RA treatment for all parameters (snout, head and trunk lengths) in seahorses, even at the first sampling. Nevertheless, at the end of the study, no significant (P<0.05) difference was found between treatments (Fig. 3).
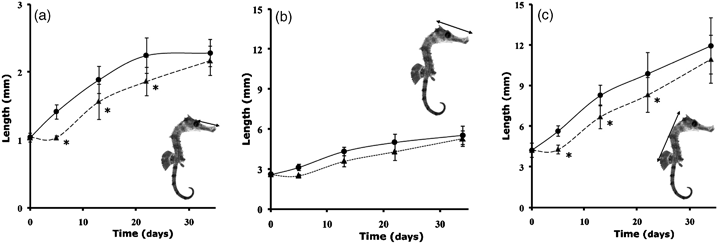
Measurements of seahorse juveniles' snout (a), head (b) and trunk (c) lengths. *Significant differences between RA (Δ) and A (O) treatments.
Both wet and dry weights obtained were significantly lower (P<0.05) with RA treatment (48.55±25.39 and 6.3±2.72 mg respectively) than with A treatment (62.64±31.70 and 12.1±5.10 mg respectively).
Lipid composition
The average crude lipid content (mean±SD) on a dry weight basis of the live prey food organisms was 32.36±1.76% for Artemia and 10.24±0.07% for rotifers. Concerning seahorse juveniles, the average crude lipid percentage (mean±SD) of wet weight for treatments RA and A was 2.78±0.14 and 3.27±0.23% respectively at 5; and 2.88±0.14% for RA and 2.88±0.08% for A at the end of trial. No significant difference was found in the lipid composition between treatments (P<0.05).
Concerning the fatty acid composition of Artemia and rotifers (Table 2) fed to the seahorses, our results show that the crustacean was characterized a high palmitic (16:0), oleic (18:1n-9) and linoleic (18:2n-6) acids, denoting its geographical origin. Besides, they also showed a higher content than the rotifers in ARA (20:4n-6), EPA (20:5n-3) and DHA (22:6n-3), which are considered as essential fatty acids (EFA) for marine fish (Tandler, Watanabe, Satoh & Fukusho 1989; Izquierdo 1996; Rodríguez, Pérez, Izquierdo, Cejas, Bolaños & Lorenzo 1996; Chang & Southgate 2001).
| Treatment | A | RA | A | RA |
|---|---|---|---|---|
| Day | 5 | 5 | 34 | 34 |
| 14:0 | 1.8 | 1.63 | 1.47 | 1.53 |
| 16:0 | 15.8 | 17.6 | 13.9 | 13.4 |
| 16:1n-9 | 0.62 | 1.52 | 1.27 | |
| 16:1n-7 | 1.9 | 1.87 | 1.8 | 3.05 |
| Me16:0 | 0.91 | 0.27 | 0.14 | |
| 17 | 1.1 | 0.75 | 0.72 | 0.72 |
| 16:3n-4 | 0.6 | 0.16 | 0.05 | |
| 16:3n-3 | 1.3 | 2.1 | 1.35 | 1.15 |
| 18:0 | 12.1 | 12.2 | 11.3 | 8.04 |
| 18:1n-9 | 17.4 | 14 | 16.9 | 19.5 |
| 18:1n-7 | 3.8 | 3.05 | 3.6 | 7.06 |
| 18:1n-5 | 0.1 | 0.19 | 0.15 | |
| 18:2n-9 | 0.15 | 0.37 | 0.51 | |
| 18:2n-6 | 14.7 | 3.04 | 13.8 | 6.68 |
| 18:3n-6 | 0.48 | 0.42 | 0.51 | |
| 18:3n-3 | 8.9 | 1.57 | 8.34 | 11.9 |
| 18:4n-3 | 1.7 | 0.49 | 1.67 | 2.36 |
| 20 | 0.6 | 0.42 | 0.63 | 0.26 |
| 20:1n-9 | 0.5 | 0.53 | 0.8 | 0.92 |
| 20:1n-7 | 0.22 | 0.26 | 0.16 | |
| 20:2n-6 | 0.3 | 0.26 | 0.32 | |
| 20:3n-6 | 0.16 | 0.15 | 0.2 | |
| ARA (20:4n-6) | 2.2 | 5.01 | 2.12 | 1.88 |
| 20:4n-3 | 0.4 | 0.17 | 0.44 | 0.53 |
| 20:5n-3 | 2.6 | 5.45 | 2.45 | 4.92 |
| 22:4n-6 | 0.86 | 0.38 | 0.32 | |
| EPA (22:5n-3) | 1 | 1.87 | 1.07 | 0.77 |
| DHA (22:6n-3) | 8.7 | 20 | 8.96 | 6.53 |
| ∑ Saturated* | 32.7 | 33.8 | 29.2 | 25.1 |
| ∑Monoenes† | 24 | 21.7 | 26.8 | 34 |
| ∑n-3‡ | 25 | 31.9 | 24.7 | 28.2 |
| ∑n-6§ | 16.9 | 10.1 | 17.5 | 10.5 |
| ∑n-9¶ | 17.9 | 15.3 | 19.6 | 22.3 |
| ∑n-3HUFA∥ | 12.7 | 27.5 | 12.9 | 12.7 |
| DHA/EPA | 3.35 | 3.66 | 3.65 | 1.33 |
| OA/n-3HUFA | 1.37 | 0.51 | 1.31 | 1.53 |
| EPA/ARA | 1.18 | 1.09 | 1.15 | 2.61 |
- * Includes 12:0, 14:0, 15:0, 16:0, 17:0, 18:0 and 20:0.
- † †Includes 14:1n-5, 14:1n-7, 15:1n-5, 16:1n-9, 16:1n-7, 16:1n-5, 18:1n-9, 18:1n-7, 18:1n-5, 20:1n-9, 20:1n-7 and 22:1n-11.
- ‡ ‡Includes 16:3n-3, 16:4n-3, 18:3n-3, 18:4n-3, 20:3n-3, 20:4n-3, 20:5n-3, 22:5n-3 and 22:6n-3.
- § §Includes 16:2n-6, 18:2n-6, 18:3n-6, 20:2n-6, 20:3n-6, 20:4n-6 and 22:4n-6.
- ¶ ¶Includes 16:1n-9, 18:1n-9, 18:2n-9, 20:1n-9 and 20:2n-9.
- ∥ ∥Includes 20:4n-3, 20:5n-3, 22:5n-3 and 22:6n-3.
- ARA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; HUFA, highly unsaturated fatty acid.
Rotifers were mainly characterized by 16:0, 16:1n-7 and 18:1n-9 and their content in ARA, EPA and DHA were very low. They were also characterized by a higher amount of 18:2n-9 than Artemia.
After only 5 days of feeding on Artemia, seahorses showed a high content of 18:2n-6 and particularly 18:3n-3 (Table 2). On the contrary, the oleic acid content was lower in rotifer-fed seahorses (RA treatment) but ARA, EPA and DHA were higher with RA treatment than in Artemia-fed seahorses. Moreover, these fish had a low content of 16:1n-7 and 18:2n-6 despite the fact that both fatty acids were high in the rotifers. After 34 days of feeding, saturated fatty acids, particularly 16:0, 17:0 and 18:0 were reduced in seahorses in comparison with the younger stages, whereas monoenoic ones were increased Tables 1 and 2.
| Artemia | Rotifers | |
|---|---|---|
| 14 | 2.0 | 3.0 |
| 14:1n-7 | 0.5 | 2.1 |
| 14:1n-5 | 0.2 | 0.3 |
| 15:0 | 0.5 | 1.6 |
| 15:1n-5 | 0.3 | 0.2 |
| 16:0ISO | 0.1 | 0.2 |
| 16 | 16.0 | 18.0 |
| 16:1n-9 | 0.6 | 1.3 |
| 16:1n-7 | 4.2 | 15.3 |
| 18 | 5.8 | 5.3 |
| 18:1n-9 | 25.0 | 20.4 |
| 18:1n-7 | 4.9 | 4.3 |
| 18:1n-5 | 0.1 | 0.7 |
| 18:2n-9 | 0.3 | 3.7 |
| 18:2n-6 | 5.6 | 5.9 |
| 18:3n-6 | 0.4 | 0.2 |
| 18:3n-3 | 9.3 | 0.6 |
| 18:3n-1 | 0.1 | 0.1 |
| 18:4n-3 | 1.3 | 0.1 |
| 20 | 0.2 | 0.2 |
| 20:1n-9 | 2.4 | 2.6 |
| 20:1n-7 | 0.2 | 0.6 |
| 20:2n-9 | 0.0 | 0.8 |
| 20:2n-6 | 0.3 | 0.2 |
| 20:3n-9 | 0.0 | 0.6 |
| 20:3n-6 | 0.1 | 0.2 |
| ARA (20:4n-6) | 1.3 | 0.6 |
| 20:4n-3 | 0.5 | 0.3 |
| EPA (20:5n-3) | 5.5 | 1.8 |
| 22:4n-6 | 0.4 | 0.2 |
| 22:5n-3 | 0.7 | 0.7 |
| DHA (22:6n-3) | 6.6 | 2.2 |
| ∑Saturated* | 25.5 | 29.4 |
| ∑Monoenes† | 38.3 | 48.0 |
| ∑n-3‡ | 24.0 | 6.8 |
| ∑n-6§ | 8.8 | 8.2 |
| ∑n-9¶ | 28.3 | 29.5 |
| ∑n-3HUFA∥ | 13.4 | 5.0 |
| DHA/EPA | 1.2 | 1.3 |
| OA/n-3HUFA | 1.9 | 4.1 |
| EPA/ARA | 4.2 | 2.7 |
- * Includes 12:0, 14:0, 15:0, 16:0, 17:0, 18:0 and 20:0.
- † †Includes 14:1n-5, 14:1n-7, 15:1n-5, 16:1n-9, 16:1n-7, 18:1n-9, 18:1n-7, 18:1n-5, 20:1n-9 and 20:1n-7.
- ‡ ‡Includes 16:3n-3, 16:4n-3, 18:3n-3, 18:4n-3, 20:4n-3, 20:5n-3, 22:5n-3 and 22:6n-3.
- § §Includes 16:2n-6, 18:2n-6, 18:3n-6, 20:2n-6, 20:3n-6, 20:4n-6 and 22:4n-6.
- ¶ ¶Includes 16:1n-9, 18:1n-9, 18:2n-9, 20:1n-9, 20:2n-9 and 20:3n-9.
- ∥ ∥Includes 20:4n-3, 20:5n-3, 22:5n-3 and 22:6n-3.
- ARA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; HUFA, highly unsaturated fatty acid.
Anaesthetic trial
All seahorses reached Phase II during the first 5 min after anaesthetic administration. They were correctly measured without significant differences between treatments (P<0.05). Finally, the animals recovered normal activity within 30 min after introduction in the ‘recovery’ tank.
Discussion
Since the moment the seahorses are expelled from the paternal pouch, these animals have been described as active and voracious (Herald & Rakowicz 1951; Wilson & Vincent 1999; Woods 2000a) visual predators (Blaxter 1980; Martinez-Cardenas & Purser 2007; Villares, Socorro, Herrera, Otero, Izquierdo & Molina 2007), able to catch different live preys. This fact explained the gut colour of juveniles after feeding episodes in both treatments. To date, the efficiency of digestion, absorption and assimilation of proteins, lipids and carbohydrates from the moment of their birth are still unclear (Damerval et al. 2003; Warland 2003; Martinez-Cardenas & Purser 2007; Sheng et al. 2007; Villares et al. 2007). Thus, it is possible that live preys are eaten but their nutrients not completely assimilated by animals in the first days (Warland 2003).
The planktonic behaviour and ‘settlement’ agree well with that described by other authors (Damerval et al. 2003) for H. hippocampus and other seahorse species (Warland 2003; Choo & Liew 2006). A nutritional deficiency in seahorses fed rotifers compared with those fed Artemia could be explaining the delay in the settlement process. Thus, lipid analysis showed low contents in ARA, EPA and DHA in the rotifers denoting a poor enrichment of these live preys (Rodríguez et al. 1996). Beside, high values of palmitic (16:0), palmitoleic (16:1n-7) and oleic (18:1n-9) acids are principal components of the total lipids commonly found in poorly enriched rotifers (Rodríguez et al. 1996; Roo, Hernández-Cruz, Socorro, Fernández-Palacios, Montero & Izquierdo 2009b). The possible HUFA deficiency in prey could also explain some alteration in the behaviour of young seahorses, such as the reduction in swimming and in feeding activities (Izquierdo 1996; Izquierdo, Socorro, Aranztamendi & Hernández-Cruz 2000). Bubble gas problems could also be related to a deficient swim bladder inflation described also by Koven (1991) in gilthead sea bream fed n-3 HUFA-deficient rotifers, which larvae floating on the water surface. Alternatively, lipid levels in preys are directly related to enrichment conditions (Izquierdo 1988; Rainuzzo, Reitan, Jorgensen, & Olsen 1994; Rodríguez et al. 1996). In this way, Roo et al. (2009b) showed better levels of HUFA in rotifers enriched in the same circumstances. Therefore, it is hypothesized that there was a problem with an inadequate enrichment time, which can degrade HUFA in a lipid emulsion (Rodríguez et al. 1996). Regarding Artemia fatty acid composition, our values were close to those found by Roo et al. (2009b), with the same type of Artemia sp. in similar enrichment conditions. More adequate lipid composition in preys could explain the fact that fish fed Artemia presented more than double the wet weight than rotifer-fed ones at day 5. The high levels of linolenic (18:3n-3) acid in Artemia are related to a cyst origin (Webster & Lovell 1991), and markedly differed from those in rotifers (Roo et al. 2009b), whereas the high EPA (20:5n-3) denoted the success in the enrichment process.
During the first weeks, seahorses juveniles fed Artemia presented better biometric values (length and dry weight) and survival rates than those fed rotifers. In fact, the mortality peaks found in the last ones, between 5 and 9 are similar to what was expected for a fry submitted to a starvation episode until 4 (Villares 2005; Molina et al. 2007; Sheng, et al. 2007). However, we did observe seahorses ingesting rotifers during the course of the experiment. This fact was also confirmed by the higher survival rate we obtained compared with the total mortality found in starvation experiments performed in similar temperature conditions (Villares 2005; Molina et al. 2007). Nevertheless, the wet weight at 5 did not increase compared with DAB 1 for rotifer-fed seahorses. This suggests that energy ingestion in seahorses fed rotifers could be suboptimal and unbalanced with the effort of catching preys. In contrast, the high content in linoleic (18:2n-6), and particularly linolenic (18:3n-3) fatty acids in seahorses after 5 days of Artemia feeding denoted a very active predation on this crustacean, which presents high contents of these fatty acids, whereas seahorses are not rich in 18:3n-3 at early life stages (L. Molina, pers. comm.). These fish also showed ARA, EPA and DHA contents that were very similar to those of this species at birth (L. Molina, pers. comm.) and to those of 34-day-old fish. Surprisingly, feeding seahorses with rotifers markedly increased the content of these EFA; this could be related to a poor feeding activity of these fish, because ARA, EPA and DHA have been found to be retained during periods of starvation in other fish species (Koven, Kissil & Tandler 1989; Tandler et al. 1989; Ako, Kraul & Tamaru 1991; Van der Meeren, Klungsoyr, Wilhelmsen & Kvensenth 1993; Rainuzzo et al. 1994; Rodríguez 1994; Izquierdo 1996; Izquierdo et al. 2000). Moreover, the reduction in 18:1n-9 can be also related to a depletion of this fatty acid to obtain energy during a starving period (Van der Meeren et al. 1993; Izquierdo et al. 2001), because this fatty acid is one of the main substrates for β oxidation (Izquierdo 1996; Tandler et al. 1989). This fact, together with the low content in 16:1n-7 and 18:2n-6, despite the fact that these fatty acids were high in rotifers, support the low feeding activity of rotifer-fed seahorses. Besides, these live preys were EFA deficient, which also could explain these results (Izquierdo, Watanabe, Takeuchi, Arakawa & Kitajima 1989; Rodríguez, Pérez, Izquierdo, Mora, Lorenzo & Fernández-Palacios 1993, 1994; Izquierdo 1996).
In addition, it is important to note that the mortality rate of rotifers-fed seahorses was reduced and seahorse growth improved when Artemia was progressively included in the diet. At the end of the trial, both treatments presented similar measurements, as seen by Damerval et al. (2003), with the same species. Probably, the increase in HUFA dietary levels supplied with adequately enriched Artemia in RA tanks after the high mortality peaks between 5 and 8 DAB could explain these results (Izquierdo 1996). In the same way, other hypothesis as compensatory growth after starvation episodes or size selective mortality could be considered. Similarly, the lower growth of A-treated animals could mean that the Artemia concentration was not high enough for our fish. This fact underlines the major role played by food density in short-snouted seahorse (H. hippocampus) breeding.
Finally, the good percentage survival rate obtained (60%) in juveniles exclusively fed Artemia (Treatment A) was high in comparison with other reports for the same species. Damerval et al. (2003) obtained a 30–45% survival rate with H. hippocampus, using a feeding protocol, which combined rotifers (1–3) and Artemia (4–32). Similar results were found with others Hippocampus sp. of similar size (Reyes-Bustamante & Ortega-Salas 1999; Jones & Lin 2007). Wilson and Vincent (1999) achieved a 100% survival rate until day 120 with three Indo-Pacific seahorse species (Hippocampus fuscus, Hippocampus barbourii and Hippocampus kuda), but phylogenetic aspects (Casey, Hall, Stanley & Vincent 2004) or the use of copepods (Payne & Rippingale 2000) as first feeding food before Artemia, could explain these results (Gardner 2004).
Concerning the anaesthetic trial with clove oil, the results of this study have shown for the first time, the effectiveness of clove oil at a concentration of 25 ppm without negative side effects for animals at this temperature and after handling. Clove oil has been also found useful in other species of marine fish (Soto & Burhanuddin 1995; García-Gómez et al. 2002) and invertebrates (Seol, Lee, Im & Park 2007). However, it is usually used at higher concentrations (40 ppm for amberjack, common dentex, gilthead seabream, European seabass and sharp-snout seabream; García-Gómez et al. 2002 at 15–17 °C), denoting the special sensitivity of seahorses to this anaesthetic. Similarly, Soto and Burhanuddin (1995) used clove oil concentrations between 33 and 100 ppm for golden-lined spinefoot (Siganus lineatus), at temperatures from 27 to 29 °C. These differences noticed could also be due to animal size, development stage and tank temperature, as all these factors influence anaesthetic efficiency (Ross & Ross 1999).
Conclusions
The good results obtained, especially in terms of survival, showed the adequate nutritive value of enriched Artemia metanauplii administrated in these conditions. This efficient first feeding protocol with Artemia simplifies seahorse production methods and builds the principles for their culture to ornamental purposes. Nevertheless, other experiments must be performed to test different Artemia concentrations or other parameters related with rearing environment (light condition, tank wall colour, green water techniques …) during the first days of seahorse life. Rotifer enrichment procedures should also be revised in order to clarify the bad results obtained with these preys. These trials could improve the survival rates achieved until now with this species. In the same way, further experiments are being performed to confirm the importance of food quality on survival and growth in seahorse breeding.
Acknowledgments
We acknowledge the Consejería de Medioambiente del Gobierno de Canarias and the Ministerio de Educación y Ciencia for support to this projet (CGL-2005-05927-C03-02).




