Quantitative l-lysine requirement of juvenile black sea bream (Sparus macrocephalus)
Abstract
An 8-week feeding experiment was conducted to determine the quantitative l-lysine requirement of juvenile black sea bream Sparus macrocephalus (initial mean weight: 9.13 ± 0.09 g, SD) in eighteen 300-L indoors flow-through circular fibreglass tanks provided with sand-filtered aerated seawater. The experimental diets contained six levels of l-lysine ranging from 20.8 to 40.5 g kg−1 dry diet at about 4 g kg−1 increments. All the experiment diets were formulated to be isoenergetic and isonitrogenous. Each diet was assigned to triplicate groups of 20 fish in a completely randomized design. Weight gain and specific growth rate (SGR) increased with increasing levels of dietary lysine up to 32.5 g kg−1 (P < 0.05) and both showed a declining tendency thereafter. Feed efficiency ratio and protein efficiency ratio was poorer for fish fed the lower lysine level diets (P < 0.05) and showed no significant differences among other treatments (P > 0.05). All groups showed high survival (above 90%) and no significant differences were observed. The whole body crude protein and crude lipid contents were significantly affected (P < 0.05) by dietary lysine level, while moisture and ash showed no significant differences. The composition of muscle and liver also presented similar change tendency. Total essential amino acid and lysine contents in muscle both obtained the highest value when fish fed 32.5 g kg−1 lysine diet (P < 0.05). Serum protein, cholesterol and free lysine concentration were affected by different dietary treatments (P < 0.05), triacylglyceride and glucose contents were more variable and could not be related to dietary lysine levels. Dietary lysine level significantly affected condition factor and intraperitoneal fat ratio of juvenile black sea bream (P < 0.05) except for hepatosomatic index. There were no significant differences in white blood cell count and red blood cell count (P > 0.05), however, haemoglobin level was significantly influenced by different diets (P < 0.05). Analysis of dose (lysine level)-response (SGR) with second order polynomial regression suggested the dietary lysine requirement of juvenile black sea bream to be 33.2 g kg−1 dry diet or 86.4 g lysine kg−1 protein.
Introduction
It is tremendously important for aquaculture where fish meal, which makes up the bulk of the dietary protein supply and causes aquatic water nitrogen pollution (Liebert & Benkendorff 2007), has and needs to be replaced by plant proteins. The use of protein-rich plant ingredients such as soy protein derivatives, maize and wheat glutens has received increasing attention as potential substitutes for fish proteins in practical fish feeding (Thiessen et al. 2003; Tulli et al. 2007; Shafaeipour et al. 2008). However, plant proteins often show deficiencies in some essential amino acids (EAAs) such as methionine, lysine and arginine (Hardy & Barrows 2002). All fish species studied to date have been shown to require 10 EAAs in their diet for maximum growth (Wilson 1985). EAAs deficiency may cause reduced growth and poor feed conversion (Wilson & Halver 1986); therefore, satisfying the EAAs requirements of a species is very important in preparing well-balanced diets.
Of all the EAAs, lysine is often the first limiting one in the ingredients used to prepare fish diets (Forster & Ogata 1998; Small & Soares 2000). It serves along with methionine as a precursor to carnitine, which is involved in the transportation of long-chain fatty acyl groups into the mitochondria for beta oxidation (Walton et al. 1984). Carnitine also facilitates removal of short-chain organic acids from mitochondria, thereby freeing intramitochondrial coenzyme A to participate in the B-oxidation and tricarboxylic acid cycle pathways, by which could avoid accumulation of lipids in fish body (Ozorio et al. 2003). According to Baker & Han (1994), lysine should be used as the reference amino acid for estimating other amino acid requirements because lysine is the only one that does not present endogenous synthesis and, unlike the sulphur amino acids, is exclusively required for body protein deposition. Furthermore, lysine is of particular concern because it is the EAA found in the highest concentration in the carcass of many fish species (Wilson & Poe 1985; Kim & Lall 2000). Therefore, it is of high priority to evaluate the dietary lysine requirement in order to formulate a more cost-effective diet, as protein is usually the most expensive feed component.
Black sea bream (Sparus macrocephalus) is an economically important food fish cultured in Japan, China and some other countries of Southeast Asia (Ma et al. 2007; Shao et al. 2008). It grows fast and has high market value (Hong & Zhang 2003). It is a euryhaline omnivorous species and can thrive in natural waters of salinity ranging from 4.1 to 35.0 g L−1. However, traditional feed for farmed black sea bream is the limited supply of chopped or minced trash fish. This type of diet is difficult to store, has variable nutritional quality, poor feed conversion rate and easily to result in water pollution. So there is an urgent need to develop a cost-effective practical diet for grow-out production of black sea bream. It is well known that lysine plays a significant role in fish, therefore, the objective of the present investigation was undertaken to study the influence of varying dietary lysine levels in isoenergetic diets on growth performance, protein utilization, body compositions and biochemical parameters so as to determine the optimum dietary lysine requirement for black sea bream juvenile.
Materials and methods
Experiment diets
Ingredients and proximate composition of the experimental diets are presented in Table 1, amino acid compositions (g kg−1 dry diet) of dietary ingredients in Table 2 and the analysed EAA contents for each diet in Table 3. Six isonitrogenous and isoenergetic diets were formulated with graded levels of crystalline lysine and dietary lysine was quantitatively increased at the expense of glutamic acid. Experimental diets contained 380 g kg−1 crude protein, which was slightly lower than the optimum protein requirement suggested in our preliminary experiment (410 g kg−1, unpublished data) to assure maximum utilization of the limiting amino acid (Wilson 1989). The 270 g kg−1 diets protein was supplied by fish meal, soybean protein concentrate and gelatine, and the remaining by a mixture of crystalline amino acids (CAAs) without lysine to simulate an amino acid profile found in 380 g kg−1 whole body protein of black sea bream. The basal diet (diet 1) contained the minimum level of lysine from fish meal, soybean protein concentrate and gelatin, and the proximate ratios of synthetic/natural bound lysine to be zero to about 50% of total lysine in the diet. The final levels of lysine were confirmed by amino acid analysis and the values were 20.8, 25.2, 28.8, 32.5, 36.8 and 40.5 g kg−1, respectively, by adding incremental levels of crystalline l-lysine ranging from zero to 20 lysine g kg−1 diet (Table 3).
| Ingredients | Diets no. | |||||
|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | |
| Fish meal | 280 | 280 | 280 | 280 | 280 | 280 |
| Soybean protein concentrate | 120 | 120 | 120 | 120 | 120 | 120 |
| Gelatine | 20 | 20 | 20 | 20 | 20 | 20 |
| Crystalline amino acid premix1 | 90 | 90 | 90 | 90 | 90 | 90 |
| Lys | 0 | 4 | 8 | 12 | 16 | 20 |
| Glu | 20 | 16 | 12 | 8 | 4 | 0 |
| Fish oil | 50 | 50 | 50 | 50 | 50 | 50 |
| Corn oil | 80 | 80 | 80 | 80 | 80 | 80 |
| Mineral premix2 | 15 | 15 | 15 | 15 | 15 | 15 |
| Vitamin premix3 | 15 | 15 | 15 | 15 | 15 | 15 |
| Others4 | 310 | 310 | 310 | 310 | 310 | 310 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Proximate analysis (g kg−1 dry matter) | ||||||
| Crude protein | 385.0 | 383.7 | 386.4 | 384.4 | 387.3 | 387.8 |
| Crude lipid | 148.4 | 150.6 | 149.9 | 152.0 | 148.5 | 149.1 |
| Ash | 120.4 | 120.6 | 120.3 | 120.1 | 120.0 | 120.1 |
| Moisture | 68.9 | 70.9 | 70.3 | 69.1 | 69.6 | 68.4 |
| Lys | 20.8 | 25.2 | 28.8 | 32.5 | 36.8 | 40.5 |
- 1 Crystalline amino acid premix: showed in Table 2.
- 2 Mineral premix (g kg−1 of premix): Na2SiO3, 0.4; CaCO3, 350; NaH2PO4·H2O, 200; KH2PO4, 200; MgSO4·7H2O, 10; MnSO4·H2O, 2; CuCl2·2H2O, 1; ZnSO4·7H2O, 2; FeSO4·7H2O, 2; NaCl, 12; KI, 0.1; CoCl2·6H2O, 0.1; Na2MoO4·2H2O, 0.5; AlCl3·6H2O, 1 and KF, 1.
- 3 Vitamin premix (mg kg−1 diet): retinyl acetate, 40; cholecalciferol, 0.1; α-tocopheryl acetate, 80; menadione, 15; niacin, 168; riboflavin, 22; pyridoxine HCl, 40; thiamin mononitrate, 45; D-Ca pantothenate, 102, biotin, 0.4; folic acid, 10; vitamin B12, 0.04; and inositol, 450.
- 4 Others: zoelite, 30; α-starch, 200; carboxymethylcellulose, 50; sodium dihydrogen phosphate, 25; betaine, 5.
| Amino acids | Supplied by 280 g fish meal kg−1 diet | Supplied by 120 g soybean protein concentrate kg−1 diet | Supplied by 20 g gelatine kg−1 diet | Supplied by crystalline amino acids | Total | 380 g kg−1 whole body protein |
|---|---|---|---|---|---|---|
| EAAs | ||||||
| Val | 10.6 | 4.8 | 0.4 | 9.1 | 24.9 | 24.9 |
| Leu | 15.1 | 6.9 | 0.6 | 10.0 | 32.5 | 32.5 |
| Ile | 9.2 | 4.2 | 0.3 | 8.4 | 22.1 | 22.1 |
| Met | 4.4 | 1.3 | 0.1 | 8.7 | 14.5 | 14.5 |
| Phe | 8.7 | 4.7 | 0.3 | 2.3 | 16.0 | 16.0 |
| Thr | 7.1 | 2.8 | 0.4 | 8.1 | 18.4 | 18.4 |
| His | 4.4 | 2.0 | 0.1 | 0.9 | 7.4 | 7.4 |
| Arg | 13.0 | 6.0 | 1.3 | 7.4 | 27.6 | 27.6 |
| Lys | 14.7 | 5.2 | 0.7 | Variable | Variable | 32.9 |
| NEAAs | ||||||
| Glu | 24.5 | 14.5 | 2.3 | Variable | Variable | 59.5 |
| Gly | 14.7 | 3.4 | 5.0 | 1.4 | 24.5 | 24.5 |
| Ala | 13.0 | 3.5 | 2.0 | 0.5 | 19.0 | 19.0 |
| Tyr | 4.9 | 2.2 | 0.2 | 9.4 | 16.7 | 16.7 |
| Asp | 16.7 | 9.1 | 0.2 | 15.3 | 41.3 | 41.3 |
| Ser | 5.6 | 2.8 | 0.8 | 5.9 | 15.1 | 15.1 |
| Pro | 10.4 | 3.5 | 0.1 | 2.6 | 16.6 | 16.8 |
- EAAs, essential amino acids; NEAAs, non-essential amino acids.
| EAAs (g kg−1 diet) | Diets | |||||
|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | |
| Val | 21.9 | 21.5 | 21.6 | 22.2 | 22.1 | 21.9 |
| Leu | 33.6 | 32.9 | 33.2 | 33.1 | 33.9 | 33.3 |
| Ile | 22.6 | 22.3 | 21.9 | 22.2 | 22.1 | 22.1 |
| Met | 14.1 | 14.2 | 14.3 | 14.2 | 14.2 | 13.9 |
| Phe | 16.1 | 16.1 | 16.3 | 16.9 | 16.7 | 17.4 |
| Thr | 18.6 | 18.5 | 18.1 | 18.6 | 18.3 | 18.2 |
| His | 6.8 | 7.1 | 7.2 | 6.9 | 6.7 | 7.1 |
| Arg | 26.7 | 27.4 | 26.9 | 27.5 | 27.2 | 26.9 |
| Lys | 20.8 | 25.2 | 28.8 | 32.5 | 36.8 | 40.5 |
- EAAs, essential amino acids.
All diets were individually blended in a mixer and then homogenized after fish oil and corn oil were added. During the mixing 6 N NaOH was added to establish a pH level of 7–8 according to the method described by Nose (1979). Gelatin was dissolved separately in a volume of water with constant heating and stirring and then transferred to the above mixture. Distilled water was then added to achieve a proper pellet consistency, and the mixture was further homogenized and extruded through a 3-mm die. The noodle-like diets were dried at 23 °C for 72 h with air condition and electrical fan. Dried noodles were broken into particles by a food processor, sieved to remove particles above 3 mm and then stored in a refrigerator at −20 °C. A representative sample was taken for proximate analysis.
Experiment procedure
Black sea bream were obtained from Marine Fisheries Research Institute of Zhejiang Province in Zhoushan, China. Prior to initiation of the feeding trial, all fish were kept in 300-L circular fiberglass tanks and fed with diet 1 for 2 weeks. At the beginning of the experiment, 20 uniform-sized and healthy fish (initial mean weight: 9.13 ± 0.09 g, mean ± SD) were stocked in each fibreglass tank (300-L water volume). Each experimental diet was randomly assigned to triplicate tanks in a completely randomized design. Each tank was supplied with sand-filtered aerated seawater at a flow rate of 2 L min−1. Fish were maintained under a natural photoperiod, the temperature and salinity of the seawater in tanks were 28 ± 1 °C and 29 g L−1 respectively. Dissolved oxygen concentrations were above 5.0 mg L−1 at any point during the experiment by using air stones with continuous aeration. Experimental fish were fed by hand twice daily (08:00 and 16:00 hours) which were fed slowly little by little to prevent waste of dietary pellets. When the experimental feeds were supplied, the fish would swim to the water surface to ingest the feeds. As long as fish were fed to satiation, they would never come up to water surface again. Hence, their apparent satiation could be judged by feeding behaviour observation, the feed losses could also be avoided almost completely. The experiment lasted for 8 weeks and feed consumption was recorded daily. Tanks were thoroughly cleaned as needed and mortality was checked daily.
Analytical procedures
At the end of the 8-week growth trial, based on our preliminary experiment to test the response of serum free amino acid to feeding in black sea bream, the peak level was obtained at 6 h after feeding. Therefore, collection of three fish from each tank was taken 6 h after feeding to measure serum free lysine, and the samples were deproteinized with 5-sulfosalicylic acid according to the method of Cross et al. (1975) and determined using an automatic amino acid analyser (Hitachi, Model L-8500A, Hitachi, Tokyo, Japan). Then remaining fish were starved for 24 h after the last feeding, and three fish from each tank were anaesthetized (MS-222, Sigma, St Louis, MO, USA at 80 mg L−1) and then stored at −20 °C for subsequent whole body proximate analysis. Dorsal muscles and livers were obtained from all the remaining fish in each tank and stored at −20 °C for subsequent proximate analysis. Blood samples were drawn from the caudal vein of five fish per tank with a 27-gauge needle and 1 mL syringe. Aliquots of blood samples were used to determine blood characteristics [haemoglobin (Hb), red blood cell count (RBC) and white blood cell count (WBC)], and the remaining samples were centrifuged at 836 g for 10 min (4 °C) to obtain serum to measure nutrient levels (Ai et al. 2006), and serum was stored at −20 °C until use.
Pooled samples of liver, dorsal muscle and whole fish in each tank were analysed in triplicate for proximate composition. Moisture, ash, protein and lipid were determined following methods of the Association of Official Analytical Chemists (AOAC 1984). Moisture concentration was determined by drying minced samples for 6 h in a forced-air oven maintained at 105 °C. Ash content was analysed by incinerating samples at 600 °C overnight in a muffle furnace; protein was estimated as Kjeldahl-nitrogen using factor 6.25 and lipid was determined by Soxhlet extraction with petroleum ether for 6 h. The serum protein, total cholesterol, triacylglycerol, glucose concentration (GLU) in juvenile black sea bream were all measured within 3 days, using the diagnostic reagent kit purchased from Nanjing Jiancheng Bioengineering Institute (China) according to the manufacturer’s instructions. Haematological characteristics of juvenile black sea bream were determined using automated hematology analyzer (CELL-DYN, 3200, System, USA). The diets and dorsal muscle of fish used for analysis of amino acid content were freeze-dried at 55 °C for 48 h, and then hydrolyzed with 6 N HCl at 110 °C for 24 h and the chromatographic separation and analysis of the amino acids was performed after orthophthaldehyde (Sigma) derivation using reverse-phase high performance liquid chromatography (HPLC, HP1100, USA) followed the modified procedure of Gardner & Miller (1980). While for methionine and cystine, the samples were oxidized with performic acid at −10 °C for 3 h to obtain methionine sulfone and cysteic acid, then freeze-dried twice with deionized water. The freeze-dried ingredients were analysed as the process of other amino acids by a commercial laboratory using an automatic amino acid analyser (Hitachi 835–50) equipped with a column (Hitachi custom ion exchange resin no. 2619). Tryptophan could not be detected after acid hydrolysis and it was excluded from analysis at the present experiment.
Calculation and statistical analysis
The following variables were calculated:
Weight gain rate (WGR %) = 100 × (final mean weight − initial mean weight) in g/initial mean weight in g.
Specific growth rate (SGR) (% day−1) = 100 × (ln final mean weight − ln initial mean weight)/day.
Feed conversion rate (FCR) = feed intake in g/(final body weight − initial body weight).
Protein efficiency ratio (PER) = weight gain in g/protein intake in dry basis in g.
Protein productive value (PPV) = protein gain in g/protein fed in dry basis in g.
Condition factor (CF) (g cm−3) = 100 × (live weight, g)/(body length, cm)3.
Hepatopancreas index (HSI) = 100 × (liver weight, g)/(body weight, g).
Intraperitoneal fat ratio (IPR) = 100 × (intraperitoneal fat weight)/(body weight).
All data were subjected to analysis of variance and regression analysis where appropriate using spssfor windows (version 16·0, USA). Differences between the means were tested by Tukey’s multiple range test. Differences were considered significant at P < 0.05. The optimum dietary lysine requirement based on SGR, which was estimated by second-order polynomial regression analysis (Y = a + bX + cX2) (Zeitoun et al. 1976).
Results
Growth performance, body-organ indices and feed utilization for juvenile black sea bream given graded levels of l-lysine for 56 days are shown in Table 4. No evidence of outward pathological signs was noted in fish given low levels of dietary lysine in our experiment. Survival of fish fed diets with lysine from 20.8 to 40.5 g kg−1 was above 90% and showed no significant difference. The growth rates of juvenile fish fed graded levels of lysine differed significantly among treatments. WGR of fish increased with increasing levels of lysine up to 32.5 g kg−1 of diet and peaked at 313.5% (P < 0.05), beyond which it showed a declining tendency. SGR increased with increasing dietary lysine level up to 28.8 g kg−1 (diet 3) and remained nearly the same thereafter, except for diet 6 which was lower than fish fed diet 3 to diet 5 (P < 0.05). The highest SGR (2.53% day−1) was observed when dietary lysine reached 32.5 g kg−1 (diet 4). Second-order polynomial regression analysis on the basis of SGR indicated that the optimum dietary lysine requirement of juvenile black sea bream was 33.2 g kg−1 dry diet (86.4 g kg−1 of dietary protein) (Fig. 1). The most efficient FCR and the highest PPV were observed in groups fed diet 4. Fish fed the diets exceeding 32.5 g kg−1 lysine level did not show any improvement in PER whereas the efficiency of protein utilization was reduced when fish fed the diets with lower lysine level (P < 0.05). HSI was higher in fish fed lysine deficient diets than in fish fed adequate lysine levels, but differences among the treatments were not significant (P > 0.05). The lowest CF (1.93), highest IPR (2.75) were observed for fish fed the diet 1 (P < 0.05), but remained nearly the same for other treatments (P > 0.05).
| Parameters | Diets (lysine) | |||||
|---|---|---|---|---|---|---|
| Diet 1 (20.8) | Diet 2 (25.2) | Diet 3 (28.8) | Diet 4 (32.5) | Diet 5 (36.8) | Diet 6 (40.5) | |
| IBW | 9.13 ± 0.13 | 9.13 ± 0.15 | 9.12 ± 0.10 | 9.13 ± 0.08 | 9.13 ± 0.08 | 9.10 ± 0.00 |
| FBW | 32.20 ± 0.13 | 34.32 ± 0.30 | 36.35 ± 0.44 | 37.77 ± 0.67 | 37.21 ± 0.43 | 34.35 ± 0.68 |
| Survival | 96.7 ± 5.8 | 96.7 ± 5.8 | 98.3 ± 2.9 | 95.0 ± 5.0 | 100.0 ± 0.0 | 91.7 ± 2.9 |
| WG | 252.6 ± 3.8 d | 275.8 ± 4.5 c | 298.7 ± 6.6 b | 313.5 ± 4.6 a | 307.4 ± 3.8 ab | 277.4 ± 7.4 c |
| SGR | 2.25 ± 0.02 c | 2.39 ± 0.03 b | 2.50 ± 0.03 a | 2.53 ± 0.02 a | 2.51 ± 0.02 a | 2.40 ± 0.02 a |
| CF | 1.93 ± 0.04 b | 2.01 ± 0.02 a | 2.02 ± 0.03 a | 2.02 ± 0.03 a | 2.04 ± 0.02 a | 1.99 ± 0.01 a |
| HSI | 1.99 ± 0.04 | 1.94 ± 0.15 | 1.94 ± 0.12 | 1.93 ± 0.13 | 1.86 ± 0.03 | 1.85 ± 0.04 |
| IPR | 2.55 ± 0.07 a | 2.38 ± 0.23 b | 2.37 ± 0.05 b | 2.36 ± 0.07 b | 2.34 ± 0.21 b | 2.34 ± 0.17 b |
| FCR | 1.36 ± 0.02 a | 1.31 ± 0.02 a | 1.24 ± 0.02 b | 1.22 ± 0.02 b | 1.24 ± 0.02 b | 1.25 ± 0.01 b |
| PER | 1.76 ± 0.03 c | 1.84 ± 0.03 ab | 1.92 ± 0.03 ab | 1.96 ± 0.03 a | 1.94 ± 0.04 a | 1.94 ± 0.01 a |
| PPV | 0.28 ± 0.02 b | 0.29 ± 0.02 ab | 0.32 ± 0.00 ab | 0.34 ± 0.00 a | 0.33 ± 0.00 a | 0.32 ± 0.01 a |
- IBW, initial mean body weight; FBW, final mean body weight; SGR, specific growth rate; WG, weight gain; CF, condition factor; HSI, hepatopancreas index; IPR, intraperitoneal fat ratio; FCR, feed conversion rate; PER, protein efficiency ratio; PPV, protein productive value.
- Values are presented as mean ± SD (n = 3); values with different superscripts in the same row differ significantly (P < 0.05).
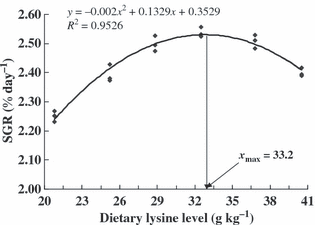
The relationship between specific growth rate (SGR, % day−1, y) and dietary lysine levels (g kg−1, x) in juvenile black sea bream.
Body compositions were significantly affected by dietary lysine level (P < 0.05). The whole body, muscle and liver protein were positively correlated with dietary lysine level, while lipid was negatively correlated with it. The ash and moisture contents were independent of dietary treatment (Table 5).
| Parameters | Diets (lysine g kg−1) | |||||
|---|---|---|---|---|---|---|
| Diet 1 (20.8) | Diet 2 (25.2) | Diet 3 (28.8) | Diet 4 (32.5) | Diet 5 (36.8) | Diet 6 (40.5) | |
| Whole body | ||||||
| Protein | 169.7 ± 1.4 c | 172.0 ± 1.8 b | 173.7 ± 1.7 b | 176.3 ± 1.7 ab | 177.5 ± 1.4 ab | 179.2 ± 2.0 a |
| Lipid | 113.8 ± 2.6 a | 111.4 ± 2.5 a | 109.5 ± 5.5 b | 103.7 ± 1.9 b | 102.3 ± 3.2 b | 101.2 ± 2.5 b |
| Ash | 51.0 ± 0.5 | 51.8 ± 0.2 | 51.6 ± 2.1 | 52.7 ± 0.9 | 52.8 ± 2. 3 | 53.9 ± 1.3 |
| Moisture | 658.0 ± 4.8 | 661.5 ± 3.7 | 658.5 ± 5.8 | 660.8 ± 4.8 | 659.8 ± 3.4 | 659.2 ± 3.5 |
| Muscle | ||||||
| Protein | 188.9 ± 0.6 bc | 197.6 ± 1.1 b | 203.6 ± 0.5 ab | 207.8 ± 1.6 a | 205.6 ± 2.1 ab | 209.0 ± 0.8 a |
| Lipid | 75.7 ± 4.3 ab | 77.9 ± 4.8 a | 72.7 ± 8.2 b | 67.7 ± 4.9 c | 68.9 ± 6.3 c | 67.1 ± 5.7 c |
| Ash | 14.5 ± 0.8 | 13.4 ± 0.1 | 14.1 ± 0.2 | 14.0 ± 0.9 | 13.7 ± 0.1 | 13.8 ± 2.0 |
| Moisture | 728.9 ± 10.4 | 713.6 ± 20.8 | 716.1 ± 19.1 | 710.5 ± 9.9 | 718.4 ± 16.7 | 713.8 ± 26.7 |
| Liver | ||||||
| Protein | 225.9 ± 4.2 c | 226.4 ± 4.9 bc | 230.8 ± 2.6 b | 241.2 ± 1.6 a | 248.5 ± 1.9 a | 247.0 ± 2.5 a |
| Lipid | 167.4 ± 0.8 a | 164.9 ± 1.2 ab | 164.7 ± 1.7 ab | 157.6 ± 2.3 bc | 149.2 ± 5.6 d | 148.8 ± 1.8 d |
| Moisture | 595.1 ± 20.9 | 599.1 ± 16.0 | 602.7 ± 11.0 | 593.7 ± 10.4 | 596.3 ± 14.4 | 595.2 ± 17.8 |
- Values are presented as mean ± SD (n = 3); values with different superscripts in the same row differ significantly (P < 0.05).
Lysine contents of fish muscle were significantly affected by dietary lysine levels (P < 0.05). Fish fed the diet with 20.8 g kg−1 lysine showed the lowest lysine content (8.99 g kg−1) in dorsal muscle, while fish fed the diet with 32.5 g kg−1 lysine had the highest value (9.69 g kg−1). The concentration of Val and His were was more variable and was not related to dietary treatment. However, other EAAs showed an increasing trend with increasing dietary lysine levels except Thr content decreased (P < 0.05). Increasing dietary lysine level enhanced EAAs contents of muscle (P < 0.05) although there was a slight decline in fish fed the diet 5 and diet 6 (Table 6).
| EAAs | Diets (lysine g kg−1) | |||||
|---|---|---|---|---|---|---|
| Diet 1 (20.8) | Diet 2 (25.2) | Diet 3 (28.8) | Diet 4 (32.5) | Diet 5 (36.8) | Diet 6 (40.5) | |
| Val | 4.07 ± 0.05 | 4.08 ± 0.02 | 4.19 ± 0.11 | 4.20 ± 0.03 | 4.23 ± 0.02 | 4.17 ± 0.06 |
| Leu | 2.49 ± 0.10 cd | 2.55 ± 0.06 bc | 2.62 ± 0.04 bc | 2.71 ± 0.07 ab | 2.56 ± 0.10 bc | 2.50 ± 0.06 cd |
| Ile | 4.47 ± 0.08 c | 4.62 ± 0.06 bc | 4.60 ± 0.08 bc | 4.63 ± 0.05 ab | 4.65 ± 0.06 bc | 4.59 ± 0.06 bc |
| Met | 2.44 ± 0.01 bc | 2.50 ± 0.05 ab | 2.57 ± 0.06 ab | 2.62 ± 0.06 a | 2.60 ± 0.14 ab | 2.40 ± 0.05 c |
| Phe | 5.59 ± 0.10 c | 5.67 ± 0.03 b | 5.83 ± 0.11 ab | 5.92 ± 0.04 a | 5.78 ± 0.10 ab | 5.76 ± 0.05 ab |
| Thr | 3.51 ± 0.06 a | 3.39 ± 0.06 ab | 3.30 ± 0.10 b | 3.46 ± 0.11 ab | 3.42 ± 0.06 ab | 3.30 ± 0.04 b |
| His | 1.50 ± 0.01 | 1.47 ± 0.03 | 1.44 ± 0.02 | 1.49 ± 0.04 | 1.44 ± 0.08 | 1.47 ± 0.06 |
| Arg | 4.11 ± 0.02 c | 4.13 ± 0.04 bc | 4.24 ± 0.03 ab | 4.29 ± 0.08 a | 4.24 ± 0.04 ab | 4.30 ± 0.03 a |
| Lys | 8.99 ± 0.03 e | 9.12 ± 0.04 cd | 9.25 ± 0.06 bc | 9.69 ± 0.06 a | 9.50 ± 0.08 ab | 9.02 ± 0.07 d |
| ∑EAA | 37.16 ± 0.23 d | 37.54 ± 0.04 cd | 38.03 ± 0.07 bc | 39.01 ± 0.27 a | 38.41 ± 0.40 ab | 37.52 ± 0.08 cd |
- EAAs, essential amino acids.
- Values are presented as mean ± SD (n = 3); values with different superscripts in the same row differ significantly (P < 0.05).
In serum profile, total protein content increased with increasing dietary lysine level (P < 0.05) (Table 7). Serum-free lysine remained at a relatively low and constant level up to fish fed diet 3. However, an increased level was observed in fish fed diet 4 and diet 5 thereafter and return to relatively low level in fish fed diet 6 similar to those observed in fish fed diet l to diet 3. TG and GLU contents were more variable and could not be related to dietary treatments. From Table 8, it can be seen that dietary lysine concentrations had no significant effect on WBC and RBC, however, Hb was significantly different among treatments and reached the top at 12.85 g 100 mL−1 when fish fed diet 4 (P < 0.05).
| Parameters | Diets (lysine g kg−1) | |||||
|---|---|---|---|---|---|---|
| Diet 1 (20.8) | Diet 2 (25.2) | Diet 3 (28.8) | Diet 4 (32.5) | Diet 5 (36.8) | Diet 6 (40.5) | |
| Total protein (g L−1) | 34.61 ± 0.43 c | 35.84 ± 1.19 bc | 37.56 ± 1.83 b | 38.33 ± 1.37 ab | 39.28 ± 2.01 a | 39.46 ± 1.11 a |
| T-CHO (mmol L−1) | 5.38 ± 0.78 a | 5.14 ± 0.49 ab | 3.57 ± 0.50 bc | 3.68 ± 0.14 bc | 3.41 ± 0.26 bc | 4.08 ± 0.36 b |
| TG (mmol L−1) | 5.96 ± 0.66 | 6.50 ± 0.88 | 5.84 ± 0.55 | 5.74 ± 0.56 | 5.58 ± 0.42 | 5.13 ± 0.88 |
| GLU (mmol L−1) | 5.52 ± 0.64 | 5.85 ± 0.66 | 5.45 ± 0.50 | 6.06 ± 0.75 | 5.82 ± 0.52 | 5.44 ± 0.36 |
| Free lysine (mg L−1) | 1.09 ± 0.04 c | 1.16 ± 0.03 bc | 1.17 ± 0.04 bc | 1.24 ± 0.02 a | 1.20 ± 0.04 a | 1.14 ± 0.03 b |
- T-CHO, total cholesterol; TG, triacylglycerol; GLU, glucose concentration.
- Values are presented as mean ± SD (n = 3); values with different superscripts in the same row differ significantly (P < 0.05).
| Parameters | Diets (lysine g kg−1) | |||||
|---|---|---|---|---|---|---|
| Diet 1 (20.8) | Diet 2 (25.2) | Diet 3 (28.8) | Diet 4 (32.5) | Diet 5 (36.8) | Diet 6 (40.5) | |
| Haemoglobin (g 100 mL−1) | 11.10 ± 0.24 c | 11.61 ± 0.21 b | 11.89 ± 0.59 b | 12.65 ± 0.15 a | 12.05 ± 0.46 ab | 11.26 ± 0.32 c |
| WBC (×109 L−1) | 4.37 ± 0.18 | 4.44 ± 0.40 | 4.88 ± 0.49 | 5.18 ± 0.32 | 4.84 ± 0.21 | 4.78 ± 0.21 |
| RBC (×1012 L−1) | 5.32 ± 0.16 | 5.28 ± 0.16 | 5.20 ± 0.22 | 5.18 ± 0.19 | 5.08 ± 0.16 | 4.92 ± 0.17 |
- WBC, white blood cell count; RBC, red blood cell count.
- Values are presented as mean ± SD (n = 3); values with different superscripts in the same row differ significantly (P < 0.05).
Discussion
The present study results indicated that lysine is essential for growth of juvenile black sea bream, and this marine omnivorous fish is able to utilize crystalline lysine. Dose–response experiments with increasing supply of amino acid are accepted in principle as a method for determining dietary amino acid requirements (Cowey 1995). The optimal dietary crystalline lysine requirement for juvenile black sea bream was estimated to be 3.35% dry diet, corresponding to 8.71% dietary protein. This value is higher to values reported for certain other species, milkfish (4.0%, Borlongan & Benitez 1990); catla (6.20%, Ravi & Devaraj 1991); African catfish (5.7%, Fagbenro et al. 1998); striped bass (4.9%, Small & Soares 2000); Indian major carp (5.75%, Ahmed & Khan 2004); grass carp (5.44%, Wang et al. 2005); black catfish (4.5%, Montes-Girao & Fracalossi 2006); grouper (5.56%, Luo et al. 2006), Japanese seabass (5.80–6.07%, Mai et al. 2006); gilthead seabream (5.04%, Marcouli et al. 2006); These wide variations observed in lysine requirements among fish species may be the result of laboratory variances: differences in dietary protein sources, the reference protein which amino acid pattern is being imitated (Forster & Ogata 1998), different diet formulation and digestible energy (Encarnação et al. 2004), different feeding regime and allowance, environmental condition, and using fish of different ages, sizes and species (strains) (Akiyama et al. 1997). Variations may also be attributed to differences in techniques used to calculate requirement (Kim et al. 1992). Some previous researches have suggested fish do not appear to utilize dietary CAAs as effectively as intact protein (Cowey and Luquet, 1983; Walton et al., 1986; Wilson & Halver 1986), because CAAs added to the diet in the free form are more readily absorbed than protein-bound amino acids (Yamada et al., 1981; Murai et al., 1982), causing an imbalance in amino acid profile in the tissue and diverting amino acids into catabolic rather than anabolic processes (Cowey & Sargent 1979). In our study, the WG and SGR varied from 252% to 313%, and 2.25 to 2.53, respectively. The growth performance seemed not inferior to those fed the intact-protein or precoating amino acids, which implied the black seabream might utilize CAAs more effectively than same fish species. Some previous studies reported that at least 90% or more of the dietary total amino acid should have been consumed up by the fish, which indicated that the leaching loss of lysine could be considered to be negligible and the relative poor growth was not due to leaching (Cheng et al. 2003; Alam et al. 2004 and Espe et al. 2007). In our present study, feeds were offered by hand carefully and slowly, in addition, the experimental black sea bream were all domesticated with high feeding activity, and the granulated feed could be scrambled and ate by fish immediately (<3 s). Thus, leaching loss of feed and crystalline lysine could be avoided almost completely. However, the total lysine concentration was partly contributed by endogenous lysine found in fishmeal, soybean protein concentrate and gelatin, when taking digestibility of the dietary ingredients into consideration, the actual lysine requirement maybe a little lower than the estimated value to formulate cost-effective and amino acid balanced diets for this fish species. Reduced growth, feed utilization, PER and PPV were observed in fish fed still higher amount of lysine (diet 5 and diet 6), as reported in other fish species such as salmonids (Anderson et al. 1993), Indian major carp (Murthy & Varghese 1997) and Japanese seabass (Mai et al. 2006). It may be due to the negative effects (lysine–arginine antagonism) of excessive amount of free lysine at this level. Dietary lysine–arginine antagonism has been well documented in poultry and rats (Jones 1964; Harper et al. 1970; Fico et al. 1982), but there are controversies in fish, further research is needed to clarify this aspect.
The negative correlation between HSI and dietary lysine level was also observed in European sea bass (Tibaldi et al. 1994) and gilthead sea bream (Marcouli et al. 2006). In our study, protein retention decreased in fish fed lower lysine diets. Peres & Oliva-Teles (2008) pointed out as lysine deficiency limited protein synthesis, AA not used for protein synthesis might have been converted after deamination to lipids or glycogen and deposited in the liver. It also possibly explained this result and IPR reducing in present experiment.
Jefferson & Kimball (2001) suggested that lack of lysine in dietary may led to intracellular accumulation of non-aminoacyl-tRNA, the activity of protein kinase A (GCN2 general control non-derepressible protein kinase) and eIF-2α eukaryotic initiation factor phosphatase were decreased, then eIF-2α phosphorylation occurred accompanied by reduced activity of eIF-2β at the same time, as the result, the peptide chain initiation inhibited. In the present study, fish fed diets with high levels of lysine showed significant increase of whole body protein when compared with that of fish fed the low lysine diets, while the highest lipid content in whole body was noted with fish fed the control diet; the variation showed an opposite trend compared to protein, which was in agreement with previous reports (Rodehutscord et al. 2000; Luo et al. 2006). The carnitine pool in fish is derived from both endogenous synthesis and diet. Carnitine is synthesized from lysine and methionine. After synthesis, the carnitine must be transported to other tissues. It is most concentrated in tissues that use fatty acids as their primary dietary fuel, such as skeletal and cardiac muscle (Harpaz 2005). Burtle & Liu (1994) reported whole body lipid content of channel catfish was reduced by supplemented dietary carnitine, lysine or both. Carnitine as well as lysine plays an important role in promoting the transport of long-chain fatty acids across the inner mitochondrial membrane, resulting in extra energy from β-oxidation. Therefore, dietary lysine supplements should enhance the oxidation of these fatty acids, thereby decreasing their availability or esterification to triacylglycerol and deposition in the various lipid storage tissues. The similar result was also reported in rohu carp (Keshavanth & Renuka 1998) and Atlantic salmon (Ji et al. 1996) and black sea bream (Ma et al. 2007). However, dietary lysine level had no significant effect on composition of moisture and ash, which was supported by Zarate & Lovell (1997).
Morris et al. (1999) suggested that protein deposition was regarded as the best for AA requirement studies in poultry, as well as in rainbow trout (Cho et al. 1992). The present study showed that the maximal protein deposition in tissues required 11–23% more lysine than WGR and SGR, this phenomenon have been reported in the previously studies in rainbow trout (Rodehutscord et al. 1997) and Atlantic salmon (Anderson et al. 1993; Espe et al. 2007). Dietary lysine at highest level also gave a low lipid accumulation supporting the view of a more healthy fish utilizing the dietary protein most efficiently for protein deposition and lean growth, the results were similar to Japanese seabass (Mai et al. 2006). Contrary to what was observed for WG and SGR, PPV increased with the increase of dietary lysine but plateau was not attained even at the higher lysine levels. Peres & Oliva-Teles (2008) figured an estimation of lysine requirement for maximum nitrogen retention was not possible. It suggested that higher dietary lysine levels may be required for maximum nitrogen gain than for weight gain as was observed in rainbow trout and Atlantic Salmon (Rodehutscord et al. 1997; Encarnação et al. 2004; Espe et al. 2007). Indeed, Hauler & Carter (2001) concluded that using protein deposition as response criteria results in higher lysine requirement estimates than weight gain in fish.
A correlation between muscle or whole body amino acid profile and amino acid patterns for fish has been demonstrated by several investigators (Wilson & Poe 1985; Borlongan & Coloso 1993; Mambrini & Kaushik 1995). In the present study, the results showed that the accumulation of free lysine in muscle may support growth data when estimating lysine requirements. The difference of total lysine in the muscle was significant, but still small, and it is probably due to the difference in free lysine in the amino acid pool of the muscle as monitored in the cases of rainbow trout and gilthead sea bream (Yamamoto et al. 2000) and Japanese seabass (Mai et al. 2006). The results of this study, EAAs retention in muscle showed marked differences among the lysine levels, which probably reflected differences in EAA availability and efficiency of utilization by black sea bream, as observed in white sturgeon (Ng & Hung 1995) and Japanese flounder (Alam et al. 2002). Dietary restriction of one EAA led to an increase in oxidation of other essential and non-EAAs present at normal levels in the diets (Ronnestad et al. 2000). The increasing dietary lysine intake may cause the amino acids imbalanced in fish and aggravate the deamination effect. The amino acids in excess from the disproportionate absorption rates or the part of lysine intake beyond optimal level would be no longer used for increasing lysine or other EAAs deposition in fish body but was deaminated to catabolism, affected the plasma arginine and urea levels, and ammonia excretion, or release as energy (Cowey & Sargent 1979) Akiyama et al. (1997) have stressed the usefulness of higher proportion of non-essential amino acids (NEAA) in evaluating dietary protein. Marcoul et al. (2004) also found that protein retention in gilthead seabream juveniles was significantly improved by the higher dietary EAA/NEAA ratio, which was partially in agreement with our study that ∑EAA index reflected the trend of body protein accretion.
Increasing dietary lysine content elevated protein concentration in the serum, cholesterol and triacylglycerol declined by and large among different diets, however GLU was variable and not related to dietary treatments as pointed out in groupers (Luo et al. 2006). According to Regost et al. (1999) the decrease in whole body fat content along with the decrease in plasma triacylglycerol concentrations suggests lipid mobilization in those groups exhibiting very poor growth, which was not observed in the present investigation. Tissue cholesterol concentrations are known to vary depending on the nutritional status of fish (Kaushik et al. 1995; Regost et al. 1999). Little information is currently available on the effect of lysine on blood characteristics, and more investigations are needed. To support the requirement estimated by growth and feed utilization, levels of free lysine in serum were analysed. Serum-free lysine taken after 6 h remained at a relatively low and constant level up to fish fed diet 3. However, a notably increased free lysine level was observed in fish fed diet 4 and diet 5 (P < 0.05), thereafter the values returned to relatively low level in fish fed diet 6 similar to the first three groups. Robinson et al. (1981) pointed out that serum levels of free arginine depended not only on dietary arginine concentration, but also on interrelations with other dietary amino acids. Berge et al. (1998) suggested the decreased level of lysine in plasma following high dietary supplementation may be caused by an increased oxidation of this amino acid. Oxidative enzymes do not allow the concentrations of EAA to rise in blood (Millward & Rivers 1998). The findings of present experiment indicated there may be a homeostatic mechanism for the regulation of free serum lysine in this fish species.
To our knowledge, there is little information on the effects of dietary lysine on haematological characteristics of fish. The paramount function of the RBC is generally reckoned to be oxygen carriage. RBC are both mechanical and biochemical barriers against infections, bacteria, and blood parasites. Immune reactions are regulated to ensure harmony between the RBC and WBC populations. Gray (1963) reported the lysine deficiency could result in 22% serum white bloods cells decrease of rats. Hb in aquatic animals operates over wide and independent variations in oxygen at the sites of loading and unloading and shows adaptations both to environmental conditions and to metabolic requirements, which govern oxygen availability and transport to tissues (Weber & Wells 1989). In our study, the Hb concentration was significantly (P < 0.05) higher at 32.5 g kg−1 dietary lysine inclusion compared to the other dietary levels, excepting 36.8 g kg−1 lysine which showed no difference with that of 32.5 g kg−1. WBC presented the similar trend as Hb but without significant difference (P > 0.05). The RBC declined from diet 1 to diet 6 (P > 0.05). In the study on lysine requirement of yellowtail, Hb and RBC were not influenced by dietary lysine, however, haematocrit was significantly different between fish fed different diets (Ruchimat et al. 1997). The oppression of growth performance and feed efficiency may be likely to relate with the RBC and Hb in serum, because when fish fed with excessive lysine dietary, the transport of oxygen and nutrient substance were affected by the decrease in the concentration of RBC and Hb in serum.
In conclusion, results of the present investigation indicate that the lysine requirement of juvenile black sea bream is little higher than other species. Second-order polynomial regression analysis based on SGR showed that l-lysine requirement for juvenile black sea bream (initial average weight, 9.13 ± 0.09 g) was 33.2 g lysine kg−1 of the dry diet or 86.4 g lysine kg−1 dietary protein. The level is also suitable for black sea bream protein and EAAs accretion as well as physiological parameter. The data generated in the present study would be useful in developing lysine-balanced practical diets for the intensive culture of this species. Evaluation of other EAAs requirements, the effect of coating EAAs and the interaction among EAAs should be conducted in future.
Acknowledgements
We wish to thank the Marine Fisheries Research Institute of Zhejiang Province for supplying the experimental black sea bream, allowing us to use the experimental base and their logistic support during the feeding experiment. We would also like to thank Y. J. Xu, X. C. Meng, W. X. Song and G. Y. Zhong for their assistance with caring for the fish and sampling. This research was funded by Bureau of Zhejiang Provincial Science and Technology, China.




