Effect of practical diets with different protein levels on the performance of Farfantepenaeus paulensis juveniles nursed in a zero exchange suspended microbial flocs intensive system
Abstract
Farfantepenaeus paulensis juveniles (72 ± 24 mg), were reared in a suspended microbial flocs system and fed practical diets containing increasing amounts of crude protein (250, 300, 350, 400 and 450 g kg−1 CP). Development of microbial flocs was promoted by high aeration rates and fertilization with wheat bran and molasses. Flocs were composed of detritus in the form of flocculated matter colonized by heterotrophic bacteria, cocoid and filamentous cyanobacteria, flagellate and ciliate protozoa and rotifers. Proximate composition analysis of the suspended microbial floc showed CP levels of 304 g kg−1. After 45 days, mean shrimp survival were above 89%, with no significant differences between treatments. Shrimp fed diets with 350 g kg−1 or higher CP content achieved significant higher (P < 0.05) final weight (0.66–0.68 g), weight gain (0.58–0.61 g) and instantaneous growth rate (0.049–0.050), with feed conversion rates (2.17–2.30) significantly lower (P < 0.05). Results show that, when rearing is carried out in a suspended microbial flocs system, dietary CP levels can be kept at 350 g kg−1. Furthermore, results confirm that microbial-based systems allow shrimp culture without compromising the surrounding environment and shows the possible reduction of production costs and fish meal dependence.
Introduction
In the last decades penaeid shrimp culture has increased worldwide, mainly due to the growing demand for new food supplies and the high commercial value achieved by shrimp products in the international market (FAO 2003). However, together with the rapid expansion of shrimp farming industry, concern with environmental impacts caused by these activities have also increased (Tovar et al. 2000; Jory et al. 2001).
Growing scales of shrimp farming enterprises have been blamed on a series of ecological problems especially due to conversion of coastal areas to aquaculture ponds that cause devastation of mangrove and other nursery areas that, by their turn, support ocean fisheries. Discharge of high loads of nutrients and chemicals into coastal waters, salinization of land and water and the introduction of exotic species and the spread of aquatic animal diseases also cause important ecological impacts. Among these possible negative impacts, water pollution by pond effluents and the indiscriminate use of fish meal and fish oil to produce feeds are among the most common objection to the development of the aquaculture industry (Naylor et al. 1998; Boyd & Gautier 2000; Naylor et al. 2000; Boyd 2003). Another setback brought by the intensification of shrimp production is the viral diseases that have plagued this activity in recent years (Lightner 1999; Moss 2002; Nunes et al. 2004).
Development of new technologies to grow shrimp in environmental friendly systems is required to meet the growing demand for healthy and high quality shrimp products (Subasinghe et al. 1998). In fact, this type of production systems has evolved in the United States during the 1990s when production of 5000 kg/ha of white shrimp Litopenaeus vannamei was achieved with negligible or even zero water exchange (Hopkins et al. 1995; Browdy et al. 2001). More recently, Mcabee et al. (2003) have reported productions of 3 kg m−2 in greenhouse enclosed raceways with stocking densities of 300 shrimp m−2 and survival higher than 70% and Otoshi et al. (2008) reported productions of 10.3 kg m−2 and survival of 67.9% in a recirculating aquaculture system with initial stocking densities of 828 shrimp m−2.
Previous reports have demonstrated that when shrimp production is carried out in closed systems, the suspended organic matter produced within the pond/tank may enhance shrimp growth rates allowing the use of lower protein feeds (Browdy et al. 2001; Decamp et al. 2002; Moss 2002). Along with the reduction of production costs, the use of lower protein feeds are part of environmentally sound aquaculture practices, considering that aquaculture effluents would have less nitrogenous compounds, diminishing the risks of eutrophication in adjacent coastal areas, as well as the reduction of dependence on fish meal component (Martinez-Cordova et al. 2003).
Although there is much information about the production of white shrimp L. vannamei in zero exchange production systems (Bratvold & Browdy 2001; Burford et al. 2004; Wasielesky et al. 2006), little is known about the rearing of other penaeid species in this type of system. The pink shrimp Farfantepenaeus paulensis is a cold tolerant species distributed throughout the Brazilian and north-eastern Argentine coast (D’incao 1991). Recent studies have shown its potential for aquaculture production (Peixoto et al. 2003; Wasielesky et al. 2004). However, as a highly carnivorous species, F. paulensis demands high values of crude protein (CP) in its feed. Fróes et al. (2006) demonstrated that when reared in clear water, the optimal dietary protein level for F. paulensis juveniles is 450 g kg−1. The present work aimed to evaluate the reduction of dietary protein levels for this species, when rearing outdoors in a zero exchange suspended microbial floc based intensive system. Additionally microbial composition and biomass of the flocs was investigated.
Material and methods
Culture conditions
The experiment was conducted from 4th March to 18th April 2006 at the Marine Aquaculture Station – FURG. An outdoor fibreglass tank (7 m2, 7 tons) was used for the development of microbial flocs. Water was strongly aerated with air lifts and, to promote the development of the flocs, the tank was stocked F. paulensis juveniles (3.54 ± 0.88 g) at a density of 40 shrimp m−2 and was fertilized with a commercial shrimp diet (400 g kg−1 CP), molasses and wheat bran. The nominal C : N ratio of the daily organic matter additions to the tank was approximately 20 : 1, considering the carbon and nitrogen concentration in the commercial diet and organic fertilizers (molasses and wheat bran). No water exchange was carried out during the experimental period; only dechlorinated freshwater was added to compensate for evaporation losses.
Fifteen round floating cages (0.2 m2, 1.5 mm mesh size) were installed at the internal periphery of the fibreglass tank. Three replicate cages were randomly assigned to treatments which consisted of five different dietary CP levels, i.e.: 250, 300, 350, 400 and 450 g kg−1 CP.
Diet formulation
Five practical diets containing different levels of CP (250, 300, 350, 400 and 450 g kg−1 CP) were formulated (Table 1). A mixture of fish meal, soybean bran and gelatin was used to obtain the desired protein levels. Care was taken to guarantee that at least 500 g kg−1 of the employed protein originated from fish meal, as commercial diets available for shrimp use fish meal as the main protein source. Wheat flour was utilized as the main carbohydrate source and lipid sources were fish and soybean oil. Other dietary ingredients were added to fulfill the nutritional requirements of penaeids (D’abramo et al. 1997) and were kept in the same levels in all diets.
| Ingredient | Protein level (g kg−1) | ||||
|---|---|---|---|---|---|
| 250 | 300 | 350 | 400 | 450 | |
| Fish meal1 | 195 | 250 | 290 | 330 | 360 |
| Soybean bran1 | 135 | 180 | 200 | 220 | 100 |
| Gelatin2 | 20 | 20 | 55 | 80 | 180 |
| Wheat meal3 | 410 | 310 | 200 | 120 | 70 |
| Fish oil1 | 20 | 15 | 10 | 05 | 03 |
| Soybean oil4 | 30 | 30 | 30 | 30 | 30 |
| Soybean lecithin1 | 5 | 5 | 5 | 5 | 5 |
| Cholesterol5 | 5 | 5 | 5 | 5 | 5 |
| Vitamin C6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Astaxanthin6a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Vitamin and mineral premix7 | 20 | 20 | 20 | 20 | 20 |
| BHT5a | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| BHA5b | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Filler | 159.7 | 164.7 | 184.7 | 184.7 | 226.7 |
| Proximate composition | |||||
| Dry matter | 922.8 | 921.7 | 941 | 942.6 | 918.8 |
| Crude protein | 250.4 | 295.7 | 345.7 | 396.2 | 445.4 |
| Crude fat | 81.6 | 90.1 | 77.7 | 75.5 | 78.0 |
| Crude fibre | 7.2 | 9.3 | 8.2 | 10.1 | 10.1 |
| Nitrogen-free extract8 | 446.4 | 368.1 | 303.5 | 243.5 | 164.6 |
| Ash | 214.4 | 236.8 | 264.9 | 269.5 | 301.9 |
| Calcium | 14.8 | 17.1 | 20.9 | 23.9 | 29.6 |
| Phosphorous | 8.7 | 10.3 | 11.8 | 13.7 | 15.1 |
| Protein : gross energy ratio9 | 128.8 | 120.6 | 100.4 | 97.0 | 92.8 |
- 1 Nutron Alimentos, Campinas SP, Brazil, l-ascorbyl-2-monophosphate.
- 2 Vetec Química Fina LTDA., Duque de Caxias RJ, Brazil.
- 3 Antoniazzi & Cia. LTDA., Santa Maria RS, Brazil.
- 4 Bunge Alimentos S.A., Rio Grande RS, Brazil.
- 5 Sigma Aldrich Inc., St. Louis, MO, USA.
- 5a Butyl hidroxytoluen – Sigma Aldrich Inc., St. Louis, MO, USA.
- 5b Hidroxyanisol butylate – Sigma Aldrich Inc., St. Louis MO, USA.
- 6 l-ascorbyl-2-monophosphate – Roche Ltd, Basel, Switzerland.
- 6a Roche Ltd, Basel, Switzerland.
- 7 DSM Nutritional Products, São Paulo SP, Brazil.
- 8 Nitrogen-free extract = 1000 − (protein + fat + ash + fibre).
- 9 mg kcal−1.
Dry ingredients were manually blended, following the addition of oil and water. The dough-like mixture was then pelletized using a meat grinder (Müller® Model KM 80, São Paulo, Brazil). Extruded feed were dried during 24 h at 60 °C in a parallel flux forced air drier. After drying, strings were broken and pellets were sieved in a 2 mm sieve and stored in plastic bags at −12 °C until fed.
Proximate analysis of percentage moisture, CP, and ash content of the diets were performed according to AOAC (2000). Total lipids were determined according to Folch et al. (1957). Dietary energy values, crude fibre, calcium and phosphorus content of the diets were estimated based on Tacon (1987). The percentage of nitrogen-free extract was determined by difference.
Shrimp growth monitoring and feeding management
Farfantepenaeus paulensis juveniles (72 ± 24 mg) in the intermoult period were stocked in the floating cages at a density equivalent to 250 shrimp m−2 (50 shrimp/cage). Shrimp were fed twice daily at an initial feeding rate of 10% of their biomass. Every 15 days, 30 shrimp were randomly sampled from each cage and their wet weight was individually measured to the nearest 0.01 g (electronic balance Ohaus®, model SC 2020, Florham Park, NJ, USA). After weighing, shrimp were returned to their respective cages. The amount of feed was adjusted to each treatment according to shrimp weight, feed consumption and mortalities observed in each cage.
Feed conversion rate (FCR) was estimated as the total dry weight of feed offered per cage divided by the shrimp wet weight gain. Protein efficiency ratio (PER) was calculated as the shrimp wet weight increase divided by total weight of protein fed to shrimp in each cage.

Water quality monitoring
Throughout the experimental period, water temperature (mercury thermometer, precision ± 0.5 °C), salinity (optical refractometer model RTS – 101, Atago® US, Bellevue, WA, USA, ±1 g L−1), pH (digital pH meter model Handylab 2 BNC, ±0.01 precision, Schott®, Hattenbergstr, Germany), dissolved oxygen (dissolved oxygen meter model Handylab/OXI/set ± 0.01 mg L−1 precision, Schott®) and transparency (Secchi disc, 20 cm diameter) were measured every day between 9:00 and 11:00 hours. The results of transparency were used to adjust daily organic fertilization. In order to keep microbial flocs in an acceptable density, fertilization was stopped when secchi depth values were below 9 cm, but when transparency values were higher than 13 cm fertilization was resumed.
Water samples were collected every 5 days to measure total ammonia nitrogen (TAN) (NH3 + NH4+-N; UNESCO 1983), nitrite nitrogen (NO2−-N; Bendschneider and Robinson 1952), reactive phosphorus (PO4−3; Aminot & Chaussepied 1983), chlorophyll a concentration and total suspended solids (TSS) (Strickland & Parsons 1972).
Microbial floc sampling and monitoring
Water samples from the outdoor tank were collected every 5 days and preserved with borate buffered formalin (4%v/v) for further analysis of microbial floc composition (Thompson et al. 2002). The characterization and abundance of microorganisms and rotifers were determined in 2.1 mL samples poured into sedimentation chambers using an Olympus inverted light microscope equipped with phase contrast (Utermöhl 1958). For bacterial and flagellate counts, volumes of 0.1 mL were concentrated in darkened polycarbonate membrane filters (Nuclepore, 0.2 μm), stained with the fluorochrome 4′, 6-diamin-2-phenyl-indol (DAPI) (15 μg mL−1) and counted under a Zeiss Axiolplan epifluorescence microscope (Thornwood, NY) equipped with a 487 701 light filters set (BP365/11; FT 395; LP 397) at 1000× final magnification (Porter & Feig 1980). Counts were made in at least 30 fields chosen at random.
At the middle of the experimental period (day 22) tank water was filtered through a 30 μm mesh to collect suspended material for further proximate composition analysis (AOAC 2000).
Statistical analysis
One-way anova was used to determine significant differences (P < 0.05) on shrimp performance. Tukey’s multiple-range test was applied when significant differences were detected. Survival data was analysed by a non-parametric Kruskall–Wallis anova because despite various transformations this data did not show a normal distribution.
Results
Diet characteristics
Compositions of the different experimental diets are shown in Table 1.
Water quality
Water quality parameters monitored during the experiment are shown in Table 2. The fluctuations of TAN, nitrite and orthophosphate are shown in Fig. 1, while the variations of total suspend solids and chlorophyll a are shown in Fig. 2.
| Parameter | Mean (±SD) | Minimum | Maximum |
|---|---|---|---|
| Temperature (ºC) | 21.6 ± 2.6 | 19.2 | 26.7 |
| Salinity (g L−1) | 35.5 ± 1.0 | 32 | 38 |
| Dissolved oxygen (mg L−1) | 6.5 ± 1.1 | 4.7 | 8.9 |
| pH | 6.7 ± 0.2 | 6.4 | 7.7 |
| Transparency (cm) | 11.7 ± 2.9 | 6 | 20 |
| Chlorophyll a (μg L−1) | 143.3 ± 89.6 | 48 | 254 |
| TSS (mg L−1) | 194.4 ± 115.7 | 79 | 437 |
| TAN (mg L−1) | 0.1 ± 0.1 | 0.0 | 0.3 |
| Nitrite-N (mg L−1) | 0.3 ± 0.7 | 0 | 2.2 |
| Reactive phosphorus (mg L−1) | 0.2 ± 0.4 | 0 | 1.0 |
- TSS, total suspended solids; TAN, total ammonia nitrogen.
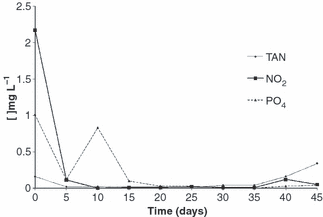
Fluctuations of total ammonia nitrogen (TAN), nitrite nitrogen (NO2−-N) and reactive phosphorous (PO4−3) concentrations during the experimental period.
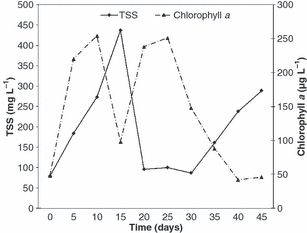
Total suspended solids (TSS) and chlorophyll a concentration of the culture water during the experimental period.
Microbial floc characterization
Proximate composition analysis of the dry suspended particulate matter (microbial flocs) is shown on Table 3.
| Proximate composition (g kg−1) | |
|---|---|
| Crude protein | 304 |
| Crude fat | 4.7 |
| Crude fibre | 8.3 |
| Nitrogen-free extract | 291 |
| Ash | 392.1 |
- Nitrogen-free extract = 1000 − (protein + fat + ash + fibre).
Microbial flocs sampled from the tank were composed of detritus in the form of flocculated matter which were colonized by attached heterotrophic bacteria, filamentous cyanobacteria, dinoflagellates, flagellates, ciliates and rotifers. The number of unicellular cyanobacteria and free living bacteria in the culture water was also determined. Mean numbers (±SD) and range values on the densities of these organisms are shown on Table 4. It was observed that cyanobacteria filaments (Fig. 3) acted like frame structures, where organic matter would aggregate, and provided shape to the flocs. A small number of pennate diatoms (<30 mL−1) was observed in the samples, while nematodes (5/ml) were only observed in samples from day 30.
| Organisms | Mean (±SD) | Minimum | Maximum |
|---|---|---|---|
| Attached bacteria (106 cells mL−1) | 4.6 ± 3.8 | 0.9 | 11.9 |
| Free living bacteria (107 cells mL−1) | 3.8 ± 1.2 | 2.3 | 6.4 |
| Unicellular cyanobacteria (103 cells mL−1) | 17.2 ± 3.2 | 13.0 | 21.8 |
| Filamentous cyanobacteria (filaments mL−1) | 218.7 ± 151.9 | 69.5 | 326.6 |
| Dinoflagellates (cells mL−1) | 182.8 ± 277.8 | 0 | 673 |
| Flagellates (104 cells mL−1) | 30.7 ± 28.2 | 16.3 | 67.4 |
| Ciliates (cells mL−1) | 78.9 ± 48.8 | 39.4 | 169.8 |
| Rotifers (organisms mL−1) | 34.6 ± 47.6 | 4.6 | 151.3 |
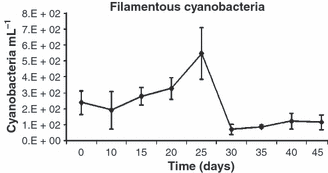
Mean number (±SD) of filamentous cyanobacteria in the microbial flocs during the experimental period.
The bulk of bacterial were free living (2.26–6.36 × 107 mL−1) (Fig. 4), whereas attached bacteria numbers ranged from 0.86 to 11.91 × 106 mL−1 (Fig. 5), which represented <20% of the total bacterial biomass.
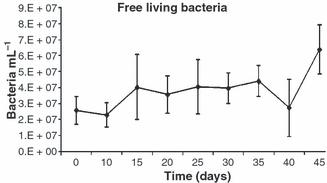
Mean number (±SD) of free living bacteria in the water during the experimental period.
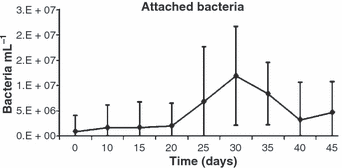
Mean number (±SD) of attached heterotrophic bacteria in the microbial flocs during the experimental period.
Shrimp performance
After 45 days, mean shrimp survival was above 89%, with no significant differences among dietary treatments. Shrimp fed diets with 350 g kg−1 or higher CP content reached significantly higher (P < 0.05) final weight, weight gain and instantaneous growth rate. For these treatments, FCR were significantly lower (P < 0.05). PER were significantly lower for shrimp fed the diet with 400 and 450 g kg−1 CP (Table 5).
| Dietary protein level (g kg−1) | Polled SEM | |||||
|---|---|---|---|---|---|---|
| 250 | 300 | 350 | 400 | 450 | ||
| S | 95.13 ± 4.21 | 95.21 ± 3.90 | 89.96 ± 8.70 | 92.73 ± 0.05 | 96.33 ± 3.65 | 2.8809 |
| F w | 0.56 ± 0.12a | 0.57 ± 0.12a | 0.68 ± 0.13b | 0.68 ± 0.11b | 0.66 ± 0.11b | 0.0688 |
| W g | 0.49 ± 0.12a | 0.50 ± 0.12a | 0.61 ± 0.13b | 0.61 ± 0.11b | 0.58 ± 0.11b | 0.0688 |
| G | 0.045 ± 0.005a | 0.045 ± 0.004a | 0.049 ± 0.004b | 0.050 ± 0.003b | 0.049 ± 0.003b | 0.0024 |
| FCR | 2.64 ± 0.002a | 2.58 ± 0.03a | 2.30 ± 0.10b | 2.22 ± 0.12b | 2.17 ± 0.06b | 0.0473 |
| PER | 1.51 ± 0.08a | 1.30 ± 0.006ab | 1.25 ± 0.18ab | 1.13 ± 0.08b | 1.02 ± 0.07b | 0.0578 |
- Different superscripts within the same row means significant differences (P < 0.05).
- FCR, feed conversion rate; PER, protein efficiency ratio.
Discussion
Microorganisms have important functions in aquaculture systems. They are responsible for primary production, nutrient cycling and are an important source of nutrition. They are also essential for the maintenance of water quality, disease control and as mediators of the environmental impact of effluents (Moriarty 1997; Decamp et al. 2002; Moss 2002; Thompson et al. 2002). Modern research has shown that even in intensive aquaculture systems, microorganisms can contribute to the maintenance of water quality standards within suitable ranges for shrimp culture (Thompson et al. 2002; Samocha et al. 2007) and that natural productivity within the culture system can promote fish/shrimp growth (Anderson et al. 1987; Avnimelech 1999; Burford et al. 2004; Wasielesky et al. 2006; Abreu et al. 2007).
Heterotrophic bacteria have the ability to synthesize protein from organic carbon and inorganic nitrogen (Avnimelech 1999; Chamberlain et al. 2001a). However, high protein diets usually employed in intensive aquaculture systems have C : N ratios of less than 10 : 1. Such proportions may slow down the rate of bacterial waste decomposition, resulting in the accumulation of inorganic nitrogen (ammonia, nitrite and nitrate) in the culture system (Mcintosh 2000a). Chamberlain et al. (2001a) recommended the use of balanced mixtures of carbonaceous and nitrogenous materials with a C : N ratio of approximately 20 : 1 to stimulate bacterial decomposition of organic wastes, while Avnimelech (1999) determined that the use of a carbon source to raise the C : N ratio within the culture system was a practical and inexpensive way to reduce inorganic nitrogen accumulation. This was demonstrated by Samocha et al. (2007), when rapid reduction of ammonium and nitrite concentrations were achieved in limited discharge nursery system for L. vannamei through the addition of molasses.
In the present experiment, the use of molasses and wheat bran as a way to raise the C : N ratio to approximately 20 : 1 was found to be efficient in maintaining low levels of nitrogen compounds throughout the experimental period. This may be the result of the high density of both heterotrophic bacteria and cyanobacteria in the water as well as heterotrophic bacteria and filamentous cyanobacteria attached to the flocs, as these microorganisms use the nitrogen available to increase their biomass (Avnimelech 1999; Ju et al. 2008). Similarly, nitrifying and denitrifying processes mediated by bacteria could also act to reduce the ammonium levels in the tanks (Schlegel 1992). Monitoring of water transparency proved to be a practical and efficient tool to manage organic fertilization and keep microbial flocs at acceptable densities within the culture water, as TSS were maintained below 500 mg L−1 as recommended by Samocha et al. (2007). Water quality parameters remained within recommended levels for shrimp culture throughout most of the experimental period. The only exception was pH, which was slightly below the range considered to be optimal. Although temperature was also below the recommended range for the culture of penaeids suggested by Van Wyk and Scarpa (1999), growth of F. paulensis is only highly affected at levels lower than 20 °C (Wasielesky 2000).
The most important protein source for aquafeeds are derived from aquatic animals, and, among these sources, fish meal is the most commonly used (Cavalli et al. 2004). According to Naylor et al. (1998), current shrimp farming systems depend on the use of nutrient rich diets containing large amounts of fish meal and fish oil from wild-caught fish. Rather than an increment to fishery resources, this practice represents a potential damage for wild fish stocks. Additionally, shortage of fish meal is expected in the near future, which will certainly result in an increase in fish meal costs (Naylor et al. 2000). As protein is the most expensive part of the diet and feeding is responsible by approximately 50–60% of the total production costs in shrimp culture (Akiyama et al. 1992), this increase may negatively affect the feasibility of current production systems. For this reason, the use of alternative protein sources is a constant concern for the development of the aquaculture industry.
Chamberlain et al. (2001b) mentioned the attempts made to produce bacterial protein as ‘single-cell protein’ and the promising results achieved with the partial fishmeal replacement in studies with rainbow trout. Although successful, this method was abandoned because of the limited economic viability of the process employed to concentrate and dry this product. Conversely, in zero exchange suspended microbial flocs based systems, microbial protein is produced and consumed in situ by the reared organisms, representing a high nutritional source available 24 h a day (Avnimelech 1999). Mcintosh (2000b) achieved L. vannamei production yields over 13 000 kg ha−1 in lined ponds without water exchange using a grain-based pellet diet (180 g kg−1 protein) with a C : N ratio of 20: 1. This author suggests that a moderate C : N ratio helps stimulate the growth of heterotrophic bacteria specific to breaking down and recycling shrimp feed and associated wastes. During the process of recycling wastes and incorporating inorganic nitrogen available in the culture water, bacteria produce microbial protein which can be consumed directly by the reared animals. Consequently, feed wastes become available to shrimp as microbial protein, improving the overall protein conversion efficiency in shrimp culture systems from the conventional 20–25% to about 40–45%. This may not only save the most expensive feed ingredients but also contribute to preventing the predicted global scarcity of feed protein sources (Avnimelech 2000). Even though high stocking densities were applied in the present work, FCR (2.17–2.64) were much lower than determined in previous studies with the same species. For instance, Jensen et al. (2004) reported values of 3.36, 3.71 and 3.91 for F. paulensis reared at 30, 60 and 90 shrimp m−2, respectively, and Pissetti (2004) reported FCR of 4.05 for F. paulensis reared at 40 shrimp m−2. Besides decreasing production costs, lower dietary protein levels also have the advantage of reducing the risks of ammonia build up within the culture system (Hopkins et al. 1995).
Reduction of dietary protein levels without affecting shrimp growth has been reported by several authors and microbial recycled protein has been cited as an important source of protein available for shrimp in these systems (Hopkins et al. 1995; Mcintosh 2000b; Decamp et al. 2002). Moss (2002) reported that L. vannamei ranging in weight from <0.1 to 32 g can benefit from the growth-enhancing particles suspended in shrimp pond water. Decamp et al. (2002) found that the performance of L. vannamei reared in unfiltered pond water and fed either a 25% CP diet (complete) or a 35% CP diet with/without mineral or vitamin premixes showed no significant differences, demonstrating that it is possible to reduce or even remove trace elements and/or vitamins from diets and also reduce protein content without affecting shrimp growth performance.
The results of the present experiment are in accordance with those results achieved for other penaeids. Even though F. paulensis is considered a more carnivorous species with a higher protein requirement (Fróes et al. 2006), we were able to reduce the dietary protein content up to 10% without impairing shrimp performance. This was probably due to the provision of in situ recycled protein from the microbial flocs. Another evidence of the importance of microbial flocs as a protein source is that shrimp fed the lowest CP diet (250 g kg−1 CP) had a higher PER.
The microbial flocs from the present experiment contained CP levels similar to those reported by Mcintosh (2000a), Tacon et al. (2002) and Wasielesky et al. (2006) and this confirms that microbial flocs may be an important source of protein for cultured shrimp. Microorganisms observed in the flocs are usually found in the natural diet of penaeids (Allan & Maguire 1992; Allan et al. 1995; Nunes et al. 1997). Densities of heterotrophic bacteria in the present study are similar to those reported by Burford et al. (2003, 2004), however only about 20% of total (free + attached) bacteria were associated with flocculated matter in comparison to about 40% mentioned in those studies. This discrepancy was, probably, due to problems in enumerating bacteria attached to the flocs, because of clumping. The number of flagellate and ciliate protozoa, dinoflagellates and rotifers is in the same range determined by Burford et al. (2003). Grazing of protozoa by rotifers was observed in fresh samples, confirming that these organisms are important links in the microbial food web in the suspended microbial floc culture system. However, just before the collection of the microbial flocs for their proximate composition analysis, filamentous cyanobacteria and flagellates were the most abundant microorganisms on the flocs. Silva et al. (2008) observed that filamentous cyanobacteria are closely related to the amount of lipids in biofilm, while diatoms and nematodes were responsible for the bulk of protein. It is quite possible that filamentous cyanobacteria and flagellates were the main contributors to protein content of the flocs, however further studies are necessary comparing the protein and lipids evolution in the aggregates with the microbial succession in order to better determine which microorganisms most contribute to the nutritional quality of flocs, as clear changes in the floc composition were observed during the study.
It is well established that the microorganisms belonging to the floc community, along with organically rich detritus, serve as an important food source for shrimp (Moss 2002). Burford et al. (2004) cited that microorganisms present in the water may be too small for shrimp to capture. Avnimelech (1999) suggests that relative large cell clusters are formed in the floc aggregates, favouring cell uptake by fish/shrimp. Additionally, these microorganisms are recognized not only as important sources of protein, but also to fulfill lipid, mineral and vitamin requirements of shrimp and a potential source of exogenous enzymes that aid digestion (Moss et al. 2001; Decamp et al. 2002; Thompson et al. 2002; Moss et al. 2006).
In a recent study, Silva et al. (2008) identified heterotrophic bacteria, filamentous cyanobacteria and heterotrophic flagellates as important lipid sources. Furthermore, it was already established that ciliate and flagellate protozoa have a higher protein to energy ratio and are able to synthesize long chain polyunsaturated fatty acids by feeding on bacteria, enriching the nutritional quality of microbial aggregates (Zhukova & Kharlamenko 1999). Abreu et al. (2007) determined through the stable isotope techniques that the microbial biofilm composed by nematodes, diatoms, filamentous cyanobacteria and ciliate may contribute to around 49% of the carbon and 70% of the nitrogen responsible for F. paulensis juveniles growth. In the present work, only a small number of diatoms and nematodes, which are considered important nutritional sources for shrimp (Schlechtriem et al. 2004, 2005; Ballester et al. 2007), were found in the microbial floc community. Thus, future research should focus on the manipulation of microbial succession in order to improve the nutritional quality of the flocs, which may further increase shrimp performance.
Conclusion
The results of this study confirmed the importance of microbial flocs as a nutritionally rich food source for F. paulensis. Microorganisms present in the flocs play important roles in the maintenance of water quality and in the provision of essential nutrients for shrimp. Most importantly, the use of microbial-based culture systems may allow the development of shrimp culture without compromising the surrounding environment and it demonstrates the possibility of reducing production costs and fish meal dependence. Further research should focus on the improvement nutritional quality of the microbial flocs through the manipulation of the microbial community in the culture system.
Acknowledgements
The authors acknowledge the financial support provided by Brazil’s Council for Scientific and Technological Development – CNPq. Wilson Wasielesky Jr, Paulo César Abreu and Ronaldo Cavalli are research fellows of this agency. We are also grateful to MSc. Charles Fróes for the preparation of the experimental diets and to two anonymous reviewers for their comments and suggestions in a previous version of the manuscript.




