Recombination rates across porcine autosomes inferred from high-density linkage maps
Summary
Studies of the variation in recombination rate across the genome provide a better understanding of evolutionary genomics and are also an important step towards mapping and dissecting complex traits in domestic animals. With the recent completion of the porcine genome sequence and the availability of a high-density porcine single nucleotide polymorphism (SNP) array, it is now possible to construct a high-density porcine linkage map and estimate recombination rate across the genome. A total of 416 animals were genotyped with the Porcine SNP60BeadChip, and high-density chromosome linkage maps were constructed using CRI-MAP, assuming the physical order of the Sscrofa10 assembly. The total linkage map length was 2018.79 cM, using 658 meioses and 14 503 SNPs. The estimated average recombination rate across the porcine autosomes was 0.86 cM/Mb. However, a large variation in recombination rate was observed among chromosomes. The estimated average recombination rates (cM/Mb) per chromosome ranged from 0.48 in SSC1 to 1.48 in SSC10, displaying a significant negative correlation with the chromosome sizes. In addition, the analysis of the variation in the recombination rates taking 1-Mb sliding windows has allowed us to demonstrate the variation in recombination rates within chromosomes. In general, a larger recombination rate was observed in the extremes than in the centre of the chromosome. Finally, the ratio between female and male recombination rates was also inferred, obtaining a value of 1.38, with the heterogametic sex having the least recombination.
The recombination rate, defined as the number of crossovers per unit DNA, is a key genomic parameter that is influenced by species, population, sex, chromosome and chromosomal region (Jensen-Seaman et al. 2004; Ma et al. 2010). Several factors are considered to be affecting the patterns of recombination, such as the chromosomal size, arm size, proportional distance from the centromere, proportional distance from the centre of the chromosome and the sequence composition Guanine and Cytosine (GC) content, CpG density and repetitive elements). Analyses of this variation in recombination rate across the genome are essential for explaining variation in patterns among species, for evolutionary genomic studies and for mapping complex traits in domestic animals by increasing the power of association studies and linkage disequilibrium mapping (Arnheim et al. 2003).
Previous studies have employed a reduced number of markers to build porcine linkage maps (http://www.thearkdb.org). The highest density genetic map for the porcine species performed up to date, USDA-MARC v2 (A) (Rohrer et al. 1996), accounted for 1042 multiallelic markers distributed along the 18 autosomes and X chromosome. However, the recently completed porcine genome sequence, the genome assembly carried out by the Swine Genome Sequencing Consortium (Archibald et al. 2010) and the newly developed high-density porcine SNP array (Ramos et al. 2009) make it possible to construct high-density porcine linkage maps and to obtain more precise estimates of the recombination rates in this species.
We genotyped a total of 416 pigs of the Iberian × Landrace (IBMAP) experimental cross (Óvilo et al. 2000, 2010): 147 males and 269 females from 62 full-sib families. A total of 86 F3 animals resulted from the cross of three F2 boars with 15 F2 sows, 79 backcrossed animals resulted from the cross of four F2 boars with 22 Landrace sows and 160 backcrossed animals resulted from the cross of five F1 boars with 25 Landrace sows. In addition, F1 and F0 sires and dams were also genotyped. The animals were genotyped with the Porcine 60K SNP BeadChip (Illumina, Inc.) using the Infinium HD Assay Ultra protocol (Illumina, Inc.). Raw individual data had high genotyping quality (call rate >0.99). Clustering of the genotypic data obtained with the Illumina BeadStudio software was checked, and markers with poor clustering performance were excluded from the analysis. The total number of high-quality SNPs corresponded to 56% of the SNPs in the panel. In addition, SNPs with an allele frequency smaller than 0.15, those located on sex chromosomes or those that were not mapped on the Sscrofa10 assembly, were also discarded. A total of 14 683 SNPs were retained in the data set for linkage analyses. The linkage maps were constructed using the option ‘Fixed’ of the updated CRI-MAP v2.503 (http://www.animalgenome.org/bioinfo/tools/share/crimap/). The order in which the SNPs were assembled within chromosome followed the physical order of the Sscrofa10 assembly. To evaluate remaining genotyping errors and mapping mistakes, the ‘Chrompic’ option of cri-map was employed to reconstruct the chromosomes and carefully check double crossovers; 180 additional SNPs were removed from the maps. The average recombination rates were calculated for each autosome as the ratio between genetic and physical lengths (cM/Mb) from the first to the last marker (rC). In addition, we averaged the recombination rate values calculated along each autosome in 1-Mb non-overlapping windows (rw).
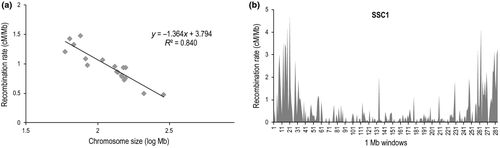
The recombination rates obtained with both procedures for each autosome and the coefficient of variation (CV) of rw are presented in Table 1. The total linkage map length was 2018.8 cM, and the estimated average recombination rates across the porcine autosomes were 0.86 and 0.87 cM/Mb respectively. These estimates are very similar to the estimate previously inferred by Rohrer et al. (1996), 0.84 cM/Mb, but with a larger number of meiosis and SNP markers. The average recombination rates per autosome ranged from 0.48 in SSC1 to 1.48 in SSC10. There was a negative relationship between the recombination rate and the chromosome size, with a regression slope of −1.36 and a Spearman's rank correlation coefficient of −0.90 (P < 0.0002; Fig. 1a). Previous studies in rodents and humans have shown that the smallest chromosomes have the highest recombination rate, with the exception of human chromosome 22 (Jensen-Seaman et al. 2004). The same is observed in this study in pigs, where chromosomes 10, 12, 17 and 18 are the smallest chromosomes (in Mb), but showed the largest recombination rates (Table 1). Analogous variations among chromosomes were observed when estimating recombination rates, taking 1-Mb sliding windows on each chromosome and estimating their coefficient of variation (Table 1). In addition, this analysis showed a large variation in recombination rate within chromosome region, which differs between chromosomes. The CV (rw) ranged between 0.78 in SSC12 and 1.67 in SSC1. An example of a chromosome with a high variation in recombination rate is C1 (Fig. 1b). This chromosome has several windows with large recombination rates (over 3 cM/Mb) in the extremes, probably corresponding to the telomeres, while in the centre of the chromosome many windows have recombination rates close to zero. This variation could be explained by the telomere and centromere effects (Nachman & Churchill 1996), which would result in a higher recombination rate near the telomeres and a reduced recombination rate near the centre of the chromosome, as demonstrated by others studies of the human, mouse, rat and porcine X chromosome (Yu et al. 2001; Kong et al. 2002; Jensen-Seaman et al. 2004; Ma et al. 2010). However, it should be noted that the distribution of the SNPs along the chromosome is not uniform, which could limit the successful capture of recombination events happening in these regions.
| SSC | Number of SNPs | Physical length (Mb) | Genetic length (cM) | Recombination rate, r (cM/Mb) | ||
|---|---|---|---|---|---|---|
| Chromosomal average | Averaged 1-Mb non-overlapping windows | |||||
| r C | r W | CV (rW) | ||||
| 1 | 2117 | 288.85 | 139.72 | 0.48 | 0.49 | 1.66 |
| 2 | 844 | 156.43 | 118.58 | 0.76 | 0.77 | 1.32 |
| 3 | 636 | 135.91 | 118.02 | 0.87 | 0.85 | 1.21 |
| 4 | 1132 | 138.50 | 119.70 | 0.86 | 0.86 | 1.16 |
| 5 | 566 | 108.35 | 115.81 | 1.07 | 1.08 | 1.25 |
| 6 | 538 | 156.97 | 148.01 | 0.94 | 1.06 | 1.48 |
| 7 | 859 | 131.86 | 125.94 | 0.96 | 0.98 | 1.22 |
| 8 | 690 | 147.14 | 118.64 | 0.81 | 0.83 | 1.44 |
| 9 | 815 | 151.45 | 141.92 | 0.94 | 0.98 | 1.23 |
| 10 | 410 | 75.84 | 111.91 | 1.48 | 1.53 | 0.90 |
| 11 | 608 | 82.42 | 89.59 | 1.09 | 1.09 | 1.06 |
| 12 | 374 | 63.85 | 91.46 | 1.43 | 1.43 | 0.78 |
| 13 | 1261 | 210.60 | 106.10 | 0.50 | 0.49 | 1.65 |
| 14 | 1510 | 153.45 | 112.15 | 0.73 | 0.74 | 1.44 |
| 15 | 825 | 147.44 | 116.31 | 0.79 | 0.80 | 1.43 |
| 16 | 512 | 84.87 | 82.88 | 0.98 | 0.98 | 1.02 |
| 17 | 454 | 68.14 | 90.52 | 1.33 | 1.32 | 1.03 |
| 18 | 352 | 59.26 | 71.53 | 1.21 | 1.20 | 0.85 |
| Total | 14 503 | 2361.36 | 2018.79 | 0.86a | 0.817a | |
- SNP, single nucleotide polymorphism; CV, coefficient of variation.
- a Weighted by the physical length (Mb).
In addition, the ratio between female and male recombination rates was inferred; sex-specific linkage maps were built using the same data set. The total linkage map length was 2353.14 cM for females and 1704.62 cM for males. This ratio of 1.38 between female and male recombination rates is supported by previous studies in mammals (Gyapay et al. 1994; Archibald et al. 1995; Mellersh et al. 1997; Neff et al. 1999) that showed that the sexual rates lay between 1.0 and 2.0, with the heterogametic sex having the least recombination.
Finally, it should be noted that although we have removed several markers from the linkage analyses owing to possible assembly errors, we cannot discard other undetected mapping errors. Therefore, improvements in the accuracy of these estimates will be expected with each new iteration of the porcine genome assembly and subsequent SNP annotation. More thorough analyses of the reasons underlying the variation observed among chromosomal regions will be possible with improvements to the porcine genome assembly, allowing for the linking structural regions of the chromosome with recombination rate variations.
Acknowledgements
This work was funded by MICINN projects AGL2008-04818-C03/GAN and CSD2007-00036. María Muñoz was funded by an FPI PhD grant from the INIA institution. Yuliaxis Ramayo-Caldas was funded by an FPU PhD grant from Spanish Ministerio de Educación. We want to thank the huge effort of Swine Genome Sequencing Consortium on Sscrofa10 assembly and particularly Dr Martien Groenen for the SNP annotation. We are also grateful to Anna Mercadé for her technical assistance with the SNP genotyping and to the reviewers for their useful comments.




