Association analysis between canine behavioural traits and genetic polymorphisms in the Shiba Inu breed
Summary
The relationships between behavioural trait data and the genotype of 15 polymorphisms in eight neurotransmitter-related genes were analysed in 77 dogs of the Shiba Inu breed, an indigenous Japanese dog. The data were obtained from a 26-item questionnaire on the dog’s behaviour, distributed to the dog’s owners, through veterinary hospitals and the Shiba Inu breed magazine. A factor analysis of the questionnaire items extracted eight factors accounting for 66.8% of the variance. An association analysis between these factors and genetic polymorphisms indicated that the polymorphism of c.471T>C in the solute carrier family 1 (neuronal/epithelial high-affinity glutamate transporter) member 2 (SLC1A2) gene was significantly associated with Factor 1, referred to as ‘aggression to strangers’. This association remained stable in separate analyses of data from surveys obtained from the hospitals and those obtained from the magazine. The results suggest that the c.471T>C polymorphism is associated with some types of aggressive behaviour in the Shiba Inu. Further studies using other dog breeds are necessary to extend these findings to dogs in general.
Introduction
Dogs have been associated with humans longer than any other domestic animal and play a number of important roles in modern society. Many researchers are working to localize genes of interest in dogs, particularly disease-related genes, for possible relevance in understanding some genetically related diseases in humans and to guide breeding programmes in dogs to improve health and welfare. A draft sequence of the canine genome was unveiled on the web site of the National Center for Biotechnology Information in July 2004 (http://www.ncbi.nlm.nih.gov/genome/guide/dog/). Subsequently, researchers working on the canine genome have pointed out that the dog is an ideal animal for clarifying the genomic background of behavioural traits as well as diseases (Lindblad-Toh et al. 2005; Houpt 2007; Spady & Ostrander 2008). Thus far, however, there have been only a few studies on the genetic aspects of canine behavioural traits (Hejjas et al. 2007; Maejima et al. 2007; van den Berg et al. 2008; Takeuchi et al. 2009).
Recently, research into the genetic basis of emotional characteristics in humans has drawn a great deal of attention. Research related to the heredity of personality in monozygotic twins has shown that individual personalities are determined by both hereditary and environmental factors and that most personality traits have heritabilities of 30–50% (Plomin 1990). A meta-analysis of many studies on identical twins determined that the heritability of the traits of interpersonal affiliation, aggressiveness and social anxiety was 70%, 58% and 65% respectively (Beatty et al. 2002). One of the earliest hereditary factors related to behaviour is the polymorphism of the dopamine D4 receptor (DRD4) (Benjamin et al. 1996; Ebstein et al. 1996). People with a high number of repeats of a certain sequence in exon 3 of this gene are likely to be highly ‘novelty seeking’.
In previous research, we reported the involvement of the c.471T>C polymorphism in the solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter) member 2 (SLC1A2) gene, and the c.216G>A polymorphism in the catechol-O-methyltransferase (COMT) gene, in behavioural patterns related to activity level of guide dogs for the blind (Takeuchi et al. 2009). In this study, we followed the experimental design of other investigators of gene-related behaviour in dogs, by employing working dogs with assessment of canine temperament by professional trainers, under the presumption that behavioural assessment by such handlers would be more reliable than that by dog owners (Hejjas et al. 2007; Maejima et al. 2007). However, we found a relative uniformity of behavioural traits in the working dog colony as well as a stratification problem derived from the litter effect (Hirschhorn & Daly 2005). These difficulties in using working dog colonies for behaviour-genetic analyses profiled the possible value of using pet dogs as a source to investigate the genetic background of behavioural traits in domestic dogs. Other investigators have demonstrated the validity of owner-reported behavioural data in describing behavioural traits (Hsu & Serpell 2003).
As a first step in using pet dogs of a specific breed to analyse the genetic basis of various behavioural traits, we used the Shiba Inu breed. We selected the Shiba Inu because of the ease of sample collection in Japan and its genetic diversity relative to other breeds, a result of its short history of artificial selection. The Shiba Inu is an indigenous Japanese dog, one of the most popular dogs in Japan, and was only recently registered by the Kennel Club (UK) and the American Kennel Club (USA). The Shiba Inu was originally bred for hunting; they were often kept as watchdogs until recently and are still used as watchdogs in rural areas. However, they are now becoming a companion animal, living inside homes in urban areas. One study reveals that they tend to be more aggressive to strangers, other dogs and children than other breeds (Takeuchi & Mori 2006). This might result from the Shiba Inu being one of the breeds most closely related, genetically, to wolves (Parker et al. 2004). In this study, we analysed the relationships between various behavioural traits, including several types of aggressive behaviour, and several polymorphisms in neurotransmitter-related genes.
Materials and methods
Animals and sample collection
Seventy-seven presumably unrelated Shiba dogs were recruited for this study through 11 veterinary hospitals in the Kansai, Kanto and Chu-bu areas of Japan (n = 39) in 2000–2002 and through an advertisement in the bimonthly Shiba Inu breed magazine, Shi-ba, (n = 38) in 2003. The mean age of these dogs was 4.7 years (range 1–14 years), comprising 27 intact males, 15 castrated males, 15 intact females and 20 spayed females. Of these subjects, 44 were mainly inside dogs, 15 inside and outside dogs and 18 mainly outside dogs.
Blood samples were collected at veterinary hospitals or hair samples were sent to our laboratory directly from the owners who participated via the magazine. We, the investigators, or the veterinarians, asked the dog owners to fill out a form with general information about age, sexual status and housing situation, as well as to complete a 26-item questionnaire about the dog’s behavioural traits (Table S1). The behavioural questions each had six possible responses based on the frequency of each behaviour. Detailed criteria are shown in Table S1.
Genotyping of polymorphisms
Genomic DNA was extracted from the blood and hair samples using a QIAamp blood or tissue kit (Qiagen). For screening candidate genes, based on previous reports, we selected 19 polymorphisms on the exons of nine neurotransmitter-related genes: (i) c.97C>T, c.168G>A, c.180G>A and c.264C>T in the tyrosine hydroxylase (TH) gene (Takeuchi et al. 2005); (ii) c.789C>A and c.1819A>G in the dopamine beta-hydroxylase (DBH) gene (Takeuchi et al. 2005); (iii) c.808C>A in the 5-hydroxytryptamine (serotonin) receptor 1A (5HTR1A) gene (van den Berg et al. 2003); (iv) c.246G>A, c.660C>G, c.955T>C and c.1146G>C in the 5HTR1B gene (Masuda et al. 2004b); (v) the short interspersed nuclear element (SINE) in the dopamine receptor D2 (DRD2) gene (Jeoung et al. 2000); (vi) the insertion–deletion in exon 1 and variable number of tandem repeats in exon 3 in the DRD4 gene (Niimi et al. 1999; Ito et al. 2004); (vii) c.216G>A and c.482G>A in the COMT gene (Masuda et al. 2004a); (viii) c.199T>C in the monoamine oxidase B (MAOB) gene (Hashizume et al. 2005); and (ix) c.129C>T and c.471T>C in the SLC1A2 gene (Ogata et al. 2006). The methods used to genotype these polymorphisms have been described elsewhere (Niimi et al. 1999; Jeoung et al. 2000; van den Berg et al. 2003; Ito et al. 2004; Masuda et al. 2004a,b; Hashizume et al. 2005; Takeuchi et al. 2005; Ogata et al. 2006). Of these 19 polymorphisms, the frequencies of minor alleles of c.180G>A in TH, the insertion/deletion in exon 1 in DRD4, and c.216G>A and c.482G>A in COMT were extremely low or non-existent as in our previous study; thus, these polymorphisms were excluded from further analysis. All screened genotype frequencies were in Hardy–Weinberg equilibrium in this study. The distribution of genotypes and allele frequencies of the polymorphisms examined in this study are presented in Table S2.
Statistical analyses
All data analyses were performed using statview 5j for Macintosh (SAS Institute). The two-tailed significance level was set at 0.05 for all statistical tests.
To quantify the data from the individual items on the questionnaire, a factor analysis was performed using the principal factor method for factor extraction and the Varimax rotation for orthogonal transformation. Of the 26 items, ‘housetraining success’ and ‘housetraining period’ had lower answer rates than the other 24 items, and so these items were excluded from the factor analysis. These 24 items are listed in Table 1. Only factors with an Eigenvalue of at least 1.0 were considered to determine which factors would be used to explain most of the behavioural variance. There were eight factors, which met this criterion. Only the factors, which explained at least 10% of the variance, were used for analyses with regard to relationship to the genetic elements of interest. Two factors, referred to as ‘aggression to strangers’ and ‘reactivity’, met this criterion.
| Factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Aggression to guests | 0.768 | |||||||
| Aggression to children | 0.686 | |||||||
| Inter-dog aggression | 0.592 | |||||||
| Watchdog barking | 0.543 | |||||||
| Excitability | 0.717 | |||||||
| Possessive aggression | 0.677 | |||||||
| Excessive barking | 0.420 | 0.521 | ||||||
| Response to strange toys | 0.764 | |||||||
| Aggression to owner | −0.541 | 0.418 | −0.428 | |||||
| Destructive motivation | 0.727 | |||||||
| General activity | 0.699 | 0.415 | ||||||
| Playfulness | 0.611 | |||||||
| Friendly response to strangers | 0.467 | |||||||
| Panting or shivering before owner’s departure | 0.875 | |||||||
| Avoidance of unpleasant places | −0.402 | 0.594 | ||||||
| Excessive barking after owner’s departure | 0.494 | |||||||
| Over-attachment to the owner at home | 0.458 | 0.482 | ||||||
| Over-attachment to the owner in unfamiliar places | 0.764 | |||||||
| Affection demand | 0.515 | |||||||
| Timid with strangers | 0.481 | 0.512 | ||||||
| Obedience without reward | 0.851 | |||||||
| Obedience with reward | 0.507 | |||||||
| Timid with a loud noise | 0.852 | |||||||
| Begging | ||||||||
| Eigenvalue | 4.34 | 2.62 | 2.17 | 1.75 | 1.55 | 1.35 | 1.17 | 1.09 |
| Contribution ratio | 18.1 | 10.9 | 9.0 | 7.3 | 6.5 | 5.6 | 4.9 | 4.5 |
| Cronbach’s α reliability coefficients | 0.661 | 0.603 | 0.484 | 0.565 | 0.634 | 0.519 | 0.472 | – |
| Expected factor name | Aggression to strangers | Reactivity | Investigation | Activity | Anxiety | Attachment | Trainability | Noise phobia |
- The absolute value, which was <0.4, was not present in the table.
The relationships between each of the eight factors and information on, age, sex and housing situation were examined by a three-way analysis of variance (anova). The relationships between each of the two factors and each genotype used were analysed using one-way anova. If the number of animals having a particular genotype was less than six, we excluded that genotype from the analysis.
To avoid Type I errors derived from multiple statistical tests, we adopted the Bonferroni correction, which uses a modified significance criterion (α/κ where κ is the number of statistical tests conducted on the given data) and the significance level was diminished according to the repetitive use of data (Bland & Altman 1995).
Results
Behavioural characteristics
As mentioned, factor analysis of the 24 questionnaire items from both the hospitals and the breed magazine resulted in eight main factors with Eigenvalues >1.0. Altogether, the eight factors accounted for 66.8% of the variance. Those questionnaire items in which the loading on a factor was at least 0.4 (positive or negative loading) are shown in Table 1 for each factor, along with the Eigenvalue, contribution ratio, Cronbach’s α coefficients and factor label. Cronbach’s α reliability coefficients ranged from 0.472 to 0.661. The three-way anova revealed a significant relationship between Factor 4, activity and age [F(1,65) = 14.24, P = 0.0003] after the Bonferroni correction. No other significant relationships or interactions among age, sex and housing situation were found.
Relationships between Factor 1 or 2 and genetic polymorphisms
The association analysis between the two factors which contributed to more than 10% of the variance and the genetic polymorphisms showed that c.471T>C in the SLC1A2 gene was significantly related to Factor 1 [anova: F(1,71) = 12.88, P = 0.0006] after the Bonferroni correction (Table 2, Fig. 1). The dogs whose genotype was CC were significantly less aggressive than dogs whose genotype was TC. The TT genotype of this polymorphism was omitted from the analysis because of the small sample size (n = 4; mean ± SE of these four Factor 1, scores is 0.17 ± 0.68). To examine for possible differences in the relationship between Factor 1 and c.471T>C as a function of the source of the data, data from the surveys were divided into two subgroups according to those recruited from hospitals and those recruited from the magazine. The separate analyses showed virtually identical results, as shown in Fig. 1.
| Polymorphisms | Factors | References | |
|---|---|---|---|
| 1 | 2 | ||
| TH | |||
| c.97C>T | 0.1753 | 0.2535 | Takeuchi et al. (2005) |
| c.168G>A | 0.5198 | 0.9032 | |
| c.264C>T | 0.0070 | 0.1325 | |
| DBH | |||
| c.789C>A | 0.2972 | 0.1530 | Takeuchi et al. (2005) |
| c.1819A>G | 0.5597 | 0.6179 | |
| 5HTR1A | |||
| c.808C>A | 0.9367 | 0.5375 | van den Berg et al. (2003) |
| 5HTR1B | |||
| c.246G>A | 0.3782 | 0.7687 | Masuda et al. (2004b) |
| c.660C>G | 0.0606 | 0.5424 | |
| c.955T>C | 0.4211 | 0.2228 | |
| c.1146G>C | 0.6816 | 0.1265 | |
| DRD2 | |||
| SINE | 0.8739 | 0.8888 | Jeoung et al. (2000) |
| DRD4 | |||
| Exon 3 | 0.0115 | 0.9270 | Niimi et al. (1999) |
| MAOB | |||
| c.199T>C | 0.7700 | 0.2042 | Hashizume et al. (2005) |
| SLC1A2 | |||
| c.129C>T | 0.0137 | 0.3131 | Ogata et al. (2006) |
| c.471T>C | 0.0006* | 0.6006 | |
- Each P-value was from anova. The values that were <0.05 were given in bold. The reference in parentheses refers to the original description of the polymorphism.
- *The value that reached significant level (P < 0.00167 = 0.05/15 polymorphisms/2 factors) after Bonferroni correction.
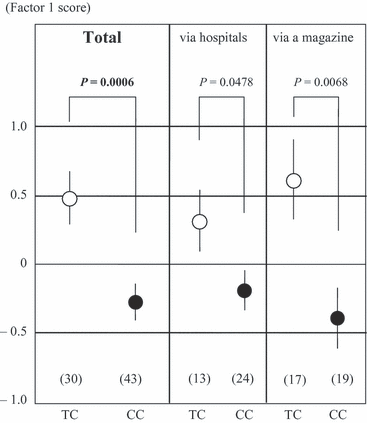
Relationship between Factor 1 and the c.471T>C polymorphism in the SLC1A2 gene. The number of animals with each genotype is shown in parentheses. Data designated by open circles (TC genotype) and closed circles (CC genotype) represent mean ± SE. The TT genotype was omitted from the analysis because of the small sample size (n = 4).
Discussion
In this study, data regarding behavioural characteristics of 77 dogs of the Shibu Inu breed, derived from a 24-item questionnaire filled out by the owners, were first subjected to a factor analysis. Eight factors with an Eigenvalue of at least 1.0 accounted for 66.8% of the variance, of which only the first two factors each accounted for at least 10% of the variance. Using these two factors to associate with the genetic polymorphisms examined, we found, after a Bonferroni correction, that c.471T>C in the SLC1A2 gene was significantly related to Factor 1, a factor labelled ‘aggression to strangers’ (P = 0.0006). The items that loaded on this factor were ‘aggression to guests’, ‘aggression to children’, ‘inter-dog aggression’, ‘watchdog barking’ and ‘excessive barking’. These highly significant results strongly suggest that this polymorphism is related to some types of aggression, at least in some breeds of dogs.
In humans, the SLC1A2 gene is thought to be involved in neurological disorders such as stroke, trauma, Alzheimer’s disease, amyotrophic lateral sclerosis and Huntington disease (Kanai et al. 1993). Based on our results, the association between the SLC1A2 gene polymorphism and aggressive traits in humans should also be examined. The SLC1A2 gene product actively removes the excitatory neurotransmitter glutamate from the extracellular space and contributes to the maintenance of low extracellular glutamate concentrations (Kanai et al. 1993; Tanaka et al. 1997; Trotti et al. 1998). Glutamate concentrations are thought to play a role in aggression in humans (Swann 2003) and in cats as well (Siegel & Schubert 1995). How the polymorphism affects the function of this gene should also be examined given that the polymorphism does not seem to express its effects through its location or type (Ogata et al. 2006).
Our rationale of using the data on behaviour from surveys completed by dog owners rather than the data from experimentally designed tests was addressed in the Introduction. In essence, data from dog owners has been shown to have high validity when compared with diagnoses by canine behaviour experts (Hsu & Serpell 2003). Separate evaluations on dogs recruited through veterinary hospitals and those recruited through a breed journal gave similar results, acting as confirmation of the relative reliability of our data set, as shown in Fig. 1.
Because the study just analysed the association of behavioural traits with genetic polymorphisms in one breed, the likelihood of association of behavioural traits with genetic elements was perhaps less than if more than one breed had been used. The polymorphism c.471T>C in the SLC1A2 gene is found in at least four other breeds (Ogata et al. 2006), and given the importance of understanding the genetic basis of aggressive behaviour, our results linking this polymorphism to aggression should be confirmed in other breeds with a larger sample size, possibly to extrapolate the findings to the canine species in general. A future study should also take the pedigree of subject dogs into account to confirm the relationship herein identified. In the present study, we could not obtain such information.
Given the documented behavioural variability among breeds of dogs (Hart & Hart 1988; Takeuchi & Mori 2006), clearly some inconsistency is to be expected in the relationship of the polymorphisms to breed-specific behaviour. For example, the DRD4 polymorphism in dogs of the Shiba Inu breed has a longer construction in this domain (exon 3) than in dogs of the Golden Retriever breed (Niimi et al. 1999). With the recognizable differences in behaviour between these two breeds, a breed-related difference in the polymorphism seems likely to correspond to one or more behavioural differences. In German Shepherd Dogs used in police work, this polymorphism was related to an ‘activity-impulsivity’ phenotype (Hejjas et al. 2007).
Aggressive behaviour in dogs is a complex behavioural issue with many forms of aggression that encompass various aspects of fear, activity level and territoriality (Hart et al. 2006). For example, the SLC1A2 polymorphism was recently found to be related to activity level in Labrador Retrievers in a guide dog colony. Those whose genotype was TT were significantly more active than dogs whose genotype was TC or CC (Takeuchi et al. 2009). An illustration of the complexity in uncovering genetic elements of aggression is our finding in this study that ‘possessive aggression’ loaded primarily on Factor 2 (reactivity) and ‘aggression to owners’ loaded primarily on Factor 3 (investigation) rather than on Factor 1. Only through a comprehensive set of analyses, involving several carefully selected dog breeds, can a more complete understanding of the genetic basis of canine aggressive behaviour be obtained.
Finally, because dog breeds represent the most diverse array of behavioural, as well as morphological and physiological, traits of any species available for study, and because of the relative ease of obtaining reliable behavioural and genetic data, this species represents a rich opportunity for further work in this field.
Acknowledgements
The authors thank the Drs Norio Kogure, Kazue Igarashi, Hisao Imoto, Reiko Usui, Keiko Uchida, Tetsuyasu Uno, Ayako Kakinuma, Shoji Satoh, Masami Takebe, Makoto Tatematsu and Kaori Murata, and the staff of their veterinary hospitals, for their cooperation in collecting canine blood samples and administering the survey questionnaires to the dog owners. This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Sports, Culture, and Technology, Japan and, in part, from the University of California, Davis, Center for Companion Animal Health (allocation #03-65-F).




