QTL for resistance to Salmonella carrier state confirmed in both experimental and commercial chicken lines
Summary
The ability of chickens to carry Salmonella without displaying disease symptoms is responsible for Salmonella propagation in poultry stocks and for subsequent human contamination through the consumption of contaminated eggs or meat. The selection of animals more resistant to carrier state might be a way to decrease the propagation of Salmonella in poultry stocks and its transmission to humans. Five QTL controlling variation for resistance to carrier state in a chicken F2 progeny derived from the White Leghorn inbred lines N and 61 had been previously identified using a selective genotyping approach. Here, a second analysis on the whole progeny was performed, which led to the confirmation of two QTL on chromosomes 2 and 16. To assess the utility of these genomic regions for selection in commercial lines, we tested them together with other QTL identified in an [N×61] × N backcross progeny and with the candidate genes SLC11A1 and TLR4. We used a commercial line divergently selected for either low or high carrier-state resistance both in young chicks and in adult hens. In divergent chick lines, one QTL on chromosome 1 and one in the SLC11A1 region were significantly associated with carrier-state resistance variations; in divergent adult lines, one QTL located in the major histocompatibility complex on chromosome 16 and one in the SLC11A1 region were involved in these variations. Genetic studies conducted on experimental lines can therefore be of potential interest for marker-assisted selection in commercial lines.
Introduction
Salmonellosis is a zoonotic disease due to the Gram-negative enteric bacterium Salmonella. More than 2500 serotypes are described, with most belonging to the same species S. enterica (Brenner et al. 2000). Whereas some of these serotypes are species-specific, some others, like S. Typhimurium and S. Enteritidis, are able to infect a broad range of domestic animals and cause mild to severe disease symptoms. Salmonella typhimurium and S. Enteritidis are able to infect both poultry and man. They can be transmitted to humans, especially from poultry products, through the consumption of contaminated eggs or meat, and are responsible of most cases of human food poisoning. Salmonella Enteritidis in particular, which possesses the ability to develop in eggs, is responsible of one third of the human food poisoning cases in France (Bouvet et al. 2002). Even when efficient prophylactic measures and vaccination are applied in poultry stocks, asymptomatic carriers of Salmonella can cause an invisible propagation of the bacteria. Selecting chicken more resistant to Salmonella carrier state could be a way of decreasing the Salmonella propagation in poultry stocks and thus decreasing subsequent food poisoning. Prévost et al. (2006) demonstrated in a simulation study that having animals with a better bacterial clearance ability, i.e. a better resistance to carrier state, combined with an enhanced immune response (i.e. vaccination), would lead to a large decrease in Salmonella propagation in industrial hen houses. Since the beginning of the 20th century, breeders have considered selection for better general disease resistance to reduce animal losses, but a better resistance to carrier state would probably be more effective at improving food safety.
Between-line differences were demonstrated in many studies for general Salmonella resistance (for instance Roberts & Card 1935; Bumstead & Barrow 1988, 1993; Duchet-Suchaux et al. 1997) and more recently for the carrier-state resistance of young chicks (Guillot et al. 1995; Berthelot et al. 1998; Beaumont et al. 1999; Girard-Santosuosso et al. 2002) and adult hens (Lindell et al. 1994; Protais et al. 1996). Several studies then identified either candidate genes or quantitative trait loci (QTL) responsible for a part of the observed Salmonella resistance variation. Only a few of these studies focused on carrier-state resistance. Beaumont et al. (2003) demonstrated the role of the genomic region carrying the candidate gene SLC11A1 and suspected the role of TLR4 in the persistence of the bacteria several weeks after inoculation. On the other hand, several QTL for resistance to carrier state were identified in two populations both derived from crosses between the White Leghorn inbred lines N and 61 [back-cross (BC) and F2] using a selective genotyping approach (Tilquin et al. 2005). In this latter study, carrier-state resistance to either S. Enteritidis (F2) or S. Typhimurium (BC) was assessed by counting bacteria from cloacal swabs performed 2 weeks post-inoculation (pi) for the BC progeny, and 4 and 5 weeks pi for the F2 progeny. Bacterial count in caeca was also considered in the F2 progeny. Thirty-three F2 individuals with extreme phenotypes were genotyped with 103 microsatellite markers, and 46 BC individuals were genotyped with 135 markers. One genome-wide significant QTL was identified on chromosome 2, and five chromosome-wide significant QTL were found on chromosomes 1 (2 QTL), 5, 11 and 16.
The aims of this study were: (1) to confirm the F2 QTL previously described (Tilquin et al. 2005) in the same F2 progeny, by extending the genotyping to the whole progeny and adding new markers; (2) to test the QTL identified in the BC and F2 progeny studied by Tilquin et al. (2005) and the TLR4 and SLC11A1 candidate genes in a different genetic background, i.e. divergent inbred lines derived from commercial lines. This second analysis was performed to evaluate the transferability of results obtained on experimental lines to commercial materials.
Materials and methods
Animals
Divergent selection of commercial lines for carrier-state resistance
Two divergent selection experiments were carried out from a base population of 79 animals sampled from a commercial layer-type line for either a decreased or an increased level of carrier-state resistance, on young chicks or adult hens (Beaumont et al., in press). A total of four lines were finally obtained after selection was conducted at each generation for six generations. For the first 3 years, selection was performed simultaneously for chicks and adults using the combined data resulting from experimental evaluations conducted at both ages, thus generating two divergent lines (low/high carrier-state resistance). For the last 3 years, selection was performed independently for chicks and adults, so that two series of lines were selected. Lines selected at a young age were named SALy+ and SALy−, while the lines selected at an adult age were named SALa+ and SALa−, where + and − respectively stand for a lower or a higher resistance to carrier state (more bacteria or less bacteria respectively). Pathogenic challenges were performed at each generation on both young chicks and adult hens, as detailed below. Infected animals could not be conserved for reproduction, so selection was conducted on siblings. Data collected from pathogenic challenges were used both for the selection experiments and to study the association between carrier-state variations and genomic regions identified in other genetic backgrounds.
F2 progeny
N and 61 are White Leghorn experimental inbred lines from the USDA Avian Disease and Oncology Laboratory, East Lansing, MI. They were provided by the Institute for Animal Health (IAH), Compton, UK. As described previously (Tilquin et al. 2005), 185 F2 progeny derived from crosses between six sires and 18 dams were reared.
Salmonella pathogenic challenges and measures of carrier-state resistance
F2 progeny
As described previously (Tilquin et al. 2005), 1-week-old birds were orally inoculated with 5 × 104 bacteria from the S. Enteritidis phage type 4 (PT 4) strain 1009, which is a spontaneous nalidixic acid (NA) and streptomycin (SM) resistant mutant of the strain 5556 used to inoculate the divergent adult lines. To assess the number of bacteria excreted, two cloacal swabs were made at weeks 4 and 5 pi. Chicks were slaughtered 5 weeks pi and weighed, and caeca were removed from each animal. The number of Salmonella colony-forming units (cfu) was counted in each caeca. Caeca bacterial counts were expressed in log (cfu) per gram of caeca (CAEC). In case of negative results, caeca were placed in an enriched solution, to discriminate weakly contaminated, positive after enrichment caeca from negative after enrichment caeca, which could be considered as Salmonella free. Blood was also taken from each bird for DNA extraction and genotyping.
Divergent lines
Resistance of young chicks was assessed as described in Duchet-Suchaux et al. (1995). For each selection experiment, 1-week-old chicks were orally inoculated with 5 × 104 cfu of the S. Enteritidis PT 4 NA and SM resistant strain 1009. Birds from the same generation originated from the same hatching batch. They were divided into four groups taking into account the bird parental origins and reared in four different cages, to prevent confusion between cage and family effects. Birds were slaughtered 5 weeks pi, and bacteria were counted in the removed caeca. Caeca bacterial counts were expressed in log (cfu) per gram of caeca (CAEC), except for two hatching lots for which the Salmonella counts were very low. In the latter case, the caeca contamination rate Caeca0–1 was considered (Table 1).
| Lines | Inoculation | Phenotyping | Traits | ||
|---|---|---|---|---|---|
| Time | Dose (cfu) | Time | SE count in | ||
| SALy+/SALy chicks | 1 week | 5 × 104 | 5 weeks | Caeca | CAEC: log (cfu) per gram caecaCaeca0–1: presence/absence of SE in caeca |
| SALa+/SALa− adults | Peak of lay | 109 | 4 weeks | LiverSpleenOvaryCaeca | Organ0–1: presence/absence of SE in organAdult0–1: 0 vs. at least one organ contaminatedAdult0–4: number of organs contaminated (0–4) |
| F2 N×61 progeny adult hens | 1 week | 5 × 104 | 4 and 5 weeks 5 weeks | Gut (cloacal swabs)Caeca | CSW41: log (cfu)/swabCSW5: log (cfu)/swabCAEC: log (cfu)/g caeca |
- SE, Salmonella enteritidis.
- 1CSWx: cloacal swab performed at the xth week after inoculation.
Resistance of adult hens was assessed as described in Protais et al. (1996). Hens were orally inoculated at their peak of lay with 109 cfu of the S. Enteritidis PT 4 strain 5556. Birds of each generation derived from two or three hatching batches and were divided, as described for the chicks, into four groups. Hens were slaughtered 4 weeks pi and their liver, spleen, caeca and ovary removed for Salmonella counting. The presence/absence of Salmonella in each organ (Organ0–1), the number of organs contaminated Adult0–4 and the global contamination rate Adult0–1 were considered for further analyses (Table 1).
Genotyping
F2 progeny
The F2 progeny and their F1 and F0 parents and grandparents were typed for a new set of 32 microsatellite markers chosen to cover previously uncovered genomic regions on the chromosomes 10, 20 and 22 and to perform a targeted, finer mapping of four of the five regions (QTL) previously identified on the chromosomes 1, 2, 5 and 16. The QTL on chromosome 11 was considered as probably spurious in the previous analysis (Tilquin et al. 2005) and was therefore not targeted in this study. Among the 32 markers used, 20 were new while 12 markers had been already used in the previous analysis to type animals with extreme phenotypes (Tilquin et al. 2005). Genetic distances between markers were inferred from the distances indicated in the chicken consensus genetic map (Groenen et al. 2000).
Divergent lines
Blood was taken from the phenotyped animals for two (chicks) or three (adults) generations for DNA extraction and typing (2003, 2004 and 2005 for adults, and 2004 and 2005 for chicks). Marker genotypes were obtained as described in Tilquin et al. (2005). Animals were typed for seven microsatellite markers (Table 2) located close to the QTL detected either in the F2 or the BC progeny (Tilquin et al. 2005). In addition, animals were typed with two SNP markers previously identified in the candidate genes SLC11A1 and TLR4, which were found significantly associated with carrier-state variation (Beaumont et al. 2003). Marker genotypes were obtained through PCR-SSCP (TLR4) or PCR-RFLP (SLC11A1) as in Beaumont et al. (2003).
| Chr | Marker | Original locus | |||||
|---|---|---|---|---|---|---|---|
| Name | Genomic position (bp) | Genetic position (cM) | Locus type | Genetic position | Trait/locus name | Cross | |
| 1 | LEI0194 | 28 787 247 | 81 | QTL | 85 | CSW5 | F2 |
| MCW018 | 65 699 816 | 203 | QTL | 207 | CSW2 | BC | |
| 2 | GCT0025 | 17 978 987 | 71 | QTL | 87 | CSW4 | F2 |
| LEI0117 | 17 958 970 | 70 | QTL | 87 | CSW4 | F2 | |
| 5 | LEI0145 | 33 917 854 | 98 | QTL | 111 | CAEC; CSW5 | F2 |
| QTL | 100 | CSW2 | BC | ||||
| 7 | SLC11A1 | 24 098 492 | 84 | CG | 84 | SLC11A1 | – |
| MCW0316 | 34 526 547 | 127 | CG | 84 | SLC11A1 | – | |
| 16 | LEI0258 | 101 458 | 50 | QTL | 50 | CAEC; CSW5 | F2 |
| 17 | TLR4 | 4 064 109 | 43 | CG | 43 | TLR4 | – |
- The QTL and candidate genes (CG) targeted by these markers are listed, with their positions and the trait with which they were originally identified (or the locus name, for the candidate genes). Positions on chromosomes are indicated in cM.
Statistical analyses
QTL analysis in the F2 progeny
QTL were searched in the N×61 F2 progeny using the same analysis method as used previously (Tilquin et al. 2005), i.e. the F2 analysis option of the qtlexpress software (http://qtl.cap.ed.ac.uk/), without covariates (as gender and cage effect were found to be non-significant). The bacteria counts in caeca in log (cfu) per gram of caeca (CAEC) and in cloacal swabs performed 4 and 5 weeks pi (CSW4 and CSW5) were used for this purpose.
Single marker analyses in the divergent lines
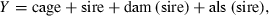
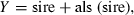
Results
QTL confirmation in the F2 N×61 progeny
Adding new microsatellite markers allowed a finer mapping of the targeted regions on chromosomes 1, 2, 5 and 16 and a mapping of previously uncovered regions on chromosomes 10, 20 and 22 (Fig. 1). While the QTL on chromosomes 1 and 5 that were previously identified with the selective genotyping approach were not confirmed in the whole-progeny analysis, one genome-wide significant QTL was detected on chromosome 2, in the same region (78 vs. 87 cM in the previous analysis) and for the same trait (CSW4) as in the former analysis (Table 2). Similarly, one chromosome-wide significant QTL was identified on chromosome 16 in the same region (7 vs. 2 cM) and with the same trait (CAEC) as previously; this QTL was also detected using CSW5 (Table 3). For each QTL, the percentage of the genetic variance explained by the QTL was calculated as a percentage of the difference between the residual sum of squares (RSS) of the reduced model (without including a fixed QTL effect) and the full model, divided by the full model RSS.
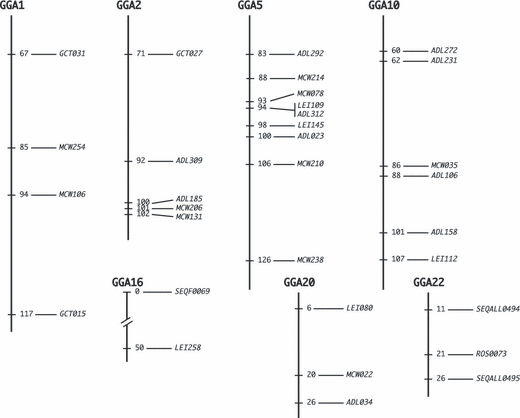
New genetic map used for the confirmation of QTL in the F2 N×61 progeny. Distances are indicated in cM. Chromosomes are not fullys represented.
| Analysis | Trait | Chr2 | Position (cM) | Threshold P-value3 | P-value1 | F max | a2 | d3 | % var4 | Marker |
|---|---|---|---|---|---|---|---|---|---|---|
| Selective | CSW41 | 2 | 87 | 0.0063 | 0.0032** | 15.15 | 2.10 | −4.70 | 7.0 | ADL185 |
| Confirmation | CSW4 | 2 | 78 | 0.0063 | 0.0050** | 9.78 | 1.67 | −2.87 | 40.3 | GCT027 |
| Selective | CAEC | 16 | 2 | 0.0008 | 0.0012* | 9.76 | −0.50 | −2.30 | 0.7 | LEI0258 |
| Confirmation | CAEC | 16 | 50 | 0.0008 | 0.0180* | 5.56 | −0.02 | −1.98 | 27.7 | LEI0258 |
| Confirmation | CSW5 | 16 | 50 | 0.0008 | 0.0080* | 7.13 | 0.60 | −0.91 | 33.0 | LEI0258 |
- The most probable location (Position) of each QTL on their chromosome (Chr) is indicated. The corresponding genome-wide threshold P-values, actual P-values, test statistics Fmax, additivity (a) and dominance (d) effects, percentages of genetic variance explained (% var) and the markers the closest from the QTL most probable locations are indicated.
- 1**/*significant at the genome-/chromosome-wide level.
- 2Computed as half the effect of the (61−N) allelic substitution.
- 3Computed as [61N−(NN+6161)/2] where 61N, NN and 6161 are the heterozygote and homozygote genotypes at the QTL.
- 4Percentage of the genetic variance explained by the QTL.
Association of markers with carrier-state resistance variation in the commercial lines
Results of the GLM analysis of the CAEC data collected on young chicks are shown in Table 4. LEI0194 on chromosome 1 is significantly associated with CAEC variations at a 5% probability threshold (P = 0.015). The SNP marker of SLC11A1 and MCW316 on chromosome 7 is almost significantly associated with CAEC variations (P = 0.088 and 0.080 respectively).
| Chromosome | Marker | Number of animals | P(als)1 |
|---|---|---|---|
| 1 | LEI0194 | 143 | 0.015 |
| 7 | SLC11A1 | 135 | 0.088 |
| 7 | MCW316 | 123 | 0.080 |
- Only significant [P(als) < 0.05] or close to significance results are shown.
- 1 P(als): probability associated with the sire allele effect.
Results of the CATMOD analysis of the data collected on adult hens are shown in Table 5. MCW316 on chromosome 7 was found significantly associated with Ovary0/1 variations (P = 0.015), and its association with Adult0/1 variations was close to significance (P = 0.061). LEI0258 on chromosome 16 was also found significantly associated with Ovary0/1 variations (P = 0.007), and its association with Liver0/1 variations was close to significance (P = 0.08). These results were confirmed with results of the GLM analysis conducted with the same data: MCW316 and LEI0258 were both significantly associated with Ovary0/1. MCW316 was also associated with Adult0/1 variations and LEI0258 with Liver0–1 variations with probabilities close to significance (data not shown).
| Chromosome | Marker | Trait | Number of animals | P(als)1 |
|---|---|---|---|---|
| 7 | MCW316 | Adult0–1Ovary0–1 | 264264 | 0.0610.015 |
| 16 | LEI0258 | Ovary0–1Liver0–1 | 157157 | 0.0070.080 |
- Only significant [P(als) < 0.05] or close to significance results are shown.
- 1 P(als): probability associated with the sire allele effect.
No significant association was identified between other markers and carrier-state resistance variations in chicks and in adult hens.
Discussion
QTL confirmation in the F2 N×61 progeny
The previous QTL analysis in a F2 N×61 progeny (Tilquin et al. 2005) was conducted only with individuals showing extreme phenotypes, to decrease genotyping costs. The risk of detecting spurious QTL and of misestimating QTL effects was more important than when analysing the genotypes of all the phenotyped progeny. Using the whole progeny with the same QTL analysis software, two out of the five QTL previously identified were confirmed, i.e. one chromosome-wide significant QTL on chromosome 16 and one genome-wide significant QTL on chromosome 2 (Table 3). Both QTL explain a much higher proportion of the genetic variance (R2) than in the previous analysis (40.3 vs. 7.0% on chromosome 2 and 27.7 vs. 0.7% on chromosome 16). Results of the whole progeny analysis are expected to be more reliable as a higher number of animals were considered. Nevertheless, due to the non-normal distribution of phenotypes in the progeny, with a majority of resistant animals, the QTL effects might have been overestimated. The non-confirmed QTL on chromosomes 1 and 5 were only chromosome-wide significant in the previous analysis and could therefore be spurious.
The QTL confirmed on chromosome 16 has been identified with both CAEC and CSW5, while it was detected only with CAEC in the previous analysis. Extreme individuals were not strictly the same for CAEC and CSW5, which could explain this discrepancy. The most probable location of the QTL on chromosome 16 is around the microsatellite LEI0258, which is embedded in the major histocompatibility locus (MHC). The involvement of this locus in the variations of resistance to various viral or bacterial diseases in chicken has been demonstrated (Kaufman et al. 1999). The QTL identified on this chromosome might correspond to one or several genes belonging to the MHC locus.
The QTL confirmed on chromosome 2 is apparently only involved in the control of Salmonella excretion (CSW4). Interestingly, the dominance effect associated with this QTL is negative, while the additive effect is positive, which could explain why it was not detected in the BC progeny: heterozygous (N61) and homozygous (NN) individuals at the QTL in the BC progeny might very well display the same resistance level, or only weak differences that made QTL detection impossible. It would be relevant to analyse data collected on a reciprocal [(N×61) × 61] BC progeny to validate this hypothesis.
QTL testing in commercial lines
The genes causing phenotypic variation and the favourable alleles at those genes could be different between highly inbred lines like N and 61 and the commercial lines used in industry. A validation step is therefore required before any attempt at selection in commercial lines is made based upon the QTL identified in the N and 61 lines. We indeed found differences between both genetic backgrounds. Three out of the nine markers tested, which were all associated with QTL in the N×61 F2 or BC progeny, were actually associated with carrier-state resistance variation in the commercial lines: LEI0194 (chromosome 1) and MCW316 (chromosome 7) for the chicks (Table 4), and LEI0258 (chromosome 16) and MCW316 for the adult hens (Table 5). The other genomic regions targeted might not be involved in variation of carrier-state resistance in this genetic background, with the infection protocol used and the phenotypes observed. This does not mean that they are not involved in phenotypic variations in other conditions, which were not tested here. TLR4 was initially identified for its association with mortality rate in young chicks inoculated with S. Typhimurium (Hu et al. 1997), i.e. for a phenotype which was not assessed in this study. Its association with the number of contaminated organs in adult hens was only suspected (Beaumont et al. 2003). It must nevertheless be noted that TLR4 was significantly associated with Spleen0–1 variations in adult hens in this study, but only in 2003 (CATMOD analysis, P = 0.04). Therefore, the possibility of its implication in carrier-state resistance variations in the conditions tested should not completely be ruled out. Similarly, both QTL on chromosome 1 and the QTL on chromosome 2 have been originally detected using CSW4, which has not been measured here. Nevertheless, it must be recognized that these results might also be false negatives, as the lack of marker informativity led to a stringent reduction of the available data.
Interestingly, LEI0194, which is close to the proximal QTL on chromosome 1, was found associated with carrier-state resistance, whereas this QTL could not be confirmed in the whole-progeny QTL analysis. This strengthens the hypothesis of a lack of power in the whole progeny analysis. MCW316, which is linked to SLC11A1, was associated with carrier-state resistance variation in commercial lines at both ages. The SNP marker of SLC11A1, although much less informative than MCW316, was also almost significantly associated with carrier-state resistance variations in chicks. Several studies mention the probable association of SLC11A1 with resistance to salmonellosis in different genetic backgrounds: in the same lines (Girard-Santosuosso et al. 2002), in crosses between the W and C inbred lines (Hu et al. 1997), in several groups of meat-type chickens (Kramer et al. 2003) and in a resource population derived from outbred broiler sires and three inbred dam lines (two MHC-congenic Leghorn and one Fayoumi line) (Liu et al. 2003). Its association with variations for carrier-state resistance in adults was also demonstrated (Beaumont et al. 2003). This result confirms the interest of the region carrying SLC11A1 for selection. Further studies are still needed to demonstrate that SLC11A1 is the actual causal gene and to investigate whether alleles coding for resistance to disease and to carrier state are the same. It has been already demonstrated in mice that the SLC11A1 allele is associated with higher Salmonella counts in the spleen in the early phase of infection, and is also associated with a higher bacterial clearance ability (Caron et al. 2002). In addition, as the genetic correlation between resistance in young chicks and in adults was estimated as negative (Beaumont et al., in press), the question of whether the favourable allele is the same at both ages should be investigated.
More generally, results obtained in the divergent lines strengthen the hypothesis of a partly different genetic control of Salmonella carrier-state resistance between young and adult animals, which was already suggested by the negative genetic correlation estimated by Beaumont et al. (in press). The selection of more resistant animals by breeders should take this difference into account.
Acknowledgements
We thank members of the teams ‘Signalisation, Portage et Virulence Bactérienne’ and ‘Plate Forme d’Infectiologie Expérimentale’ of INRA, Nouzilly, for the experimental infections of the divergent lines. Frédéric Lecerf and Julie Demars have been supported by a fellowship from the European SABRE (sustainable animal breeding) project (EC number FOOD-CT-2006-01625). Genotyping was performed in LABOGENA (Jouy-en-Josas, France) and on the genomic platform of the Genopole Toulouse-Midi-Pyrénées, France.




