Recent advances in the molecular pathology of soft tissue sarcoma: Implications for diagnosis, patient prognosis, and molecular target therapy in the future
Abstract
In the present paper, recent advances in the molecular pathology of soft tissue sarcomas (STS) and the implications for their prognostic value are reviewed, and the potential targets of molecular therapy are discussed. According to the molecular genetic aspect, STS are divided into two groups: chromosome translocation-associated sarcomas and sarcomas without specific translocation. In the former group, specific fusion transcripts, such as SS18–SSX, EWS–FLI1, and PAX3–FKHR, could be detected in synovial sarcoma, Ewing's sarcoma and primitive neuroectodermal tumor, and alveolar rhabdomyosarcoma, respectively. The direct or indirect interactions between these fusion transcripts and cell cycle regulators have been elucidated by several investigators. Therefore, these fusion transcripts are promising candidates as molecular targets. As evaluated in carcinomas, alterations of several tumor-suppressor genes and adhesion molecules and overexpression of growth factors and their receptors have been extensively assessed in STS. In mixed-type STS, epidermal growth factor receptor overexpression was associated with decreased overall survival, suggesting the beneficial role of epidermal growth factor receptor inhibitors in STS. In malignant rhabdoid tumor and epithelioid sarcoma, frequent alteration of the SMARCB1/INI1tumor-suppressor gene and the loss of its protein have been demonstrated, indicating that this molecule could be an effective target of these sarcomas. In sarcomas with epithelioid differentiation, such as synovial sarcoma and epithelioid sarcoma, overexpression of dysadherin, which downregulates E-cadherin expression, was a poor prognostic factor. In conclusion, further studies are necessary to search for effective and specific molecules for the inhibition of tumor growth in each type of STS, especially in sarcomas without specific translocation. (Cancer Sci 2009; 100: 200–208)
Soft tissue sarcomas are relatively rare malignant neoplasms compared with carcinomas and other neoplasms, and they constitute less than 1% of all cancers. STS are composed of many histological subtypes, such as pleomorphic undifferentiated sarcoma or malignant fibrous histiocytoma, leiomayosarcoma, and liposarcoma. In addition to their wide variety of subtypes, it is often difficult to make an accurate histological diagnosis because certain kinds of STS share similar morphological features. However, it is important to make a definitive diagnosis so that adequate therapy can be administered in each case. Recently, several reciprocal chromosomal translocations and fusion transcripts have been proven to be characteristic of particular histological types (Table 1; Fig. 1). The detection of these fusion transcripts is useful for the differential diagnosis of histologically peculiar cases.
| Histological type | Chromosomal translocation | Fusion transcript |
|---|---|---|
| Ewing's sarcoma | t(11;22)(q24;q12) | EWS–FLI1 (85%)(1) |
| Primitive neuroectodermal tumor | t(21;22)(q22;q12) | EWS–ERG (10–15%) |
| t(7;22)(p22;q12) | EWS–ETV1 (<1%) | |
| t(17;22)(q12;q12) | EWS–E1AF (<1%) | |
| Synovial sarcoma | t(X;18)(p11.2;q11.2) | SS18–SSX1 (66%)(1) |
| SS18–SSX2 (33%) | ||
| SS18–SSX4 (<1%) | ||
| Myxoid/round cell liposarcoma | t(12;16)(q13;p11) | FUS–DDIT3 (>90%)(1) |
| t(12;22)(q13;q12) | EWS–DDIT3 (rare) | |
| Clear-cell sarcoma | t(12;22)(q13;q12) | EWS–ATF1 (94%) |
| t(2;22)(q34;q12) | EWS–CREB1 (6%) | |
| Angiomatoid fibrous histiocytoma | t(2;22)(q34;q12) | EWS–CREB1 |
| t(12;22)(q13;q12) | EWS–ATF1 | |
| Extraskeletal myxoid chondrosarcoma | t(9;22)(q22;q12) | EWS–NR4A3 (67%) |
| t(9;17)(q22;q11) | RBP56–NR4A3 (17%) | |
| Desmoplastic small round cell tumor | t(11;22)(p13;q12) | EWS–WT1 |
| Alveolar rhabdomyosarcoma | t(2;13)(q35;q14) | PAX3–FKHR (55%)(2) |
| t(1;13)(q36;q14) | PAX7–FKHR (22%) | |
| Dermatofibrosarcoma protuberans | t(17;22)(q22;q13) | COL1A1–PFGFB (74%)(3) |
| Giant cell fibroblastoma | ||
| Congenital fibrosarcoma | t(12;15)(p13;q25) | ETV6–NTRK3 |
| Cellular congenital mesoblastic nephroma | ||
| Alveolar soft part sarcoma | t(X;17)(p21;q25) | ASPL–TFE3 |
| Inflammatory myofibroblastic tumor | t(2;5)(p23;q35) | TPM3,4–ALK (38%) |
| t(2;17)(p23;q23) | CLTC–ALK (25%) | |
| Low-grade fibromyxoid sarcoma | t(7;16)(q33;p11) | FUS–CREB3L2 |

Myxoid/round cell liposarcoma (MRCLS) arising in the buttock of a 36-year-old man. (a) The tumor mainly consists of oval to fusiform cells with abundant myxoid matrix and arborizing vasculature, accompanied by a large number of mature lipoblasta (left: myxoid area). The tumor is partially composed of cellular proliferation of undifferentiated round cells with occasional multivacuolated lipoblasts (right: round cell component). (b) Cytogenetic study disclosed reciprocal chromosomal translocation between chromosome 12 and chromosome 16 in this case. (c) The reverse transcription–polymerase chain reaction method detected specific fusion transcripts for MRCLS, FUS–DDIT3 type 2 (arrow). Direct sequencing of the polymerase chain reaction product confirmed the break point between FUS exon 5 and DDIT3 exon 2. P, positive control; S, sample.
In relapsed cases of STS complete remission is difficult, despite the conventional multidisciplinary therapy. Therefore, novel therapies such as molecular target therapy are required, especially in relapsed cases. Several investigators have analyzed tumor-suppressor genes, cell cycle regulators, and growth factors in STS to find an effective molecular target similar to that in carcinomas. Certain kinds of molecules have been reported to correlate with a patient's prognosis or malignant characteristics in STS (Table 2). Moreover, detailed associations between specific fusion transcripts and cell cycle regulators have been assessed, and the possibility that the fusion transcripts could be novel molecular targets in specific types of STS has been studied (Fig. 2).
| Class | Marker | Type of sarcoma | Method | Potential prognostic value and other remarks |
|---|---|---|---|---|
| Fusion transcripts | EWS–FLI1 type 1 | ES/PNET | RT-PCR | Better prognosis(4,5), low proliferative rate(6) |
| SS18–SSX1 | Synovial sarcoma | RT-PCR | Poor prognosis(7), high proliferative rate(8) | |
| PAX3–FKHR | Alveolar RMS | RT-PCR | Poor prognosis(2) | |
| Tumor-suppressor genes | p53 and MDM2 coexpression | Mixed STS | IHC | Poor prognosis(9) |
| Dedifferentiated LS | IHC | Dedifferentiation(10) | ||
| p53 | Mixed STS | IHC | Poor prognosis(11) | |
| Mixed STS | Mutation | Poor prognosis(12) | ||
| Leiomyosarcoma | IHC, mutation | Poor prognosis in deep tumor(13) | ||
| Synovial sarcoma | IHC | Poor prognosis(14) | ||
| Myxoid/round cell LS | IHC | Poor prognosis(15) | ||
| Myxoid/round cell LS | IHC, mutation | High histological grade, poor prognosis(16) | ||
| ES/PNET | IHC | Poor prognosis(17) | ||
| DFSP | Mutation | Malignant transformation to fibrosarcoma(18) | ||
| MDM2 mRNA | Mixed STS | qRT-PCR | Poor prognosis(19) | |
| p16INK4a | Leiomyosarcoma | IHC | Poor prognosis(20) | |
| p14ARF | Myxoid/round cell LS | IHC | Poor prognosis(16) | |
| RB | Dedifferentiated LS | IHC, LOH, mutation | Dedifferentiation(21) | |
| DAP kinase, p53 | Leiomyosarcoma | MSP, mutation | High histological grade, poor prognosis(22) | |
| RASSF1A | Mixed STS | MSP | Poor prognosis, frequent in leiomyosarcoma(23) | |
| Cell cycle regulators | CHFR | MPNST | IHC | Poor prognosis(24) |
| p21WAF1 | Myxofibrosarcoma | IHC | High histological grade, poor prognosis(25) | |
| Growth factors and receptors | EGFR | Mixed STS | IHC | Poor prognosis(26) |
| HGF/MET coexpression | Synovial sarcoma | IHC | Poor prognosis(27) | |
| Multidrug resistance | P-glycoprotein | Mixed STS | IHC | Poor prognosis(28) |
| Mixed STS | IHC | Poor response to chemotherapy(29) | ||
| Mixed STS | IHC | Large tumor size, high stage(30) | ||
| MDR1/MRP1, coexpression | Mixed STS | RT-PCR | High histological grade(31) | |
| YB-1 | Synovial sarcoma | IHC | Poor prognosis, high proliferative rate(32) | |
| Embryonal RMS | IHC | High proliferative rate(33) | ||
| Adhesion molecule | E-Cadherin | Synovial sarcoma | IHC | Poor prognosis(34) |
| α-Catenin | Synovial sarcoma | IHC | Poor prognosis(34) | |
| β-Catenin | Synovial sarcoma | IHC | Poor prognosis, high proliferative rate(34) | |
| Dysadherin | Epithelioid sarcoma | IHC | Poor prognosis, proximal type(35) | |
| Synovial sarcoma | IHC | Poor prognosis(36) |
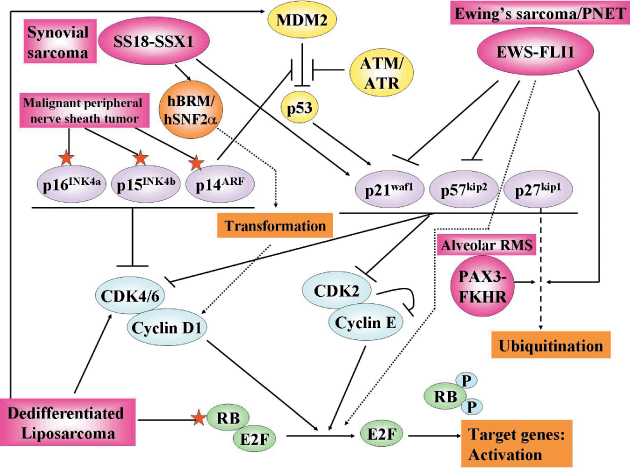
Interactions between the fusion transcripts SS18–SSX1, EWS–FLI1, and PAX3–FKHR, and G1–S checkpoint cell cycle regulators. Molecular alterations in malignant peripheral nerve sheath tumor and dedifferentiated liposarcoma are also demonstrated. ––, direct stimulatory modification; - - -, tentative stimulatory modification; ––|, direct inhibitory modification; ⋆, gene alterations.
The present review outlines investigations involving the following molecules in STS: (i) specific fusion transcripts; (ii) fusion transcripts and cell cycle regulation; (iii) tumor-suppressor genes, oncogenes, and cell cycle regulators; (iv) SMARCB1/INI1; (v) growth factors and their receptors; (vi) multidrug resistance; (vii) adhesion molecules (E-cadherin, β-catenin, and dysadherin); and (viii) Wnt–β-catenin signaling. We also introduce our recent investigations concerning these molecules.
Specific fusion transcripts
Within the past two decades, several reciprocal chromosome translocations and their concordant fusion genes have been identified in specific types of STS, as summarized in Table 1. Interestingly, certain types of sarcoma and other types of malignant tumor have the same fusion genes: ASPL–TFE3 in both alveolar soft-part sarcoma and childhood renal cell carcinoma; ETV6–NTRK3 in both congenital fibrosarcoma and cellular congenital mesoblastic nephroma; and various ALK fusions in both inflammatory myofibroblastic tumor and anaplastic large-cell lymphoma. Among these specific fusion transcripts, close correlations between fusion type and prognosis have been demonstrated in ES and PNET, synovial sarcoma, and alveolar rhabdomyosarcoma. EWS–FLI1 is the most predominant fusion and is found in approximately 85% of ES/PNET patients.(1) The most common fusion is EWS–FLI1, which is mainly composed of EWS exon 7 and FLI1 exon 6, so-called type 1 fusion.(4) Some investigators demonstrated that type 1 EWS–FLI1 is a significant positive predictor of survival compared with other types of fusion transcripts.(4,5) Type 1 tumors also show lower proliferative rates than other types of tumors, probably mediated by different regulation of the insulin-like growth factor receptor pathway.(6) Synovial sarcoma usually has two major types of fusion transcript, SS18–SSX1 and SS18–SSX2. The SS18–SSX1 subtype has been reported to show poorer prognosis in multi-institutional studies compared with the SS18–SSX2 subtype.(7) Moreover, significantly higher proliferative activities have been demonstrated in SS18–SSX1 tumors in comparison with SSX2 tumors.(8) The distinction of ARMS from embryonal rhabdomyosarcoma is very important because the alveolar type shows more aggressive biological behavior than the embryonal type. In most ARMS patients either PAX3–FKHR or PAX7–FKHR fusion transcripts are detected. According to the large series of Sorensen et al. tumors with PAX3–FKHR correlated with poor prognosis, whereas tumors with PAX7–FKHR showed favorable outcomes.(2) Concerning MRCLS, three major types of recurrent FUS–DDIT3 fusion transcripts have been reported as types I, II, and III (Fig. 1).(15) However, in contrast to other tumors with specific translocation, such as ES/PNET, synovial sarcoma, and ARMS, the variant of FUS–DDIT3 did not affect patient survival in MRCLS.(15)
Fusion transcripts and cell cycle regulation
The interactions between major fusion transcripts and cell cycle regulators are illustrated in Figure 2. In synovial sarcoma, Nagai et al. demonstrated the association of SS18–SSX1 chimeric protein with the chromatin remodeling factor hBRM–hSNF2α, which regulates transcription and corresponds with the transformation of fibroblasts through downregulation of deleted in colon cancer (DCC) levels.(37) It has also been documented that SS18–SSX1 induces growth suppression through induced p21 expression in a hBRM-independent manner.(38) Other investigators reported a close correlation between SS18–SSX1 and increased expression of cyclin A and D1, which may influence the more aggressive behavior of this phenotype compared with the SS18–SSX2 phenotype.(39)
As for ES/PNET, inhibition of p21 induction by EWS–FLI1 via suppression of the histone acetyltransferase activity of p300 has been reported.(40) Moreover, oncogenesis of EWS–FLI1 through a decrease in the stability of p27 protein due to increased action of Skp2-mediated 26S proteasome degradation,(41) and inhibition of the retinoblastoma (RB) family protein by EWS–FLI1,(42) have been demonstrated. On the other hand, Zhang and Wang demonstrated that PAX3–FKHR also reduced the expression of p27kip1 protein via elevated Skp2 and 26S proteasome-dependent degradation.(43)
Tumor-suppressor genes, oncogenes, and cell cycle regulators
In STS, p53 pathway alterations have been demonstrated as p53 point mutations, homozygous deletions of CDKN2A (encoding both p14ARF and p16INK4a), and MDM2 amplification. STS without specific translocations, such as malignant fibrous histiocytoma, leiomyosarcoma, well-differentiated or dedifferentiated liposarcoma, MPNST, adult fibrosarcoma, and angiosarcoma, show more frequent p53 pathway alterations, whereas such alterations are rare events in the already-mentioned sarcomas with specific translocations.(44) However, when present, such alterations have been demonstrated as a strong adverse prognostic factor in synovial sarcoma,(14) MRCLS,(15) and ES/PNET.(17) We also analyzed p53 pathway alterations in 90 cases of MRCLS and demonstrated that the reduction of p14ARF protein expression and p53 mutation were related to poor prognosis. These alterations are correlated with the presence of the round cell component, which is a strong adverse prognostic factor. Accordingly, the p14ARF–p53 pathway may contribute to the presence of the round cell component and malignant progression in this tumor.(16)
In several kinds of STS, nuclear overexpression of p53 protein(11) and mutation of the p53 gene(12) have been reported as poor prognostic factors. As for MDM2 alterations, overexpression of p53 and MDM2 protein expression in the same tumor are of high prognostic relevance in mixed-type STS.(9) High MDM2 mRNA level has also been reported as a predictive adverse prognostic factor.(19) In leiomyosarcoma, we found that p53 gene alteration is a useful prognostic factor in deeply situated tumors.(13) We also demonstrated that decreased expression of p16INK4a protein is a significantly poor prognostic factor in both univariate and multivariate analysis in leiomyosarcoma.(20) Promoter methylation was shown to have a strong association with the inactivation of p16INK4a in leiomyosarcoma. DAP kinase was recently characterized as an upstream regulator of p53. We analyzed DAP kinase alteration in leiomyosarcoma and demonstrated that DAP kinase alteration and p53 mutation had a close association with high histological grade and poor prognosis.(22) The RASSF1A tumor-suppressor gene has been reported to control the cell cycle, and several RASSF1A gene targets were identified. Seidel et al. demonstrated that hypermethylation of RASSF1A was frequently detected in leiomyosarcoma and closely associated with poor prognosis in several kinds of STS.(23)
Alteration of the p53–MDM2 pathway has been reported in WDLS and DDLS. DDLS is defined as a malignant adipocytic neoplasm showing the transition from WDLS to non-lipogenic high-grade sarcoma. p53 and MDM2 expression, detected by immunohistochemistry, may be involved in the dedifferentiation process in DDLS because these were more prominent in the dedifferentiated high-grade components than in the well-differentiated low-grade components.(10) The phenomenon of the MDM2-mediated inactivation of p53 has been observed in retroperitoneal WDLS and DDLS, whereas p53 gene mutation appears to correlate with the dedifferentiation process in non-retroperitoneal WDLS and DDLS.(45) Recently, frequent MDM2 and CDK4 amplifications have been demonstrated in both WDLS and DDLS (Fig. 2).(46) However, no difference was detected in MDM2–CDK4 amplification status between dedifferentiated and well-differentiated components in DDLS.(46) We investigated the RB gene status in DDLS and found that the dedifferentiated component more frequently harbored loss of heterozygosity and mutation or promoter methylation of the RB1 gene, and decreased the expression of RB protein compared with the well-differentiated component. Our results suggest that the RB protein has a major role to play in dedifferentiation (Fig. 2).(21)
Malignant peripheral nerve sheath tumor often occurs in association with NF-1. We compared immunohistochemical p53 expression between MPNST and its concordant neurofibroma. The expression of p53 was higher in the areas of MPNST than in the areas of neurofibroma in eight patients with NF-1.(47) Inactivation of one or more genes of p15INK4b, p14ARF, and p16INK4a has been demonstrated in 77% of sporadic and NF-1-related MPNST (Fig. 2).(48) We also demonstrated that decreased expression of CHFR was significantly correlated with a high mitotic count, a high Ki-67 labeling index, and a poor prognosis in MPNST. These results support the assertion that CHFR functions as an inhibitor of tumor proliferation.(24)
In terms of malignant transformation of DFSP, 10–20% of DFSP cases have a fibrosarcomatous area in either primary or recurrent tumors. DFSP with fibrosarcoma has metastatic potential. We found that microsatellite instability and p53 mutations are involved in the tumor progression of DFSP to fibrosarcoma as early and late events, respectively.(18)
We have also carried out extensive investigations concerning the altered expression of cell cycle regulatory genes and proteins in several kinds of STS and the following results have been obtained. In ASPS, the inactivation of hMLH1 DNA mismatch repair genes seems to have an important role to play in the mutagenesis of tumor-suppressor genes such as p53 and APC in ASPS.(49) As for myxofibrosarcoma, reduced expression of p21WAF1 could be considered a new parameter to be evaluated, along with classical clinicopathological prognostic factors, for identifying those at high risk of this tumor.(25) In rhabdomyosarcoma, p53 overexpression may be related to tumor progression because tumors with p53 overexpression have high proliferative activity. The alveolar type had both a significantly higher mitotic rate and significantly higher E2F-1 labeling indices when compared with the embryonal type.(50) Concerning clear-cell sarcoma, all tumors with genetic alterations of the p16INK4a, p14ARF, or p53 genes showed a high mitotic rate or tumor necrosis, which are indicators of aggressive biological behavior. Therefore, these alterations were considered to be influential in the poor prognosis of clear-cell sarcoma patients.(51)
The tumor-suppressor gene PTEN/MMAC1 was identified on chromosome 10q23.3, and a loss of heterozygosity of chromosome 10q has been described in STS.(52) We analyzed PTEN mutation in 56 cases of STS without specific chromosomal translocations. We detected PTEN mutation in only two cases (3.9%), both of which were leiomyosarcomas arising in abdominal cavities.(53) In addition to PTEN mutation, we also assessed promoter methylation and homozygous deletion of the PTEN gene in 51 cases of STS without specific translocation.(54) Promoter methylation and homozygous deletion were found in 13 and 2% of cases, respectively. Although molecular alteration did not significantly correlate with protein expression, decreased immunohistochemical expression of PTEN showed a correlation with proliferative activities. Furthermore, we also searched for PTEN mutation in 49 cases of synovial sarcoma and detected mutations in seven cases (14.7%). However, the mutation status of PTEN was not associated with overall survival rate in patients with synovial sarcoma.(55) Therefore, PTEN gene alterations seem to be relatively rare events and may play a minor role in the inactivation of PTEN in STS. Further studies are required in this field to find candidate genes for effective molecular target therapy in each subtype of STS.
SMARCB1/INI1
Malignant rhabdoid tumor is a highly aggressive renal or soft tissue tumor that occurs in children and consists histologically of characteristic rhabdoid cells. Rhabdoid cells are also focally observed in a wide variety of malignant tumors, including other STS, such as synovial sarcoma, extraskeletal myxoid chondrosarcoma, and epithlioid sarcomas, as well as carcinomas and meningiomas.(56) MRT and proximal-type epithelioid sarcoma share the same morphological findings, and their differential diagnosis is often problematic.(35,57) Initially, some investigators demonstrated abnormalities in the long arm of chromosome 22 and deletion or mutation of the SMARCB1/INI1 gene in renal and extrarenal MRT.(58) The SMARCB1/INI1 gene is a member of the ATP-dependent SWI/SNF chromatin-remodeling complex, and has been suggested as a candidate specific tumor-suppressor gene in MRT. Since then, alteration of the SMARCB1/INI1 gene and loss of immunoreactivity of its gene product have been considered to be the most characteristic molecular events in MRT.(59) However, these alterations have also been frequently demonstrated in epithelioid sarcoma,(60) as well as occasionally detected in extraskeletal myxoid chondrosarcomas.(61) Recently, we found less-frequent gene alteration of SMARCB1/INI1 in epithelioid sarcoma compared with MRT, whereas the loss of its protein was commonly observed in both tumors.(62) Considering these findings, SMARCB1/INI1 could be a molecular target in MRT and certain kinds of malignant tumors with a rhabdoid phenotype.
Growth factors and their receptors
Several growth factors and their receptors have been reported to play an important role in autocrine or paracrine stimulation of tumor growth in many kinds of carcinomas. EGFR is a representative target based on the prevalence of its overexpression in a wide variety of solid tumors. Sato et al. demonstrated that EGFR overexpression is a negative prognostic factor and is associated with high histological grade in adult STS.(26) This result indicates the benefits of treatment with biospecific inhibitors for EGFR in STS patients, as recently demonstrated in several kinds of carcinoma patients. Frequent immunohistochemical expression of EGFR without gene amplification has been reported in embryonal-type rhabdomyosarcoma(63) and SS18–SSX1-type synovial sarcoma,(64) compared with alveolar-type rhabdomyosarcoma and SS18–SSX2 synovial sarcoma, respectively. Moreover, the combination of EGFR blockage and conventional chemotherapy has been reported to inhibit the growth of STS cells in vitro and in vivo.(65)
The receptor tyrosine kinase MET and its ligand HGF have been implicated in cellular proliferation, cell movement, and invasion in several human malignancies. Frequent expression of HGF and MET has been reported in MPNST,(66) and it was demonstrated in vitro and in vivo that the HGF–MET pathway plays an important role in the malignant phenotype of rhabdomyosarcoma.(67) In MPNST associated with NF-1, we also compared the expression of TGF-β1, TGF-β receptor type II, HGF, and MET between MPNST and concordant neurofibroma components.(47) The expression of these growth factors and receptors was higher in the MPNST areas than in the neurofibromatous area. We also analyzed HGF and MET immunohistochemical expression in 69 cases of synovial sarcoma and found that coexpression of HGF and MET was associated with high proliferating activity and significantly adverse prognosis.(27)
Recently, several clinical trials have assessed the usefulness of imatinib mesylate therapy in STS expressing PDGF and PDGFR, such as aggressive fibromatosis(68) and DFSP.(69) Aoki et al. demonstrated frequent and high expression of PDGFR-β and EGFR at both the mRNA and protein levels in MPNST compared with those in benign peripheral nerve sheath tumors.(70) They also clearly indicated the possibility of the therapeutic potential of imatinib in suppressing the invasion and growth of MPNST. It has been proposed that the abnormal fusion transcript COL1A1–PDGFB in DFSP probably causes PDGFB and PDGFRB autocrine stimulation and cell proliferation, which are responsible for the development of this tumor. We recently demonstrated PDGFB gene amplification and coexpression of PDGFB and PDGFRB mRNA in tumor tissue of DFSP.(3) Moreover, the PDGFB and PDGFRB mRNA expression levels showed a positive correlation. These results indicate that the COL1A1–PDGFB fusion protein, which is processed by the COL1A1–PDGFB transcript, can serve as a functional ligand for PDGFRB in DFSP.
Multidrug resistance
Intrinsic or acquired MDR is a major problem in the chemotherapy of primary or relapsed cancer. ABC transporters, including MDR1 and P-gp, the MRP family, and MVP, a ribonucleoprotein particle, are the main contributors to MDR. YB-1 has been identified as a transcription factor and has been reported to regulate MDR1 and MVP.
Several investigators have demonstrated that overexpression of P-gp is associated with poor prognosis in several kinds of STS,(28) as well as with poor pathological response to chemotherapy.(29) Coexpression of MDR1 and MRP1 mRNA was significantly correlated with tumor grade in STS.(31) We recently demonstrated that P-gp expression is closely correlated with large tumor size and advanced stage.(30) We also found that the expression of MDR1 and MDR3 mRNA in MPNST was significantly higher than in other STS.(30) Overexpression of the mRNA of these two types of ABC transporter may play an important role in the frequent poor chemoresponse in MPNST.
In synovial sarcoma, YB-1 nuclear expression has been reported to correlate with P-gp and topoisomerase IIα expression, MRP1 mRNA expression, and high proliferative activity.(32) Moreover, YB-1 nuclear expression proved to be an independent poor prognostic factor by multivariate analysis. In rhabdomyosarcoma, nuclear expression of YB-1 protein was correlated with P-gp and MVP expression and a higher proliferative activity in the embryonal type; however, in the alveolar type no such relationships were observed.(33) Our results suggest that YB-1 may be a candidate molecular target in synovial sarcoma and embryonal-type rhabdomyosarcoma therapy.
Adhesion molecules (E-cadherin, β-catenin, and dysadherin)
E-cadherin and its cytoplasmic binding proteins α-catenin, β-catenin, and γ-catenin, are essential for intercellular junctions and their reduced expression has been found to correspond to poor morphological differentiation, high metastatic potential, and poor prognosis in epithelial malignancies. We analyzed E-cadherin and catenin alterations, and the mechanism of E-cadherin inactivation in STS with epithelial differentiation, such as synovial sarcoma and epithelioid sarcoma. Synovial sarcoma has two main histological subtypes: spindle cell sarcoma with glandular epithelial differentiation (biphasic type) and pure spindle cell sarcoma (monophasic type). In synovial sarcoma, reduced expression of E-cadherin and α-catenin and aberrant nuclear β-catenin expression, detected by immunohistochemistry, were significantly poor prognostic factors.(34) Mutation of exon 3 of the β-catenin gene was detected in 4 out of 50 cases (8.2%), and two out of these four cases died within 1 year. E-cadherin mutation was observed in 12 out of 42 analyzed cases (24.5%), and its mutation was correlated with decreased immunohistochemical expression and spindle morphology in monophasic fibrous tumor.(71) These results suggest that E-cadherin gene mutation may be a determinant of histological subtype in synovial sarcoma. Several investigators have demonstrated that the SS18–SSX1 phenotype has a close correlation with glandular epithelial differentiation compared with the SS18–SSX2 phenotype.(7) We demonstrated that E-cadherin membranous expression is correlated with the SS18–SSX1 phenotype and biphasic glandular morphology.(72) ELF3, a transcription factor associated with epithelial differentiation, was more highly expressed at the mRNA level in the biphasic type compared with the monophasic type. Moreover, mRNA expression of E-cadherin and its transcriptional repressor Snail showed a significant inverted correlation. Recently, Saito et al. demonstrated the interaction between Snail and Slug in the inactivation of E-cadherin and their correlation with the SS18–SSX phenotype and the morphological subtype.(73)
Dysadherin is a cancer-associated cell membrane glycoprotein that downregulates the expression of E-cadherin and promotes metastasis. Dysadherin expression was found to be an independent poor prognostic factor in ES(35) and synovial sarcoma.(36) In ES, dysadherin expression was frequently observed in the proximal type, which has been reported as a more aggressive tumor compared with the distal type.(35) In synovial sarcoma, we also demonstrated that E-cadherin dysfunction caused by dysadherin expression is associated with E-cadherin repression and morphological change from the epithelioid to the spindle phenotype.(36)
Concerning the diagnostic utility of adhesion molecules in STS, membranous expression of β-catenin(57) and dysadherin(35) is useful in distinguishing proximal-type ES from MRT. All the examined cases of proximal-type ES showed diffuse and strong membranous expression of β-catenin and dysadherin, whereas no cases of MRT had immunoreactivity for these proteins. Furthermore, established cell lines from proximal-type ES revealed significantly higher levels of dysadherin mRNA expression compared with the levels seen in MRT cell lines.
Wnt–β-catenin signaling
Accumulated free cytoplasmic or nuclear β-catenin has been reported to behave as an oncoprotein through the alteration of the APC protein–β-catenin–Tcf pathway in colorectal carcinoma and desmoid tumor. We searched for the APC mutation in synovial sarcoma and detected it in only 8.2% of the examined cases.(74) All of the mutated cases were of the monophasic fibrous type and showed nuclear β-catenin accumulation. Frequent hypermethylation of the APC promoter has been demonstrated in MRCLS.(75)
Desmoid tumor has no metastatic potential; however, it is categorized as an intermediate, locally aggressive tumor, according to the recent World Health Organization classification.(1) Nuclear β-catenin immunoreactivity is constantly observed in desmoid tumor and this finding is useful to distinguish desmoid tumor from other spindle-cell sarcomas.(76) We found that nuclear β-catenin accumulation had a significant correlation with cyclin D1 overexpression and high proliferative activities in sporadic desmoid tumors.(77) This close relationship between nuclear β-catenin accumulation and cyclin D1 expression was also recognized in other spindle and pleomorphic sarcomas, but not in synovial sarcoma.(78) Among sporadic desmoid tumors, cyclin D1 and β-catenin mRNA expression was significantly higher in the β-catenin-mutated group than in the β-catenin wild-type group.(79) This result suggests that in addition to the cyclin D1 gene, the β-catenin gene itself may be a target gene in the APC–β-catenin–Tcf pathway. Moreover, we recently demonstrated a significant correlation between MMP-7 overexpression and nuclear expression of β-catenin in sporadic desmoid tumors.(80)
Conclusions
Soft tissue sarcomas comprise various histological subtypes. In terms of molecular genetics, they can be divided into two groups: translocation-associated sarcomas and sarcomas without specific translocation. The translocation-associated sarcomas are morphologically made up of a diffuse proliferation of small, uniform, rounded or spindle-shaped tumor cells. Small round-cell sarcomas such as ES/PNET and rhabdomyosarcoma show a good response to conventional chemotherapy and effective regimens have been established. However, it is difficult to treat relapsed cases. In translocation-associated spindle-cell sarcomas such as synovial sarcoma, it is controversial whether a clinical benefit could be obtained with conventional chemotherapy. In these translocation-associated sarcomas, specific fusion transcripts in each histological subtype are promising candidates for molecular targets because several investigators have demonstrated the interactions between fusion transcripts and cell cycle regulators (ES/PNET, EWS–FLI1; SS, SS18–SSX1; RMS, PAX3–FKHR) or growth factors (DFSP, COL1A1–PDGFB). Novel drugs to effectively inhibit these fusion transcripts are desirable, as shown by the effectiveness of imatinib mesylate for the treatment of chronic myeloid leukemia, which is also a bcl–abl translocation-associated tumor. In sarcomas without specific translocation, further studies are required to search for important molecules corresponding to tumor progression because no definitive key molecules have been identified in this heterogeneous group. Several studies have demonstrated that the SMARCB1/INI1 tumor-suppressor gene is involved in key biochemical pathways implicated in cell growth and development. Therefore, in MRT and ES, SMARCB1/INI1 may be promising as a molecular target because of the frequent loss of its protein in these tumors. In desmoid tumors, the Wnt–β-catenin signaling pathway is considered to be an important cascade because this pathway is closely correlated with proliferation (cyclin D1) and invasiveness (MMP-7) in this intermediate tumor. Moreover, concerning multidrug resistance, YB-1 may be an effective molecular target because it is correlated with high proliferative activity and induces the overexpression of MDR-related molecules such as P-gp, MRP1, or MVP in STS, as previously reported in other human malignant tumors.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (18590332), Tokyo, Japan. The English used in this manuscript was revised by KN International (http://www.kninter.com/).
Abbreviations
ALK, anaplastic lymphoma kinase; ASPL, alveolar soft part sarcoma chromosome region candidate 1; ATF, activating transcription; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3-related protein; CDK, cyclin dependent kinase; CDKN2A, cyclin-dependent kinase inhibitor 2A; CLTC, clathrin heavy chain; COL1A1, collagen type I alpha 1; CREB1, cAMP responsive element binding protein 1; CREB3L2, cAMP responsive element binding protein 3-like protein 2; DAP, death-associated protein; DDIT, DNA-damage-inducible transcript; ELF3, ETS-related transcription factor Elf-3; ETV, ETS translocation variant; EWS, Ewing sarcoma breakpoint region 1; FKHR, forkhead box; FLI1, friend leukemia integration 1 transcription factor; hMLH, human MutL homolog; INI1, integrase interactor 1; MDM, murine double minute; MET, mesenchymal-epithelial transition factor; MMP, matrix metalloproteinase; NR4A3, nuclear receptor subfamily 4, group A, member 3; NTRK, neurotrophic tyrosin kinase; PAX, paired box; PDGF, platelet-derived growth factor; PDGFB, platelet-derived growth factor beta chain; PDGFR, platelet-derived growth factor receptor; PDGFRB, platelet-derived growth factor receptor beta; PGK, phosphoglycerate kinase; PNET, primitive neuroectodermal tumor; PTEN, phosphatase and tensin homolog; RASSF, Ras association domain family; RB, retinoblastoma; RMS, rhabdomyosarcoma; SMARCB1 SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1; SS18, synovial sarcoma translocation, chromosome 18; SSX, synovial sarcoma, X breakpoint; TFE3, transcription factor E3; TPM, tropomyosin; WT, Wilms tumor.




