Cytokine profiles of bronchoalveolar lavage fluid in patients with pneumocystis pneumonia
ABSTRACT
The clinical features of PCP differ according to the factors responsible for the predisposing immunosuppression. Although the diagnosis of PCP often requires BAL, the profiles of the inflammatory mediators in the BAL fluid are not thoroughly documented. The aim of the current study was to characterize the profiles of inflammatory mediators in BAL fluid during PCP in patients with underlying autoimmune diseases, malignancies, or AIDS. The medical records of 14 patients with autoimmune diseases, 10 with malignancies, and 8 with AIDS, all of whom had been diagnosed with PCP by microscopic examination of BAL fluid, were reviewed. The concentrations of TNF-α, MCP-1, HMGB1, IL-8, IL-6, IL-10, and IFN-γ in the BAL fluid that had been obtained for the diagnosis of PCP were measured. The concentrations of MCP-1, IL-8, and IL-6 differed according to the underlying disease, tending to be higher in patients with autoimmune diseases and lower in those with AIDS. The concentrations of HMGB1, IL-8, and IL-6 were positively correlated with the proportion of neutrophils in BAL fluid and inversely with the oxygenation index. Although the serum concentrations of CRP and LDH were positively correlated with those of IL-8 and MCP-1, none of the mediators in BAL fluid was correlated with the serum β-D-glucan concentration. The production of inflammatory mediators in the lung differed between the patient groups with different underlying disorders. The modest upregulation of IL-8 and IL-6 might be associated with the milder clinical manifestations of PCP in AIDS patients.
List of Abbreviations:
-
- AIDS
-
- acquired immunodeficiency syndrome
-
- BAL
-
- bronchoalveolar lavage
-
- CRP
-
- C-reactive protein
-
- NF-κB
-
- nuclear factor-κB
-
- IFN-γ
-
- γ-interferon
-
- HMGB1
-
- high mobility group box 1
-
- IL
-
- interleukin
-
- IQR
-
- interquartile range
-
- LDH
-
- lactate dehydrogenase
-
- MCP-1
-
- monocyte chemotactic protein-1
-
- PCP
-
- pneumocystis pneumonia
-
- P. jirovecii
-
- Pneumocystis. jirovecii
-
- TNF-α
-
- tumor necrosis factor-α
-
- WBC
-
- white blood cells
PCP remains one of the most frequent and serious opportunistic infections in immunocompromised patients, including those with AIDS (1). PCP can also occur in patients with malignant disease, especially when they have been undergoing treatment with anti-neoplastic agents, corticosteroids, or other immunosuppressive agents (1, 2).
The clinical characteristics of PCP are known to vary according to the underlying disorders responsible for the predisposing immunosuppression (3). In particular, differences in the clinical course of PCP between AIDS and non-AIDS patients have been described (3–5). At presentation, the latter typically have a shorter duration of symptoms and more severe oxygenation impairment compared with the former (3). The prognosis of PCP also differs between non-AIDS patients, only 40–70% of whom survive, and AIDS patients, who have survival rates as high as 90% (3). Among non-AIDS patients, the prognosis of those with cancer has been reported to be the worst (4, 5). It has previously been reported that PCP in AIDS patients is characterized by greater numbers of organisms and fewer inflammatory cells in BAL fluid samples than are found in other immunocompromised patients (6). It has been suggested that, in non-AIDS patients, fewer organisms are able to induce severe lung inflammation, leading to respiratory impairment, and high rates of complications and death, although other factors may account for the observed differences (6). In addition, it has been demonstrated that the radiological features of PCP differ depending on whether the patients have AIDS, rheumatoid arthritis, or malignancy (7, 8). Since the radiological findings demonstrate the severity and extent of inflammation during PCP, we hypothesized that cytokine profiles in the lung might differ between patients with AIDS, autoimmune diseases, and malignancies.
Although the human pulmonary epithelial cell line A549 secretes the proinflammatory cytokines IL-6, IL-8, and MCP-1 following stimulation with pneumocystis (9, 10), the profile of inflammatory mediators in the lungs during PCP has rarely been reported. It has been shown that production of pulmonary IL-10 and CC chemokine are markedly defective in AIDS patients with PCP, while pulmonary TNF-α and IL-6 concentrations are normal (11). However, it remains to be determined whether the profile of mediators in BAL fluid is influenced by underlying disorders or is correlated with other clinical parameters. The primary goal of the present study was to evaluate the pulmonary production of inflammatory and anti-inflammatory mediators during PCP in patients with various underlying diseases. We also examined whether the concentrations of the mediators in BAL fluid correlated with serum markers, oxygenation, and total and differential cell counts in peripheral blood and BAL fluid.
METHODS
The study protocol was approved by the ethical committee of Keio University School of Medicine.
Patients
We retrospectively evaluated data from 32 consecutive patients with autoimmune diseases (n= 14), malignancies (n= 10), or AIDS (n= 8) who had been diagnosed with PCP after BAL at Keio University Hospital (Tokyo, Japan) during the period between May 2003 and May 2009. Six nonsmoking healthy volunteers served as controls. All subjects provided written informed consent.
A diagnosis of PCP was made when the patient had symptoms such as fever, cough and progressive dyspnea, associated with bilateral infiltrates on chest radiography, and when P. jirovecii was detected in a respiratory specimen by either Grocott-Gomori methenamine or Calcofluor white staining.
The underlying autoimmune diseases included systemic lupus erythematosus (n= 7), pemphigus vulgaris (n= 3), rheumatoid arthritis (n= 2), mixed connective tissue disease (n= 1), and Behçet's disease (n= 1). The underlying malignancies included non-Hodgkin lymphoma (n= 4), acute lymphoblastic leukemia (n= 2), lung cancer (n= 2), malignant melanoma (n= 1), and choriocarcinoma (n= 1). Most of the patients with malignancy were immunocompromised as a result of preceding antineoplastic or corticosteroid therapy. None of the patients with AIDS had been diagnosed as HIV-positive prior to the episode of PCP.
Data collection
We reviewed the medical records of all the patients and evaluated the clinical data at the time when the PCP was first recognized, as well as their clinical course. In the sera, the concentrations of CRP, KL-6, LDH, and β-D-glucan were examined. The counts of total WBC, neutrophils, and lymphocytes in peripheral blood were also evaluated. The oxygenation index was determined from the arterial oxygen tension and inspiratory oxygen concentration values.
BAL procedure and fluid processing for analysis
In most cases, BAL was targeted toward affected lung segments as noted on chest computed tomography and performed with 50 ml of 0.9% saline solution per lavage. Usually, three lavages were performed, and the lavage fluid immediately placed on ice. The BAL fluid was pooled, filtered through sterile gauze to remove mucous strands, and centrifuged at 200 g for 5 min at 4°C. The cell pellets were used for the differential counts on Wright-Giemsa-stained preparations.
For the detection of P. jirovecii, a 10-ml aliquot of BAL fluid was centrifuged at 1875 g for 10 min, and a smear microscopically examined for the presence of P jirovecii with Grocott-Gomori methenamine and Calcofluor white stains (Fungifluor, Polysciences, Warrington, PA, USA), following the recommendations of the manufacturer. The supernatants were divided into aliquots and frozen at −80°C until measurement of the mediators.
Measurement of inflammatory mediators
The concentrations of TNF-α, IL-6, IL-8, IL-10, IFN-γ, and MCP-1 in BAL fluid were measured using sandwich enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA). The minimum detectable amounts of TNF-α, IL-6, IL-8, IL-10, IFN-γ, and MCP-1 were 0.19 pg/ml, 0.11 pg/ml, 3.5 pg/ml, 3.9 pg/ml, 3.5 pg/ml, and 5 pg/ml, respectively. HMGB1 concentration in BAL fluid was measured using an HMGB1 ELISA Kit II (Shino-test, Kanagawa, Japan). The detection limit for HMGB1 was 1 ng/ml.
The relationships between the cytokine concentrations and other clinical parameters (underlying disease, differential cell counts in BAL fluid and peripheral blood, oxygenation impairment, blood counts, and serum markers) were analyzed subjectively.
Statistical methods
SPSS 15.0 software (SPSS, Chicago, IL, USA) was used for statistical analyses. Data are presented as median scores with the IQR in parentheses. Among the patient groups with different underlying diseases, differences in variables were compared by the Kruskal-Wallis test. Since the data were not normally distributed, comparisons of parameters between AIDS and non-AIDS patients were performed using the non-parametric Mann-Whitney U test. For IL-10, a χ2 test was performed to assess whether the observed frequencies of detection differed significantly from the expected frequencies. The relationships between variables were analyzed by the Spearman rank-order correlation test. Statistical significance was defined as P < 0.05.
RESULTS
Patient characteristics
The patient characteristics and laboratory data are shown in Table 1. The age of the patients was not statistically different between the patient groups. There were no female patients among the AIDS patients.
| Autoimmune disease (n= 14) | Malignancy (n= 10) | AIDS (n= 8) | P value* | |
|---|---|---|---|---|
| Mean age, yr | 58 (37–68) | 37 (29–46) | 35 (29–44) | 0.081 |
| Male/female | 6/8 | 4/6 | 8/0 | – |
| Blood count, ×103/mm3 | ||||
| WBC | 7.5 (3.7–10.0) | 4.8 (3.2–6.8) | 6.0 (5.3–7.5) | 0.206 |
| Neutrophil | 6.3 (3.4–8.3) | 4.3 (2.3–6.4) | 5.0 (3.7–6.8) | 0.418 |
| Lymphocyte | 0.5 (0.2–1.0) | 0.3 (0.2–0.4) | 0.8 (0.5–1.1) | 0.103 |
| Serum markers, | ||||
| CRP mg/dl | 8.0 (3.6–13.0) | 5.7 (1.3–12.1) | 2.8 (1.1–5.1) | 0.069 |
| LDH, IU/l | 528 (326–719) | 373 (297–612) | 441 (301–549) | 0.563 |
| KL-6, U/ml | 1,420 (642–2,440) | 1,000 (390–1,086) | 596 (544–1,010) | 0.340 |
| β-D-glucan, pg/ml | 231 (86–446) | 261 (184–405) | 80 (61–252) | 0.243 |
| PaO2/FIO2, mmHg | 220 (155–270) | 322 (160–415) | 338 (237–425) | 0.030 |
- Data are presented as median score with interquartile range in parentheses.
- *: Kruskal-Wallis test
Blood counts
The peripheral total WBC, neutrophil, and lymphocyte counts are shown in Table 1. WBC counts were within the normal range in most patients. No significant differences were observed in WBC, neutrophil, and lymphocyte counts between the three groups of patients with different underlying diseases. Even when AIDS and non-AIDS patients were compared, there were no significant differences in total WBC and differential counts.
Serum markers
We compared the serum concentrations of KL-6, LDH, CRP, and β-D-glucan among the patient groups (Table 1). Serum concentrations of the markers were increased in most of the patients, but there was no significant difference between the three groups in any of the serum markers examined. However, the serum CRP concentration in 24 non-AIDS patients was 6.4 (IQR: 2.9–12.6) mg/dl, which was significantly greater than in the AIDS patients (P < 0.05). There were no differences between the AIDS and non-AIDS patients in the other three markers.
Oxygenation index
Oxygenation indices are shown in Table 1. There were significant differences in the oxygenation index between the three groups (P < 0.05). The oxygenation indices in 24 non-AIDS patients and 8 with AIDS were 226 (IQR: 158–318) and 338 (IQR: 237–425) mmHg, respectively. There was a significant difference in the oxygenation index between the AIDS and non-AIDS patients (P < 0.05).
Total and differential cell counts in BAL fluid
The recovery rate, total and differential cell counts in BAL fluid are summarized in Table 2. There was no significant difference between the three groups in total cell counts in BAL fluid, but the total cell count in 24 non-AIDS patients was 8.0 (IQR: 3.0–11.2) × 105/ml, which was significantly greater than in the AIDS patients (P < 0.05). The percentage of macrophages was significantly different among the three groups (P < 0.05), although the macrophage numbers did not differ. The proportions and numbers of neutrophils, lymphocytes, and eosinophils in BAL fluid did not differ among the three groups. In 24 non-AIDS patients, however, the neutrophil count in BAL fluid was 0.55 (IQR: 0.25–1.42) × 105/ml, which was significantly greater than in the 8 patients with AIDS (P < 0.05).
| Autoimmune disease (n= 14) | Malignancy (n= 10) | AIDS (n= 8) | P value* | |
|---|---|---|---|---|
| Fluid recovery, % | 40 (29–55) | 38 (30–55) | 52 (42–57) | 0.612 |
| Cell concentration, 105/ml | 8.2 (3.7–16.0) | 4.1 (2.2–8.6) | 2.9 (2.0–4.2) | 0.052 |
| Macrophages,% | 25 (15–28) | 56 (25–75) | 48 (24–69) | 0.033 |
| Macrophages, 105/ml | 2.1 (0.9–3.3) | 1.9 (0.8–3.6) | 1.4 (0.9–2.0) | 0.583 |
| Lymphocytes,% | 54 (27–71) | 26 (20–58) | 33 (11–65) | 0.460 |
| Lymphocytes, 105/ml | 4.4 (1.6–7.5) | 0.8 (0.6–5.1) | 1.0 (0.3–2.4) | 0.052 |
| Neutrophils,% | 12 (3–40) | 6 (2–15) | 6 (4–13) | 0.559 |
| Neutrophils, 105/ml | 0.5 (0.2–2.0) | 0.6 (0.2–1.1) | 0.2 (0.1–0.7) | 0.118 |
| Eosinophils,% | 0 (0–2) | 0 (0–2) | 1 (0–6) | 0.787 |
| Eosinophils, 105/ml | 0.1 (0.0–0.2) | 0.0 (0.0–0.0) | 0.0 (0.0–0.2) | 0.581 |
- Data are presented as median score with interquartile range in parentheses.
- *: Kruskal-Wallis test
Concentrations of inflammatory mediators in BAL fluid
The concentrations of the mediators examined are summarized in Table 3. TNF-α, IL-6, IL-8, IFN-γ, HMGB1, and MCP-1 were detected in all of the BAL fluid samples collected from the patients with PCP.
| Autoimmune disease (n= 14) | Malignancy (n= 10) | AIDS (n= 8) | P value* | |
|---|---|---|---|---|
| TNF-α, pg/ml | 6.8 (2.3–13.7) | 2.4 (0.5–5.1) | 4.1 (2.7–6.0) | 0.196 |
| MCP-1, pg/ml | 2,252 (1,758–2,826) | 379 (143–2,072) | 715 (473–1,049) | 0.003 |
| HMGB1, ng/ml | 18.7 (12.3–32.4) | 15.7 (3.2–20.6) | 16.5 (12.7–25.5) | 0.457 |
| IL-8, pg/ml | 206 (103–473) | 70 (34–122) | 54 (30–66) | 0.001 |
| IL-6, pg/ml | 281 (63–622) | 53 (3–228) | 35 (19–57) | 0.012 |
| IFN-γ, pg/ml | 14 (9–47) | 26 (6–165) | 117 (12–249) | 0.417 |
- Data are presented as median score with interquartile range in parentheses.
- *: Kruskal-Wallis test
The TNF-α concentrations in BAL fluid did not differ between the three groups of patients with different underlying diseases. There was no difference in TNF-α concentrations even when the 8 patients with AIDS and 24 without AIDS were compared (P= 0.931). TNF-α was not detected in any of the control subjects.
MCP-1 concentrations in BAL fluid differed between the three groups (P < 0.01). The MCP-1 concentration was increased to as much as 2,252 (IQR: 1,758–2,826) pg/ml in BAL fluid collected from the patients with autoimmune diseases. MCP-1 concentrations in the 24 non-AIDS patients and 8 with AIDS were 2,054 (IQR: 576–2,769) and 715 (IQR: 473–1,049) pg/ml, respectively. There was a significant difference between the AIDS and non-AIDS patients in MCP-1 concentrations in BAL fluid (P < 0.02). MCP-1 was detected in three of six control subjects, and the concentration did not exceed 30 pg/ml in any of them.
The HMGB1 concentrations in BAL fluid did not differ between the three groups of patients with different underlying diseases (Table 3). There was no difference in HMGB1 concentrations even when the 8 patients with AIDS and 24 without AIDS were compared (P= 0.828). HMGB1 was not detected in any of the control subjects.
IL-8 concentrations in BAL fluid differed between the three groups (P < 0.01). The IL-8 concentration was increased to as much as 206 (IQR: 103–473) pg/ml in BAL fluid collected from the patients with autoimmune diseases. IL-8 concentrations in the 24 non-AIDS patients and 8 with AIDS were 127 (IQR: 69–451) and 54 (IQR: 30–66) pg/ml, respectively. There was a significant difference between AIDS and non-AIDS patients in IL-8 concentrations in BAL fluid (P < 0.01). The IL-8 concentrations in BAL fluid were significantly higher in the patients with autoimmune disease than in those with malignancies (P < 0.05). IL-8 was not detected in any of the control subjects.
IL-6 concentrations in BAL fluid differed between the three groups (P < 0.02). The IL-6 concentration was increased to as much as 281 (IQR: 63–622) pg/ml in BAL fluid collected from the patients with autoimmune diseases. IL-6 concentrations in the 24 non-AIDS patients and 8 with AIDS were 156 (IQR: 39–392) and 35 (IQR: 19–57) pg/ml, respectively. There was a significant difference between AIDS and the non-AIDS patients in IL-6 concentrations in BAL fluid (P < 0.01). The IL-6 concentrations in BAL fluid were significantly higher in the patients with autoimmune disease than in those with malignancies (P < 0.05). IL-6 was not detected in any of the control subjects.
IL-10 was not detectable in BAL fluid of any of the AIDS patients but it was detected in 3 out of 10 patients (30%) with malignancy and 9 out of 14 (64%) with autoimmune diseases. When the AIDS patients and those without AIDS were compared, IL-10 was detected in 12 (50%) of the non-AIDS patients whereas it was detectable in none of the AIDS patients. χ2 analysis revealed a significant difference between the AIDS and non-AIDS patients in the frequencies of detection of IL-10 in BAL fluid (P < 0.02). IL-10 was not detected in any of the control subjects.
The IFN-γ concentrations in BAL fluid did not differ between the three groups of patients with different underlying diseases (P= 0.417). There was no difference in the IFN-γ concentrations even when the 8 patients with AIDS and 24 without AIDS were compared (P= 0.222). IFN-γ was not detected in any of the control subjects.
Correlation between inflammatory mediators and other clinical parameters
We examined whether the cytokine concentrations in BAL fluid were correlated with other clinical parameters, such as the cell counts in BAL fluid, oxygenation index, and the concentrations of serum markers.
The total cell count in BAL fluid did not correlate with any of the mediators examined. The proportion of BAL fluid neutrophils, however, revealed significant correlation with the concentrations of HMGB1 (ρ= 0.609, P= 0.002) (Fig. 1a), IL-8 (ρ= 0.450, P= 0.015) (Fig. 1b), and IL-6 (ρ= 0.483, P= 0.009). There were significant correlations between the neutrophil counts in BAL fluid and the concentrations of HMGB1 (ρ= 0.409, P= 0.041), IL-8 (ρ= 0.527, P= 0.005), and IL-6 (ρ= 0.503, P= 0.007). In contrast, the proportion and number of BAL fluid lymphocytes did not correlate with the mediator concentrations in BAL fluid. There was significant correlation between the neutrophil number in BAL fluid and oxygenation index (ρ=−0.495, P= 0.015), suggesting that neutrophil accumulation might be associated with oxygenation impairment during PCP.
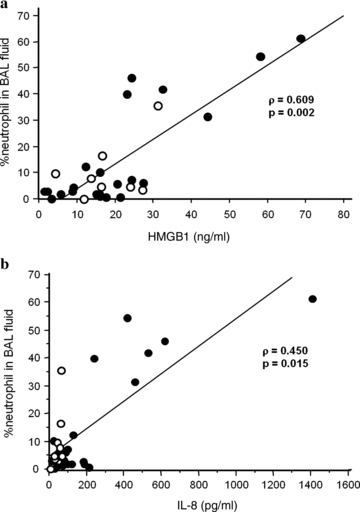
Relationships between neutrophil accumulation and cytokine concentrations in the alveolar space. (a) HMGB1 concentration is significantly correlated with neutrophil percentage in BAL fluid (ρ= 0.609, P= 0.002). (b) IL-8 concentration is significantly correlated with neutrophil proportion in BAL fluid (ρ= 0.450, P= 0.015). Closed circles, non-AIDS patients; open circles, patients with AIDS.
The oxygenation index did not correlate with TNF-α, MCP-1, IL-10, and IFN-γ, whereas it revealed significant inverse correlations with HMGB1 (ρ=−0.555, P= 0.002) (Fig. 2a), IL-8 (ρ=−0.709, P < 0.0001) (Fig. 2b), and IL-6 (ρ=−0.694, P < 0.001) (Fig. 2c). In AIDS patients, there was significant correlation between the HMGB1 concentration and oxygenation index (ρ=−0.905, P= 0.017).
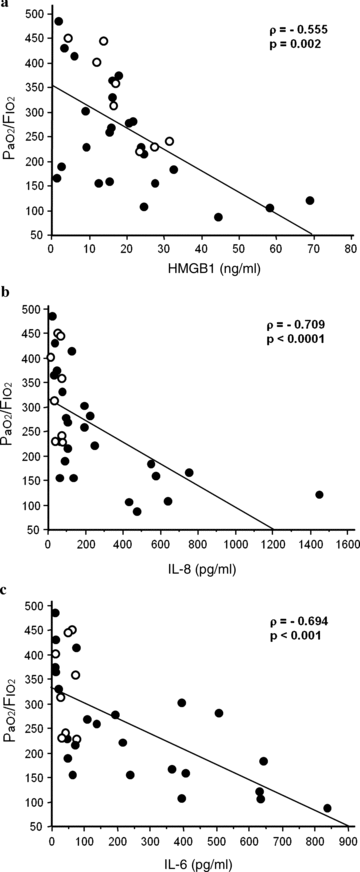
Relationships between oxygenation index and cytokine concentrations in the alveolar space. (a) HMGB1 concentration in BAL fluid is inversely correlated with the oxygenation index (ρ=−0.555, P= 0.002). (b) IL-8 concentration in BAL fluid is inversely correlated with the oxygenation index (ρ=−0.709, P < 0.0001). (c) IL-6 concentration in BAL fluid is inversely correlated with the oxygenation index (ρ=−0.694, P < 0.001). Closed circles, non-AIDS patients; open circles, patients with AIDS.
Serum concentrations of LDH were significantly correlated with those of IL-8 (ρ= 0.415, P= 0.021) in BAL fluid (Fig. 3a). Serum CRP concentrations showed significant correlation with those of MCP-1 (ρ= 0.549, P= 0.002), IL-8 (ρ= 0.638, P < 0.001) (Fig. 3b), and IL-6 (ρ= 0.522, P= 0.004). In patients with AIDS, the IL-8 concentration in BAL fluid was positively correlated with serum CRP concentration (ρ= 0.905, P= 0.017). There was no significant correlation between the serum concentrations of KL-6 and β-D-glucan and those of mediators in BAL fluid. Peripheral WBC and differential counts were not correlated with the mediator concentrations in BAL fluid.
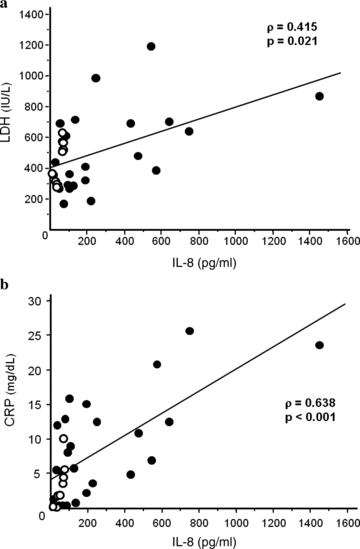
Relationships between serum indicators and cytokine concentrations in the alveolar space. (a) IL-8 concentration in BAL fluid is significantly correlated with the serum concentration of LDH (ρ= 0.415, P= 0.021). (b) IL-8 concentration in BAL fluid is significantly correlated with the serum concentration of CRP (ρ= 0.638, P < 0.001). Closed circles, non-AIDS patients; open circles, patients with AIDS.
DISCUSSION
The clinical features of PCP are known to differ according to the underlying factors responsible for the predisposing immunosuppression (1, 4). In the present study, we evaluated cytokine concentrations in BAL fluid in PCP patients with various underlying diseases. The major findings included differences between the patient groups with different underlying diseases in the concentrations of MCP-1, IL-8, and IL-6 in BAL fluid. Concentrations of HMGB1, IL-8, and IL-6 in BAL fluid were significantly correlated with clinical parameters, such as the oxygenation index, neutrophil count in BAL fluid, and serum concentrations of LDH and CRP. As described in AIDS patients, it has been suggested that these mediators in the lungs might be associated with lung inflammation and tissue damage during PCP (12). To the best of our knowledge, this is the first report that describes the cytokine profiles in BAL fluid and compares PCP patients with different underlying diseases. We speculated that differences in cytokine production in the lungs might be associated with the variety of clinical features of PCP.
In this study, the MCP-1 concentration in BAL fluid was greater in patients with autoimmune diseases than in those with AIDS. It has previously been reported that MCP-1 concentration is elevated in BAL fluid collected from patients with interstitial pneumonia associated with collagen vascular diseases (13). In addition, Wang and colleagues have reported that pneumocystis stimulates MCP-1 production by alveolar epithelial cells (14). We believe that PCP might enhance the MCP-1 production that has been associated with the underlying autoimmune diseases.
HMGB1 is a late inflammatory mediator of sepsis or endotoxin lethality (15). Recent investigations have shown that HMGB1 plays a critical role in the pathogenesis of severe pneumonia and lung injury by inducing neutrophil accumulation, lung edema, and cytokine release (16, 17). In this study, we found that HMGB1 concentrations in BAL fluid were associated with the proportion of neutrophils in BAL fluid and the oxygenation index. We speculated that upregulation of HMGB1 might be responsible for neutrophil recruitment and lung injury during PCP.
Severe PCP is characterized by intense neutrophilic pulmonary inflammation associated with diffuse alveolar damage, gas exchange impairment, and respiratory failure (18). The extent of neutrophilic inflammation has been shown to be more predictive of respiratory failure and death than organism burden during infection (6, 19). Besides HMGB1, neutrophil accumulation in the alveolar space is also associated with the concentrations of IL-8 and IL-6 in BAL fluid. Chollet-Martin and coworkers have reported that the concentrations of IL-8, a strong chemotactic factor for neutrophils, were elevated in the alveolar spaces and related to the clinical severity in patients with pneumonia, although PCP was not included (20). IL-6 has also been reported to increase in BAL fluid and serum during bacterial pneumonia (21). In this study, the concentrations of IL-6 and IL-8 were significantly higher in patients with autoimmune disease than in those with malignancies. However, since increased cytokine concentrations have been reported in interstitial pneumonia associated with collagen vascular diseases, the increase in cytokines might be owing to the underlying autoimmune disease (22, 23). We consider that IL-8 and IL-6 might contribute to the development of systemic and local inflammation during PCP through recruitment of neutrophils, especially in patients with autoimmune diseases.
Previous investigation by Israël-Biet and colleagues has shown that pulmonary IL-10 production is markedly defective in HIV-infected patients (11). In the present study, IL-10 was not detectable in any of the AIDS patients during PCP, whereas it was detected in half of the non-AIDS patients. We believe that this finding is compatible with those of previous studies. Since the IL-10 concentration in BAL fluid did not correlate with other clinical parameters, it remains unclear whether the impaired IL-10 production modifies the disease course of PCP in patients with AIDS.
We have previously reported that serum LDH concentration is correlated with oxygenation impairment during PCP, suggesting that LDH might be a good marker for organ damage (24). Quist and Hill have reported that serum LDH concentration was increased in all of the PCP patients examined and that an elevated LDH concentration is likely to be a reflection of pulmonary inflammation (25). In the present study, the serum LDH concentration was significantly correlated with that of IL-8 in BAL fluid. Since IL-8 is a major neutrophil chemotactic factor, IL-8 might be responsible for the neutrophil accumulation which leads to lung tissue damage during PCP.
β-D-glucan is one of the major components of the yeast cell wall and its concentration in serum is known to be a reliable indicator for detecting P. jirovecii infection (24). Although PCP in HIV-negative patients is reported to be characterized by a lower organism burden (6), we did not observe a difference between AIDS and non-AIDS patients in serum β-D-glucan concentrations. We concluded that β-D-glucan can most likely not be applicable to the quantitative estimation of organism burden.
The mechanism by which pneumocystis organisms induce pulmonary inflammation remains incompletely elucidated. Wang and coworkers have reported that pneumocystis activates NF-κB signaling in alveolar epithelial cells (26). The activation of NF-κB is known to be a critical step for the expression of inflammatory mediators, including inflammatory cytokines, chemokines, and adhesion molecules (27). We therefore surmised that pneumocystis might directly alter the gene expression of alveolar epithelial cells in a manner that promotes pulmonary immune and inflammatory responses. β-D-glucan has been implicated in strongly promoting pulmonary inflammation (28). Evans and colleagues have reported that β-D-glucan induces chemokine production by alveolar epithelial cells through activation of NF-κB (29). However, since there was no correlation between serum β-D-glucan concentration and cytokine concentration in BAL fluid, we speculated that β-D-glucan might not be the only factor that induces cytokine production in the lung during PCP.
One of the major limitations of the present study is the lack of sequential assessment of the mediators in BAL fluid. Since we could only perform BAL once in the course of PCP, it remains unclear whether the time course of mediator concentrations is affected by the underlying disease or by treatment including corticosteroids. In addition, serum concentrations of the cytokines were not evaluated. We think that these could be the subjects of future investigations.
In conclusion, the upregulation of MCP-1, IL-8, and IL-6 in the alveolar space during PCP differed between patient groups with different underlying disorders. The concentrations of HMGB1, IL-8, and IL-6 in BAL fluid were significantly correlated with neutrophil accumulation in the lungs, oxygenation index, and serum indicators of inflammation and tissue damage. The modest upregulation of IL-8 and IL-6 in AIDS patients might be associated with the milder clinical manifestations of PCP. Although further investigation is necessary, the profile of inflammatory mediators in BAL fluid might be predictive of the severity and outcome of PCP.
ACKNOWLEDGMENTS
The authors are very grateful to Miyuki Yamamoto for her excellent technical assistance.




