Leuconostoc citreum HJ-P4 (KACC 91035) regulates immunoglobulin E in an ovalbumin-induced allergy model and induces interleukin-12 through nuclear factor-kappa B and p38/c-Jun N-terminal kinases signaling in macrophages
ABSTRACT
Leuconostoc citreum (L. citreum) HJ-P4 (KACC 91035) is one of the major predominant species in kimchi fermentation in Korea. The purpose of the present study was to test the immunomodulatory capacity of L. citreum to modulate the IgE-mediated allergic response and to examine the involvement of NF-κB and MAPK in IL-12 production in macrophages. Balb/c mice were sensitized with OVA/alum and oral administration of L. citreum to the mice began before or after the OVA sensitization. Protein and mRNA expression of Th1 cytokines in splenocytes by L. citreum in vitro was measured. The role of NF-κB and MAPK such as p38, ERK1/2 and JNK in L. citreum-induced IL-12 was investigated in peritoneal macrophages and RAW264.7 cell lines. L. citreum inhibited the serum levels of total IgE, IgG1 and IgG2a altogether and increased OVA-specific IFN-γ production in splenocytes from pre- and post-sensitized animals. However, the downregulation of IL-4 and IL-5 production was observed only in the pre-sensitization group. The ability of L. citreum to stimulate IFN-γ was dependent on its induction of IL-12. NF-κB, p38 and JNK were mainly involved in L. citreum-induced IL-12 production. In conclusion, the current study demonstrated that L. citreum is able to regulate serum IgE generation at the induction and effector phases of allergic response through overall control over antibody production and that its involvement of IL-12 production was mediated through NF-κB and p38/JNK. Taken together, the use of L. citreum can be useful in preventing the development and progression of IgE production.
List of Abbreviations:
-
- APC
-
- antigen presenting cells
-
- CFU
-
- colony forming units
-
- ERK
-
- extracellular signal-regulated kinases
-
- IFN
-
- interferon
-
- IgE
-
- immunoglobulin E
-
- IL
-
- interleukin
-
- JNK
-
- c-Jun N-terminal kinases
-
- MAPK
-
- mitogen-activated protein kinases
-
- NF-κB
-
- nuclear factor-kappa B
-
- OVA
-
- ovalbumin
As the incidence of allergic diseases has increased over decades, it has been widely accepted that decreased exposure to microbial infection in infancy due to higher hygienic standards and more frequent antibiotic treatments might contribute to a defect in the maturation of Th1-dependent immune responses (1–4). The increased generation of IgE, which is one of the typical features of allergy, has been explained by the insufficient population of Th1 cells to reciprocally inhibit Th2 cells (5, 6). Therefore, it has been suggested that boosting the Th1 response can be beneficial in modulating the immune system against the Th2-type allergic disease (7).
Consumption of lactic acid bacteria-containing food is expected to be useful in restoring the imbalance of Th1/Th2 cytokine profiles observed in allergic diseases. The ability of lactic acid bacteria to induce the Th1 response depends on the production of innate cytokines such as IL-12, IL-18 and type I interferon by APC such as macrophages and dendritic cells (8–10). A number of studies have demonstrated the benefits of lactic acid bacteria, which enables the immune system to deviate away from the Th2-type allergic phenotype (11–14). It has been well documented that several strains of lactic acid bacteria can inhibit serum IgE through the modulation of Th1/Th2 responses. However, few data are available on whether treatment with lactic acid bacteria given at the induction phase or at the effector phase of allergic response can regulate IgE generation.
The NF-κB and MAPK signaling pathways are necessary for the production of immediate inflammatory cytokines in response to microbial stimuli (15, 16). Under normal conditions, NF-κB is sequestered in the cytoplasm by its inhibitory protein, IκB, but, upon stimulation, IκB is phosphorylated on serine/threonine residues, subsequently ubiquitinated and degraded by a proteosome complex, allowing the translocation of NF-κB into the nucleus where it activates the expression of several cytokine genes. MAPK such as JNK, p38 and ERK are involved in multiple steps of signaling pathways by phosphorylating target intracellular proteins and subsequently affecting gene expression and protein production (17).
Leuconostoc citreum (L. citreum) is one of the predominant species in the early phase of kimchi (a general term for fermented vegetable foods in Korea) fermentation (18). This strain, classified as a psychotroph (a bacterium which can grow at low temperatures but has a higher optimum growth temperature), is able to grow even at 2°C and still shows high enzyme activity (19). In the current study, we investigated whether treatment with L. citreum HJ-P4 was beneficial in regulating IgE in an OVA-induced allergy model from preventive and therapeutic perspectives. Also, we tried to explore the signaling mechanism behind the L .citreum-induced Th1-type response.
MATERIALS AND METHODS
Animals
Male Balb/c mice and C57BL/6 mice at 8 weeks of age were purchased from Charles River Korea (Orient, Korea) and maintained with rodent chow and water ad libitum in a temperature- and humidity-controlled pathogen-free animal facility at Kyunghee University. Mice were cared for according to the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, USA). All experiments were approved by our institutional ethical committee for animal welfare.
Preparation of microorganisms
Leuconostoc citreum HJ-P4 (KACC 91035) was provided by Professor Nam Soo Han at Chungbuk National University. L. citreum was cultured in MRS broth (DIFCO, Detroit, MI, USA) for 16 hr at 30°C, collected by centrifugation, washed several times with sterile distilled water and lyophilized. For in vitro assays, the microorganism was heat killed.
Cell lines
Mouse macrophage RAW264.7 cells were obtained from the Korean Cell Line Bank. Cells were cultured at 37°C in DMEM (WelGENE, Daegu, Korea) containing 10% fetal bovine serum (Sigma, St Louis, MO, USA) and 1% antimycotic-antibiotic (Gibco-BRL, Grand Island, NY, USA) in a humidified atmosphere of 5% CO2.
Animal experiments: Treatment protocol
Viable L. citreum was suspended in 9% skim milk to 2 × 108CFU/ml. Each mouse received this suspension by oral gavage in a volume of 100 μl per animal three times per week. Untreated and control mice were given an equal volume of skim milk. Each experimental group consisted of seven to eight mice.
Mice were sensitized by i.p. injection with 1 μg OVA grade V (Sigma) dissolved in a mixture of an equal volume of Al(OH)3 gel (alum) and PBS once per week for 3 weeks. Untreated mice were injected with PBS.
Experiments consisted of the pre-sensitization and post-sensitization protocols. For the effect of L. citreum on the induction of OVA-induced Th2 response (pre-sensitization), oral administration began 1 week before the first sensitization and lasted until the third sensitization. For its effect on the effector phase of OVA-induced Th2 response (post-sensitization), L. citreum treatment was applied immediately after the final sensitization and continued for 2 weeks. By the end of the post-sensitization experiment (on days 23, 25 and 28), mice received 20 μl of 0.01% of OVA solution to each nostril. Mice were killed at the end of the experiments. Serum and spleens were obtained from each mouse individually. The procedures are summarized in Figure 1.
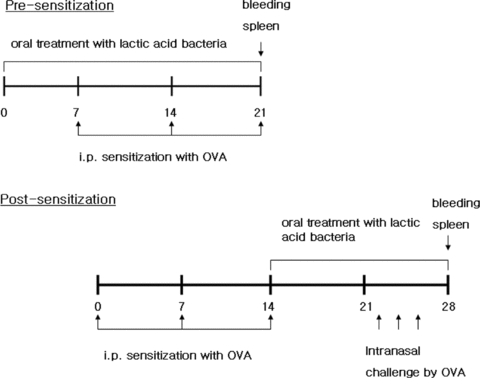
Experimental procedures. Day 0 is defined as the start of the experiment. For the effect of Leuconostoc citreum (L. citreum) on the induction phase of OVA-induced allergic response, Balb/c mice were orally treated with L. citreum 1 week before the first sensitization and continued until the end of the experiment (pre-sensitization). For the effect of L. citreum on the effector phase of OVA-induced allergic response, oral treatment with L. citreum began immediately after the third sensitization and intranasal treatment of OVA solution was carried out on days 23, 25 and 28. Spleens and blood were obtained at the end of the experiment.
In vitro OVA restimulation of in vivo sensitized splenocytes
Splenocytes from OVA-sensitized mice were prepared by disrupting the spleen between glass slides in RPMI-1640 (WelGENE) with 10% FBS and 1% antimycotic/antibiotics. After a 10-min centrifugation at 150 ×g to separate cells from debris, cells were washed in complete medium, followed by lysis of erythrocytes. Splenocytes were resuspended and adjusted to 5 × 106 cells/ml and cultured for 72 hr with OVA (1 mg/ml) at 37°C in a 5% CO2 incubator.
In vitro cell culture for cytokine analysis
Splenocytes from naïve Balb/c mice were isolated and stimulated with L. citreum. Cell supernatant and mRNA were obtained at 24 hr. Peritoneal macrophages were obtained from C57BL/6 mice 3 days after i.p. injection of 2 ml sterile thioglycollate medium (DIFCO). Cells were resuspended in complete DMEM and seeded at a density of 5 × 105/ml. After 90 min, non-adherent cells were removed. To assess NF-κB or MAPK-dependent cytokine production, the adherent cells were incubated with various doses of pharmacological inhibitors for NF-κB (sulfasalazine) (Sigma), p38 (SB230580) (Promega, Madison, WI, USA), ERK1/2 (U0126) (Sigma) and JNK (SP600125) (Sigma) for 30 min before stimulation with the bacteria. Sulfasalazine was dissolved in NH4OH solution and the rest of the inhibitors were dissolved in dimethylsulfoxide (DMSO). Control cells (0 μg/ml) were treated with an equal volume of DMSO or NH4OH solution. To determine IκB degradation and phosphorylation of p38, ERK1/2 and JNK, RAW264.7 cells (3 × 106 cells/ml) were stimulated with lactic acid bacteria for various time points.
ELISA
The levels of IL-4, IL-5, IFN-γ, IL-12, IgE and IgG2a were measured using a commercial OptEIA ELISA set (Pharmingen, San Diego, CA, USA). IgG1 was measured using capture antimouse IgG1 (A85-3) and biotinylated detection anti-mouse IgG1 (A85-1) (BD Pharmingen). Purified mouse IgG1 isotype (BD Pharmingen) was used as standard. Each cytokine and antibody concentration was calculated from the standard curve.
Real-time RT-PCR
Total RNA was isolated with an RNeasy Mini Kit (QIAGEN, Hilden, Germany) and cDNA was reverse-transcribed by a Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Oligonucleotide primers were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA). The GAPDH gene was used as an endogenous control to normalize the expression of target genes. Quantitative real-time PCR was carried out in triplicate using a 96-well optic tray on an ABI PRISM 7300 sequence detector (Applied Biosystems). Negative controls lacking template RNA were included in each experiment. The expressions of each mRNA were quantified by relating the PCR threshold cycle obtained from the samples to amplicon-specific standard curves and normalized to each GAPDH gene.
Western blot analysis
Cells were harvested, washed twice with ice-cold PBS and lysed on ice for 20 min using PRO-PREP protein extraction solution (iNtRon, Seongnam, Korea) containing phosphatase inhibitor (Sigma). Whole-cell lysates were centrifuged at 12 000 ×g at 4°C for 10 min and the supernatants obtained. Samples were separated on 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% skim milk in TBS-Tween (0.1%) for 1 hr and then incubated with polyclonal antibody for IκBα and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), p38, JNK, ERK, phospho-p38, phospho-JNK and phospho-ERK (Cell Signaling, Beverly, MA, USA) overnight at 4°C. After washing three times with TBS-Tween, the membranes were incubated with secondary horseradish peroxidase-conjugated antirabbit antibody (Santa Cruz) for 1 hr. Band detection was conducted using an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Uppsala, Sweden).
Statistical analysis
Comparisons between groups were determined by anova followed by Dunnet's post-hoc test. Calculations were carried out using SPSS version 12. P values less than 0.05 were considered significant.
RESULTS
Effect of L. citreum on the serum antibody response and splenocyte cytokine production from pre-sensitized mice
Allergic response consists of systemic response in the induction phase and local inflammation in the effector phase. First, we tried to measure the effect of L. citreum on the induction phase of OVA-induced Th2 response. Balb/c mice were orally given L. citreum prior to sensitization. This intervention lasted until the final sensitization and serum and splenocytes were obtained at the end of the experiment (Fig. 1) OVA-sensitized mice developed a typical Th2 response characterized by higher levels of serum IgE and IgG1. A significant reduction was observed in L. citreum-treated mice (Fig. 2a,b). Subsequently, in order to determine whether these inhibitions were indicative of overall B-cell suppression or the modulation of Th1/Th2 responses, we analyzed the levels of serum IgG2a, a Th1-type antibody. Surprisingly, a significant reduction in serum IgG2a was also elicited (Fig. 2c), suggesting that such inhibition did not happen selectively in IgE-producing B cells. We further investigated the production of Th1 and Th2 cytokines from OVA-restimulated splenocytes isolated from pre-sensitized mice. The immunomodulation of Th1/Th2 cytokine production was observed in the L. citreum-treated group: the secretion of IFN-γ was enhanced, concomitant with a decrease in IL-4 and IL-5 (Fig. 2d,e,f). These data indicate that the IgE inhibition by L. citreum does not appear to be due to a direct effect on the modulation of antigen-specific Th1 and Th2 responses.
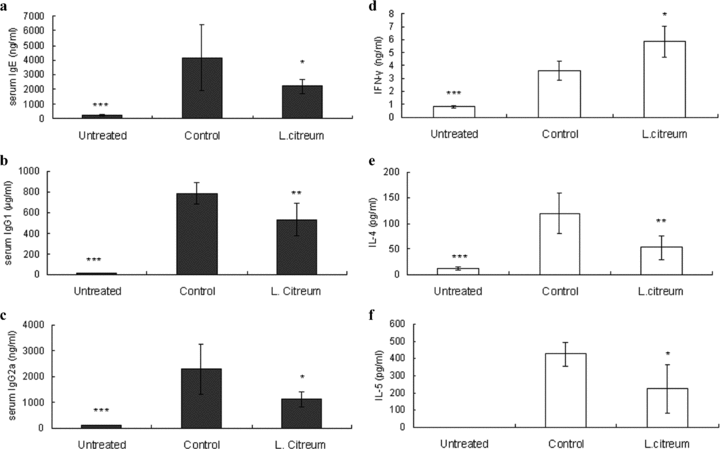
Effect of L. citreum on serum IgE, IgG1 and IgG2a and cytokine production in splenocytes from pre-sensitized mice. The immunization and treatment procedures are described in the legend of Fig. 1. Control and L. citreum-treated mice were sensitized with OVA whereas untreated mice were injected with PBS. Untreated and control mice received an equal volume of skim milk. (a–c) The concentrations of total IgE, IgG1 and IgG2a in serum were individually assayed instead of pooling serum by ELISA. (d–f) Splenocytes from pre-sensitized mice were restimulated with OVA (1 mg) for 72 hr and cytokine levels from supernatants were measured by ELISA. Data are the mean ± SD from two independent experiments. *P < 0.05, **P < 0. 01, ***P < 0.001, compared with control (anova followed by Dunnet's post-hoc test). n= 7–8/group.
Effect of L. citreum on the serum antibody response and splenocyte cytokine production from post-sensitized mice
Next, we wanted to see whether L. citreum could inhibit IgE production during the effector phase of the OVA-induced Th2 response. As shown in Figure 1, L. citreum treatment began right after the final sensitization until the end of the experiment for 2 weeks, which included OVA challenge. Like the effects observed with the pre-sensitization model, this intervention strongly inhibited the serum levels of IgE, IgG1 and IgG2a altogether, indicating that L. citreum treatment at post-sensitization also played an inhibitory role in B-cell responses (Fig. 3a,b,c). With regard to antigen-specific Th1/Th2 cytokine production, a higher level of IFN-γ was observed, but those of IL-4 and IL-5 were rather increased, although they did not reach statistical significance (Fig. 3d,e,f). Results from our two protocols suggest that regardless of the time points at which mice were sensitized, the mechanism behind the serum IgE downregulation by L. citreum might be associated with pan-B cell suppression, but not a direct result of the modulation of Th1/Th2 cytokine production.
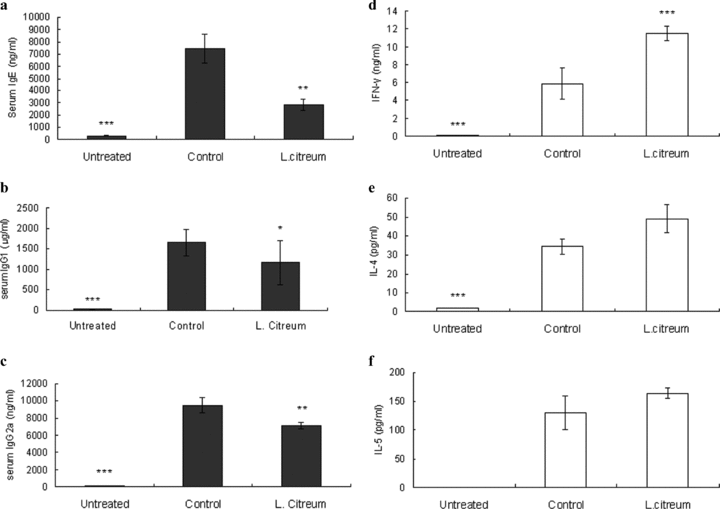
Effect of L. citreum on serum IgE, IgG1 and IgG2a and cytokine production in splenocytes from post-sensitized mice. The immunization and treatment procedures are described in the legend of Fig. 1. (a–c) The concentrations of total IgE, IgG1 and IgG2a in serum were individually assayed instead of pooling serum by ELISA. (d–f) Splenocytes from post-sensitized mice were restimulated with OVA (1 mg) for 72 hr and cytokine levels from supernatants were measured by ELISA. Data are the mean ± SD from two independent experiments. *P < 0.05, **P < 0. 01, ***P < 0.001, compared with control (anova followed by Dunnet's post-hoc test). n= 7–8/group.
Effect of L. citreum on the induction of Th1 cytokines in mouse splenocytes in vitro
In order to further characterize the ability of L. citreum to induce Th1 cytokines, mouse splenocytes were stimulated with the microorganism for 24 hr in vitro. We used heat-killed L. citreum in order to keep the cell count constant throughout all the in vitro assays. IL-12 and IFN-γ were examined at the protein and mRNA levels. IL-12 is a heterodimer of two chains of p40 and p35. Its biologically active form is p70 and expression of the p40 subunit reflects the production of IL-12 p70 while the mRNA of the p35 is relatively constant (20). Therefore, we measured IL-12 p70 and IL-12 p40 by ELISA and real-time RT-PCR, respectively. The peak of IL-12 expression occurs prior to that of IFN-γ (9). As expected, the production of IL-12 in response to L. citreum at 24 hr was more outstanding than that of IFN-γ, whereas at the mRNA level, the transcriptional activity of IFN-γ was stronger than that of IL-12 at the same time point (Fig. 4a,b). T-bet is involved in the inhibition of IgE in B cells as well as the upregulation of IFN-γ in T cells (21, 22). The induction of T-bet by L. citreum was also detected as shown by real-time RT PCR (Fig. 4b).
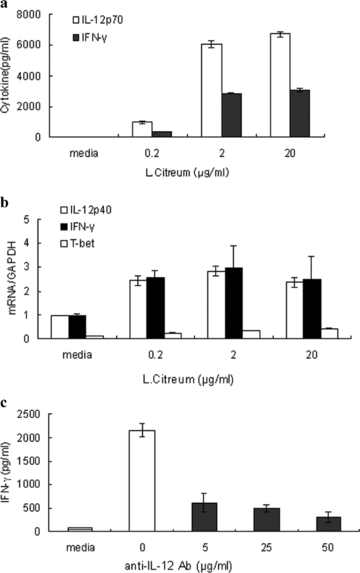
Effect of L. citreum on the expression of Th1 cytokines in vitro. (a,b) Splenocytes from naïve Balb/c mice were cultured with various concentrations of heat-killed L. citreum for 24 hr. Cytokine production was measured by ELISA (a). Gene expression was analyzed by real-time RT PCR (b). (c) Splenocytes were pre-treated with 5, 25 and 50 μg/ml neutralizing anti-IL-12 mAb for 30 min and then stimulated with heat killed L. citreum (20 μg/ml) for 24 hr and cytokine levels were measured by ELISA. Data are the mean ± SD of three independent experiments.
The expression of IFN-γ by lactic acid bacteria depends on the production of IL-12 by macrophages and dendritic cells, as the removal of these cells abrogates IFN-γ (8, 9). In an effort to identify whether L. citreum-induced IL-12 was necessary for IFN-γ production, we treated splenocytes with neutralizing anti-IL-12 mAb in the presence of L. citreum for 24 hr. Consistent with previous reports, the IFN-γ release by L. citreum was clearly decreased, but not completely (Fig. 4c). These results partly confirmed the IL-12 dependence of IFN-γ in response to the microbial stimulation.
Effect of L. citreum on NF-κB and MAPK signaling in IL-12 production in macrophages
We tried to determine the role of the NF-κB and MAPK pathways in L. citreum-induced IL-12 production in macrophages. Peritoneal macrophages from C57BL/6 mice were obtained and cultured with the bacteria in the presence of varying concentrations of specific pharmacological inhibitors for NF-κB (sulfasalazine), ERK1/2 (U0126), p38 (SB203580) and JNK (SP600125). Our results showed that these inhibitors inhibited IL-12 production in a dose-dependent manner, indicating that NF-κB, p38 and JNK clearly participated in the cytokine production (Fig. 5a). ERK1/2 seemed to play a marginal role, as the inhibition was only observed at the highest dose tested (50 μM). Next, western blotting was carried out in order to identify whether L. citreum was able to directly activate these proteins. Mouse macrophage RAW264.7 cells were stimulated with the bacteria for various time points. IκB degradation, which is indicative of nuclear translocation of NF-κB by L. citreum, was detected (Fig. 5b). We also found that p38, JNK and ERK1/2 were activated. From these results, it can be concluded that activation of p38, JNK and NF-κB signaling pathways are mainly involved in IL-12 production in L. citreum-treated macrophages.
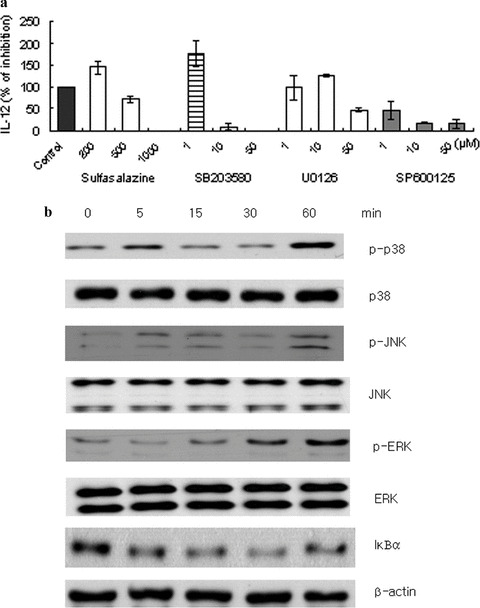
Effect of L. citreum on NF-κB and MAPK signaling in IL-12 production in macrophages (a) Peritoneal macrophages from C57BL/6 were pre-treated with various inhibitors for NF-κB (sulfasalazine), p38 (SB203580), ERK (U0126) and JNK (SP600125) at the indicated concentrations for 30 min and then stimulated with L. citreum (20 μg/ml). After 24 hr, cell culture supernatants were collected and assayed for IL-12 by ELISA. The percentage of IL-12 production was calculated as: IL-12 production (%) = (inhibitor/control) × 100. Data are the mean ± SD of four independent experiments. (b) RAW 264.7 cells were stimulated with lactic acid bacteria for the indicated time points. Whole-cell lysates were obtained and analyzed by western blotting using 10% SDS-PAGE and antibodies against the indicated proteins. Similar results were obtained in four independent experiments.
DISCUSSION
Leuconostoc citreum, one of the major species of bacteria involved in the process of kimchi fermentation, shows high acid tolerance and plays an important role in the preservation of kimchi by inhibiting other microbial growth (23). The purpose of the present study was to test the immunomodulatory capacity of L. citreum to modulate the IgE-mediated allergic response in vivo and to examine its involvement of the NF-κB and MAPK pathways in IL-12 production in macrophages.
Our in vivo allergy experiments consisted of preventive and therapeutic models by applying L. citreum treatment before or after antigen sensitization. We demonstrated that L. citreum was effective in reducing the serum levels of total IgE and IgG1 generated during the induction and effector periods of OVA-induced allergic response. However, this microorganism reduced the serum levels of IgG2a and was selective in modulating Th1/Th2 cytokine secretion depending on the timing of sensitization. Antigen-specific IFN-γ secretion was enhanced in OVA-restimulated cells from pre-and post-sensitized mice but only the pre-sensitization mode decreased antigen-specific IL-4 and IL-5. These results indicate that the inhibition of serum IgE by L. citreum is likely to be due to suppression of pan-B cell responses, but not to an alteration of Th1/Th2 cytokine profiles. Apart from the high affinity receptor for IgE (FcɛRI), which is mainly responsible for IgE-mediated mast cell degranulation (24), most mature B cells express the low-affinity receptor for IgE (CD23, FcɛRII). This receptor enables B cells to bind with IgE/antigen complexes regardless of specificity and amplify IgM-, IgG- as well as IgE- responses (25). The ability of IgE to enhance antibody responses is acquired only when it binds with the antigen (26–28). Based on this, it can be assumed that L. citreum may play some inhibitory roles in the formation of IgE/antigen complexes and thus render CD23 B+ cells less responsive to them, ultimately leading to a reduction in IgE-mediated feedback response. This can be achieved by inhibiting IgE generation in B cells. Further detailed study is required to investigate this possibility. A previous study showed that systemic administration of IFN-γ reduced OVA-specific IgE levels in serum (29). The authors demonstrated that such a reduction was not mediated via a direct effect on reduction in antigen-specific Th2 cells. Likewise, continuous exposure to the bacteria, as shown in our experiments, may achieve a similar effect exerted by IFN-γ treatment above and thus downregulate serum IgE production without affecting Th2 cytokines. In fact, IFN-γ was reported to reduce IL-4-induced germline ɛ-transcript in B cells (30).
In keeping with these observations, recent studies have demonstrated that microorganisms may use a different pathway to downregulate IgE production in B cells independently of IL-4 in vitro (22, 31). T-bet, known as the Th1 master transcription factor, has been reported to be involved in IL-4-independent IgE suppression in the presence of bacterial DNA in B cells (22). We found that L. citreum has the ability to directly stimulate T-bet expression although we did not clarify what types of cells were involved. Also, it should be confirmed whether the involvement of T-bet in IgE suppression at the cellular level can be induced only by microorganisms or by other different stimuli.
Cytokine signaling is required for the appropriate host immune response against microorganisms, whether being pathogenic or non-pathogenic. IL-12 is one of the immediate cytokines in response to microbial stimuli and augments the early phase of IFN-γ production by NK and T cells. NF-κB participates in the expression of various cytokine genes including IL-12 (32). We found that one of the pathways used by L. citreum required the activity of NF-κB. Certain strains of lactobacilli have been demonstrated to activate NF-κB whereas others negatively regulate it (33–36). The mechanisms by which non-pathogenic lactic acid bacteria selectively modulate NF-κB signaling are not yet clearly elucidated. Apart from NF-κB, the MAPK signaling pathways are also involved in bacteria-induced cytokine production signaling (37). Results from our study demonstrated that the ability of L. citreum to induce IL-12 relies mainly on p38 and JNK. Although the high dose of ERK inhibitor reduced the level of IL-12, this protein appears to be less critical.
In conclusion, the current study demonstrated that L. citreum is able to regulate serum IgE levels through overall control over antibody production generated at both the induction and effector phases of allergic response and that its involvement of IL-12 production was mediated through NF-κB and p38/JNK. Taken together, the use of L. citreum can be useful in preventing the development and progression of IgE production.