Extensive diversification of pebblesnails (Lithoglyphidae: Fluminicola) in the upper Sacramento River basin, northwestern USA
Abstract
Mitochondrial DNA sequences from the cytochrome c oxidase subunit I (COI) and cytochrome b (cytb) genes were obtained from the nine extant, previously described species of the northwestern North American freshwater gastropod genus Fluminicola (commonly known as pebblesnails) and from a large number of taxonomically undescribed populations of these animals from the upper Sacramento River basin, California and Oregon, which is composed of the Sacramento River headwaters, and the McCloud and Pit Rivers. Phylogenetic analyses of separate and combined molecular datasets yielded well-supported and largely congruent trees delineating 13 genetically divergent and morphologically distinctive upper Sacramento basin lineages, which we describe as new species. These include two groups of closely related and geographically proximal species that are further united by unique radular or shell features. Most of these novelties have narrow geographical distributions and are restricted to headspring areas, whereas several are more wide ranging and typically occupy larger, well-integrated habitats. The highly endemic fauna of upper Sacramento River pebblesnails is not a single species flock, but instead a polyphyletic assemblage spread among four separate clades. Our phylogeny, together with the application of a COI molecular clock for Fluminicola, suggests that upper Sacramento River clades originated as a result of late Neogene separation of this basin from neighbouring regions (northwestern Great Basin, Klamath River basin), which is consistent with previous biogeographical hypotheses based on the distributions of fishes. The upper Sacramento River pebblesnails evolved in association with the complex late Cenozoic history of regional landscape and drainage and diversification was also facilitated by the invasion of and adaptation to insular spring habitats. Our findings are consistent with the generally limited dispersal ability and geologically ancient (mid-Tertiary) age of this genus and imply that other portions of northwestern North America may also harbour a large number of undescribed pebblesnail species. Journal compilation © 2007 The Linnean Society of London, Zoological Journal of the Linnean Society, 2007, 149, 371–422. No claim to original US government works.
INTRODUCTION
Aquatic gastropods of the genus Fluminicola (family Lithoglyphidae), commonly known as pebblesnails, are distributed from central California to British Columbia (Hershler & Frest, 1996; Hershler, 1999), ranging from sea level to elevations in excess of 2000 m. Although pebblesnails are locally abundant and easily collected in springs, streams, and rivers throughout much of their broad range, they have been little studied and the scope and content of the genus has not been well established. Hershler & Frest (1996) reviewed Fluminicola and considered nine nominal species, all described prior to 1950, to be distinct. (Two of these have not been collected since their original descriptions and may be extinct; Hershler & Frest, 1996.) They also provided a morphology-based phylogenetic hypothesis in which Fluminicola was depicted as paraphyletic relative to the eastern North American lithoglyphid genus Somatogyrus. Two additional species of Fluminicola were subsequently described by Hershler (1999).
The nine currently recognized extant species of Fluminicola are considered to be a small fraction of actual taxonomic diversity in this genus (Hershler & Frest, 1996), which is consistent with what is known of the natural history of the genus. Pebblesnails are gill breathing and have an entirely benthic life cycle. They are restricted to clean, flowing, well-oxygenated waters and are usually confined to hard substrates, suggesting that colonies separated by dry land or reaches of unsuitable aquatic habitat (e.g. soft sediment) may be genetically isolated. Given the wide geographical range and mid-Tertiary age of Fluminicola (Hershler & Frest, 1996) it is reasonable to hypothesize that the genus has undergone extensive diversification leading to the development of a large modern fauna.
A rigorous evaluation of this hypothesis leading to an accurate description of Fluminicola diversity is especially desirable at this time because these snails are currently being incorporated into conservation initiatives in various parts of the Pacific North-west (e.g. USDA, USDI, 1994). Towards this end, we examined the evolutionary differentiation of pebblesnails in the upper Sacramento River basin, a large (c. 19 830 km2) watershed in north-eastern California and south-eastern Oregon that traverses two major physiographical regions (Modoc Plateau, High Cascades) (Fig. 1). The upper Sacramento River heads in the vicinity of Mount Shasta and flows south within a narrow gap between the Klamath Mountains and southern High Cascades. Its main tributary, the Pit River, flows westward across the Modoc Plateau (volcanic and composed of Miocene and younger lava flows; Pease, 1965) and through the Cascades between Mount Shasta and Lassen Peak, where it joins the south-flowing McCloud River just above its connection with the Sacramento River (this area of confluence is now impounded behind Shasta Dam). The Pit River headwaters consist of two forks that join in the vicinity of Alturas, California. Goose Lake, which is located in a deep, topographically closed basin in south-east Oregon, occasionally overflowed its sill to the North Fork Pit River during the late Quaternary and as recently as 1881 (Phillips & Denburgh, 1971) and thus is considered the uppermost segment of this drainage. The upper Sacramento River basin had a complex late Cenozoic geological history (Pease, 1965; Macdonald, 1966; Page, 1995), which presumably set the stage for extensive local diversification of aquatic biota. Although only two described pebblesnails live in this basin –F. seminalis (Hinds, 1842), which was historically distributed from the Pit River drainage to near the mouth of the Sacramento River, but is now extinct over much of its range (Taylor, 1981; Hershler & Frest, 1996) and F. modoci Hannibal, 1912, which was described from springs along the margin of Goose Lake (Hershler & Frest, 1996) – the two junior authors discovered many taxonomically unstudied populations during the course of a recent field survey, including representatives of a potentially large number of new species (Frest & Johannes, 1993, 1994, 1995a, 1997).
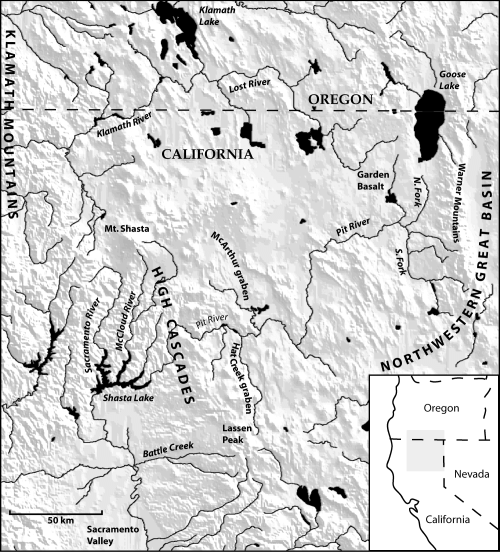
Map showing the upper Sacramento River drainage and other geographical features discussed in the text.
Previous taxonomic studies of Fluminicola have been based on gross morphology that, aside from one divergent species, F. virens (Lea, 1838), is conservative within the genus (Hershler & Frest, 1996). Molecular evidence may thus be critical to delineating species limits within Fluminicola, especially in studies such as ours that have a relatively narrow geographical focus. Here we generate phylogenetic hypotheses for the upper Sacramento River fauna and all currently recognized extant species of Fluminicola based on partial DNA sequences from two mitochondrial genes, cytochrome c oxidase subunit I (COI) and cytochrome b (cytb). Separate and combined analyses of these two mitochondrial datasets congruently depict a large number of distinct pebblesnail lineages within the upper Sacramento River basin, consistent with the previously hypothesized occurrence of substantial undescribed evolutionary diversity. Thirteen of these lineages were found to be diagnosable by differences in morphology and consequently are described as new species. We also use our molecular-based phylogenies to examine the biogeographical history of the upper Sacramento River pebblesnails, and discuss geological and biological factors that may have contributed to the extensive diversification of this fauna. This paper is the second in a series on the systematics of aquatic snails of the upper Sacramento River basin (Hershler et al., 2003).
MATERIAL AND METHODS
Specimens
Samples from the upper Sacramento River basin were relaxed with menthol crystals, fixed in dilute (4%) formalin, and preserved in 70% ethanol for morphological study. Brief locality descriptions are provided in the taxonomic section, and are followed by UTM coordinates (all from Zone 10) and elevation (in parentheses), the date of collection, and the name(s) of the collector(s). Collector abbreviations are as follows: JC, Julian Colescott; TF, Terrence J. Frest; AH, Amy Hansen; EJ, Edward J. Johannes; JJ, James E. Johannes; JL, Jacquie S. Lee; SR, Stewart Reid; DS, Donald W. Sada. For additional locality details, see Frest & Johannes (1995a, 1997). The specimens used in this study are deposited at the National Museum of Natural History, Smithsonian Institution (USNM). Portions of these lots were also catalogued into the collections of Deixis Consultants, Seattle, Washington; a list of this material may be obtained from the third author (TJF).
Small subsamples from 82 of the 120 sites in which pebblesnails were found were directly preserved in 90–95% ethanol and utilized for DNA analysis. In order to evaluate the phylogenetic relationships of the upper Sacramento River basin pebblesnails, the nine currently recognized, extant species of Fluminicola–F. coloradensis Morrison, 1940, F. dalli (Call, 1884), F. fuscus (Haldeman, 1841), F. insolitusHershler, 1999, F. modoci, F. seminalis, F. turbiniformis (Tryon, 1865), F. virens, F. virginiusHershler, 1999– were also included in the DNA study.
DNA
Laboratory methods
Genomic DNA was extracted from individual snails using a CTAB protocol (Bucklin, 1992). Amplifications were conducted in a 25 µl total volume, containing 5 µl of Invitrogen optimizer buffer F (10 mm MgCl2, pH 9.0) for COI and buffer D (17.5 mm MgCl2, pH 8.5) for cytb, 2.5 µl of dNTPs (2.5 mm each), 1.25 µl of each primer (10 µm), 1 unit Taq polymerase, 1 µl of template (c. 100 ng double-stranded DNA), and 13.8 µl of sterile water. For the COI gene, COIL1490 and COIH2198 (Folmer et al., 1994) were used to amplify a c. 710 bp fragment. For the cytb gene, Cytb424F (5′TGA GGY GCY ACK GTT ATT ACT AA3′) and MUcytbR (5′AAN AGA AAR TAY CAY TCN GGY TG3′) were used to amplify a c. 430 bp fragment. Cytb424F and MUcytbR were designed based on a set of universal cytb primers, UCTYB151F and UCYTB270R (Merritt et al., 1998), and cytb sequences from two gastropods: Littorina saxatilis (GenBank 4165511) and Cepaea nemoralis (GenBank 5835415). Thermal cycling was performed with an initial denaturation for 2 min at 94 °C, followed by 35 cycles of 1 min at 94 °C, 1 min at the gene-specific annealing temperature, 2 min at 72 °C, with a final extension of 10 min at 72 °C. Annealing temperatures were 45–50 °C for COI, 50 °C for cytb. The amplified polymerase chain reaction product was cleaned using the exonuclease I/shrimp alkaline phosphatase method. Double-stranded DNA templates were incubated at 37 °C for 30 min and then at 85 °C for another 15 min with 5 U exonuclease I and 0.5 U shrimp alkaline phosphatase. Between 1 and 5 µl (c. 10–20 ng) of the cleaned polymerase chain reaction product was used as a template for cycle sequencing reactions in a 10 µl total volume with the CEQ DTCS Quick Start Kit (Beckman Coulter). The following cycling conditions were used: 96 °C for 2 min, then 35 cycles of 96 °C for 20 s, 45–50 °C for 20 s, and 60 °C for 4 min. The cycle-sequenced products were purified using an ethanol precipitation method following the Beckman Coulter protocol and separated by electrophoresis using a Beckman Coulter CEQ8000 sequencer. Sequences were determined for both strands and were edited and aligned using SEQUENCHER™ 3.1.1 (Gene Codes Corp.).
A survey of sequence variation within several upper Sacramento River samples revealed limited divergence ranging from 0 to 0.6% (0–4 bp) for COI and from 0 to 1.7% (0–6 bp) for cytb, e.g. Elk Springs (ten specimens, 0 bp for COI, 0 bp for cytb), site 30 (ten, 0 bp, 0–5 bp), site 65 (ten, 0–3 bp, 0–5 bp), site 68 (five, 0–2 bp, 0–6 bp), site 69 (five, 0–4 bp, 1–6 bp), and site 330 (four, 0–1 bp, 0 bp). On the basis of this limited amount of within-sample haplotype variation, we typically sequenced only two specimens per population and used one sequence from each in the final set of phylogenetic analyses. Sample localities, museum voucher numbers, and GenBank accession numbers for sequenced material are given in Table 1.
| Species (lineage) | Code | Locality | Voucher | COI | Cytb |
|---|---|---|---|---|---|
| F. fremonti (1) | D45aA | Hunters Spring, Lake Co., OR | 1020662 | AY962931 | AY963038 |
| F. fremonti (1) | D45aB | Hunters Spring, Lake Co., OR | 1020662 | AY962932 | AY963039 |
| F. warnerensis (2) | F13 210B | Spring creek east of Blue Lake, Modoc Co., CA | 1020655 | AY962995 | AY963101 |
| F. warnerensis (2) | F13 217A | Parsnip Spring, Modoc Co., CA | 1020653 | AY962996 | AY963102 |
| F. warnerensis (2) | F13 351B | Soup Spring, Modoc Co., CA | 1020654 | AY962997 | AY963103 |
| F. warnerensis (2) | SP360A | Springs south-east of Hilton, Modoc Co., CA | 1020657 | AY962999 | AY963105 |
| F. warnerensis (2) | FSP387F | Springs east of Miller Gulch, Modoc Co., CA | 1020790 | AY962998 | AY963104 |
| F. warnerensis (2) | SP388A | Miller Spring run, Modoc Co., CA | 1020659 | AY963000 | AY963106 |
| F. warnerensis (2) | F10 482A | Rush Creek (source), Modoc Co., CA | 1020658 | AY962994 | AY963100 |
| F. lunsfordensis (3) | F359A | Lunsford Spring, Modoc Co., CA | 1020689 | AY962935 | AY963041 |
| F. lunsfordensis (3) | F359B | Lunsford Spring, Modoc Co., CA | 1020689 | AY962936 | AY963042 |
| F. erosus (4) | F11 200B | Smokey Charlie Spring, Modoc Co., CA | 1020667 | AY962918 | AY963025 |
| F. erosus (4) | F12 200E | Smokey Charlie Spring, Modoc Co., CA | 1020667 | AY962920 | AY963027 |
| F. erosus (4) | F10 202E | Spring south-east of Smokey Charley Spring, Modoc Co., CA | 1020664 | AY962917 | AY963024 |
| F. erosus (4) | F11 202A | Spring south-east of Smokey Charley Spring, Modoc Co., CA | 1020664 | AY962919 | AY963026 |
| F. erosus (4) | F12 202A | Spring south-east of Smokey Charley Spring, Modoc Co., CA | 1020664 | AY962921 | AY963028 |
| F. favillaceus (5) | SP364B | Ash Creek at north culvert, Lassen Co., CA | 1020672 | AY962927 | AY963034 |
| F. favillaceus (5) | SP487A | Ash Creek above FS39N50 bridge, Lassen Co., CA | 1020673 | AY962928 | AY963035 |
| F. favillaceus (5) | SP509A | Chisolm Spring, Lassen Co., CA | 1020674 | AY962929 | AY963036 |
| F. favillaceus (5) | SP510A | Ash Creek, south culvert at Ash Valley Road crossing, Lassen Co., CA | 1020669 | AY962930 | AY963037 |
| F. caballensis (6) | SP400A | Bob Creek, Lassen Co., CA | 1020678 | AY962910 | AY963017 |
| F. caballensis (6) | FSP401A | Davis Creek, Lassen Co., CA | 1020677 | AY962911 | AY963018 |
| F. caballensis (6) | SP402A | Russell Dairy Spring, Lassen Co., CA | 1020679 | AY962912 | AY963019 |
| F. caballensis (6) | SP403B | Spring run west of Russell Dairy Spring, Lassen Co., CA | 1020680 | AY962913 | AY963020 |
| F. caballensis (6) | SP404A | Second spring west of Russell Dairy Spring, Lassen Co., CA | 1020681 | AY962914 | AY963021 |
| F. neritoides (7) | F9 369B | Willow Creek at mouth of Hayden Canyon, Lassen Co., CA | 1020685 | AY962953 | AY963059 |
| F. neritoides (7) | F9 371A | Willow Creek at lower end of Lower McBride Springs, Lassen Co., CA | 1020686 | AY962954 | AY963060 |
| F. neritoides (7) | F9 372A | Willow Creek west of Hayden Hill, Lassen Co., CA | 1020687 | AY962955 | AY963061 |
| F. ahjumawi (8) | sem93C | Lost Creek (middle site), Shasta Co., CA | 1020704 | AY962894 | AY963001 |
| F. ahjumawi (8) | F10116B | Hat Creek at Bridge Picnic Area, Shasta Co., CA | 1020693 | AY962895 | AY963002 |
| F. ahjumawi (8) | F10164A | Three springs on point opposite large island in Pit River, Shasta Co., CA | 1020695 | AY962896 | AY963003 |
| F. ahjumawi (8) | F10 195C | Spring run near Pit River Hatchery, Shasta Co., CA | 1020697 | AY962897 | AY963004 |
| F. ahjumawi (8) | sem340A | Honn Creek, Shasta Co., CA | 1020698 | AY962898 | AY963005 |
| F. ahjumawi (8) | F10 344B | Spring run north of Sam Wolfin Spring, Shasta Co., CA | 1020699 | AY962899 | AY963006 |
| F. ahjumawi (8) | FSP345E | Upper Sucker Springs Creek, Shasta Co., CA | 1020791 | AY962900 | AY963007 |
| F. ahjumawi (8) | sem347B | Spring west of Thousand Spring run, Shasta Co., CA | 1020692 | AY962901 | AY963008 |
| F. ahjumawi (8) | sem349A | West spring source of Mallard Creek, Shasta Co., CA | 1020700 | AY962902 | AY963009 |
| F. ahjumawi (8) | F10 405A | Beaver Creek, Lassen Co., CA | 1020701 | AY962903 | AY963010 |
| F. ahjumawi (8) | sem408C | Burney Creek, upstream of Burney Falls, Shasta Co., CA | 1020702 | AY962904 | AY963011 |
| F. ahjumawi (8) | SP476B | Jimmerson Spring, Modoc Co., CA | 1020703 | AY962905 | AY963012 |
| F. ahjumawi (8) | Fsem535A | Lost Creek near source spring, Shasta Co., CA | 1020797 | AY962906 | AY963013 |
| F. ahjumawi (8) | Fsem536A | Lost Creek (uppermost site), Shasta Co., CA | 1020798 | AY962907 | AY963014 |
| F. umbilicatus (9) | F8 92A | Lost Creek (lowermost site), Shasta Co., CA | 1020710 | AY962987 | AY963093 |
| F. umbilicatus (9) | F8 93B | Lost Creek (middle site), Shasta Co., CA | 1020711 | AY962988 | AY963094 |
| F. umbilicatus (9) | F7 99A | Big Spring, tributary of Hat Creek, Shasta Co., CA | 1020707 | AY962989 | AY963095 |
| F. umbilicatus (9) | F7 338A | Hat Creek at Hat Creek Resort, Shasta Co., CA | 1020716 | AY962990 | AY963096 |
| F. umbilicatus (9) | F8 536A | Lost Creek (uppermost site), Shasta Co., CA | 1020799 | AY962991 | AY963097 |
| F. anserinus (10) | F6 40B | Spring near Chalk Mountain, Shasta Co., CA | 1020735 | AY962984 | AY963090 |
| F. anserinus (10) | F15 270B | Spring, Goose Valley, Shasta Co., CA | 1020729 | AY962908 | AY963015 |
| F. anserinus (10) | F6 321A | Blackberry Creek, Shasta Co., CA | 1020733 | AY962985 | AY963091 |
| F. anserinus (10) | SP395A | Rim of the Lake Spring, Shasta Co., CA | 1020730 | AY962909 | AY963016 |
| F. potemicus (11) | F2 36A | Spring near Potem Creek, Shasta Co., CA | 1020720 | AY962956 | AY963062 |
| F. potemicus (11) | F2 36B | Spring near Potem Creek, Shasta Co., CA | 1020720 | AY962957 | AY963063 |
| F. scopulinus (12) | F14 251B | Northern-most spring south-west of Popcorn Spring, Shasta Co., CA | 1020723 | AY962958 | AY963064 |
| F. scopulinus (12) | F14 303B | Northern-most spring west of Popcorn Spring, Shasta Co., CA | 1020726 | AY962959 | AY963065 |
| F. multifarius (13) | SP8B | Spring near Conant, Siskiyou Co., CA | 1020772 | AY962939 | AY963045 |
| F. multifarius (13) | F2 10B | Ney Springs, Siskiyou Co., CA | 1020757 | AY962975 | AY963081 |
| F. multifarius (13) | F1 12B | Sacramento River near Stink Creek, Siskiyou Co., CA | 1020760 | AY962976 | AY963082 |
| F. multifarius (13) | F6 27B | Crystal Spring, Siskiyou Co, CA | 1020774 | AY962940 | AY963046 |
| F. multifarius (13) | F4 30A | Rock Spring, Siskiyou Co., CA | 1020777 | AY962941 | AY963047 |
| F. multifarius (13) | F5 30A | Rock Spring, Siskiyou Co., CA | 1020778 | AY962942 | AY963048 |
| F. multifarius (13) | F3 65A | Southern-most of Shasta Springs, Shasta Co., CA | 1020781 | AY962943 | AY963049 |
| F. multifarius (13) | F4 65A | Southern-most of Shasta Springs, Shasta Co., CA | 1020780 | AY962944 | AY963050 |
| F. multifarius (13) | F5 65A | Southern-most of Shasta Springs, Shasta Co., CA | 1020782 | AY962945 | AY963051 |
| F. multifarius (13) | FSP65F | Southern-most of Shasta Springs, Shasta Co., CA | 1020792 | AY962946 | AY963052 |
| F. multifarius (13) | F4 68A | Spring north of Mossbrae Falls, Shasta Co., CA | 1020783 | AY962947 | AY963053 |
| F. multifarius (13) | F3 69A | Spring runs north of Mossbrae Falls, Shasta Co., CA | 1020785 | AY962948 | AY963054 |
| F. multifarius (13) | F10 99B | Big Springs (source), north-west of city of Mount Shasta, Shasta Co., CA | 1020755 | AY962977 | AY963083 |
| F. multifarius (13) | F1 140A | Sacramento River at Cave Springs, Siskiyou Co., CA | 1020786 | AY962949 | AY963055 |
| F. multifarius (13) | F2 143A | Spring along Sacramento River (third to the) east of Cantara Bend, Siskiyou Co., CA | 1020762 | AY962978 | AY963084 |
| F. multifarius (13) | F2 144A | Spring along Sacramento River (first to the) east of Cantara Bend, Siskiyou Co., CA | 1020764 | AY962979 | AY963085 |
| F. multifarius (13) | SP237A | Big Springs, three middle runs, Siskiyou Co., CA | 1020765 | AY962980 | AY963086 |
| F. multifarius (13) | SP238B | Big Springs, western-most run, Siskiyou Co., CA | 1020767 | AY962981 | AY963087 |
| F. multifarius (13) | F10 239B | Big Springs, eastern-most run, Siskiyou Co., CA | 1020768 | AY962982 | AY963088 |
| F. multifarius (13) | SP241A | Big Springs at west side of park, Siskiyou Co., CA | 1020769 | AY962983 | AY963089 |
| F. multifarius (13) | F10 322B | Bundoora Spring, Siskiyou Co., CA | 1020771 | AY962950 | AY963056 |
| F. multifarius (13) | F3 330A | Spring run north of Crystal Spring, Siskiyou Co., CA | 1020788 | AY962951 | AY963057 |
| F. multifarius (13) | ELKSPB | Elk Spring (lowermost), Siskiyou Co., CA | 1020801 | AY962952 | AY963058 |
| Fluminicola sp. (A) | SP113A | Springs west of Canby, Modoc Co., CA | 1020796 | AY962922 | AY963029 |
| Fluminicola sp. (B) | SP373A | Spring at west end of Upper Rush Creek Campground, Modoc Co., CA | 1020793 | AY962923 | AY963030 |
| Fluminicola sp. (A) | SP373B | Spring at west end of Upper Rush Creek Campground, Modoc Co., CA | 1020793 | AY962924 | AY963031 |
| Fluminicola sp. (A) | F2SP373A | Spring at west end of Upper Rush Creek Campground, Modoc Co., CA | 1020793 | AY962925 | AY963032 |
| Fluminicola sp. (B) | F2SP373E | Spring at west end of Upper Rush Creek Campground, Modoc Co., CA | 1020793 | AY962926 | AY963033 |
| F. coloradensis | – | Green River above Warren Bridge, Sublette Co., WY | 905307 | AY962915 | AY963022 |
| F. dalli | – | Spring west of Thunderbolt Bay, Washoe Co., NV | 1009519 | AY962916 | AY963023 |
| F. fuscus | – | Grande Ronde River, Asotin Co., WA | 1082059 | DQ372901 | DQ372902 |
| F. insolitus | – | Page Springs, Harney Co., OR | 863507 | AY962934 | AY963040 |
| F. modoci | – | Spring at Three Springs Ranch, Modoc Co., CA | 1009518 | AY962938 | AY963044 |
| F. cf. modoci | – | Link River at Klamath Falls bridge, Klamath Co., OR | 1020714 | AY962937 | AY963043 |
| F. seminalis | sem98B | Big Lake outlet near Rat Farm, Shasta Co., CA | 1020736 | AY962960 | AY963066 |
| F. seminalis | sem104B | Baum Lake (deep-water site), Shasta Co., CA | 1020737 | AY962961 | AY963067 |
| F. seminalis | sem105A | Fall River at Caltrout Public Fishing Access Area, Shasta Co., CA | 1020783 | AY962962 | AY963068 |
| F. seminalis | sem329A | Baum Lake (shallow-water site), Shasta Co., CA | 1020739 | AY962963 | AY963069 |
| F. seminalis | D38A | Battle Creek east of Coleman Fish Hatchery, Shasta Co., CA | 1020740 | AY962964 | AY963070 |
| F. seminalis | sem364A | Ash Creek at north culvert, Lassen Co., CA | 1020741 | AY962965 | AY963071 |
| F. seminalis | SP366B | Ash Creek north-west of Ash Creek Campground, Lassen Co., CA | 1020742 | AY962966 | AY963072 |
| F. seminalis | sem411A | Crystal Springs, Shasta Co., CA | 1020743 | AY962967 | AY963073 |
| F. seminalis | sem416A | Big Lake Springs, Shasta Co., CA | 1020744 | AY962968 | AY963074 |
| F. seminalis | sem425A | Pit River near confluence of Hat Creek, Shasta Co., CA | 1020746 | AY962969 | AY963075 |
| F. seminalis | sem426A | Spring Creek on south side of Spring Creek Road, Shasta Co., CA | 1020747 | AY962970 | AY963076 |
| F. seminalis | sem487A | Ash Creek above FS39N50 bridge, Lassen Co., CA | 1020748 | AY962971 | AY963077 |
| F. seminalis | Fsem532A | Lava Creek at boathouse on Hanna Estate, Shasta Co., CA | 1020749 | AY962972 | AY963078 |
| F. seminalis | Fsem546A | Spring on west side of Spring Creek, Shasta Co., CA | 1020751 | AY962973 | AY963079 |
| F. seminalis | Fsem552A | Lava Creek source spring pool, Shasta Co., CA | 1020752 | AY962974 | AY963080 |
| F. turbiniformis | – | Roaring Springs, Harney Co., OR | 883506 | AY962986 | AY963092 |
| F. virens | – | Willamette River at Canby Ferry, Clackamas Co., OR | 1020713 | AY962992 | AY963098 |
| F. virginius | – | Hardscrabble Creek, Washoe Co., NV | 1004474 | AY962993 | AY963099 |
- COI, cytochrome c oxidase subunit I; cytb, cytochrome b.
Phylogenetic analysis
Several preliminary sequence analyses were conducted prior to the phylogenetic analysis. Base compositional differences were evaluated with the χ2 test. The partition homogeneity/incongruence length difference test (Farris et al., 1994) implemented in PAUP*4.0b10 (Swofford, 2002) was used to determine whether the two datasets were consistent and could be combined for the phylogenetic analysis. The test was conducted using parsimony-informative sites only and 500 replicates. The incongruence length difference test indicated no significant incongruence between COI and cytb (P = 0.1). Because no major incongruence was found, we performed both separate and combined analyses of the two datasets.
MODELTEST (Posada & Crandall, 1998) was used to obtain an appropriate substitution model and parameter values for distance, maximum likelihood (ML), and Bayesian analyses. The TIM + I + G model was selected as the best fit for the COI dataset using the Akaike information criterion (MODELTEST 3.7; Posada & Crandall, 1998), and the GTR + I + G model was selected for the cytb and combined datasets.
Phylogenetic trees based on distance, parsimony, and ML methods were generated using PAUP* 4.0b10. A Bayesian analysis using MrBayes 3.04 (Huelsenbeck & Ronquist, 2001) was also performed as another means of estimating phylogeny. GTR distances were used to generate neighbour-joining (NJ) trees based on the clustering method of Saitou & Nei (1987). Node support was assessed by completion of 10 000 bootstrap replications (Felsenstein, 1985) in PAUP using the fast-search option. Maximum parsimony (MP) analyses were conducted with equal weighting using the heuristic search option with tree bisection reconnection branch swapping, 100 replications of random stepwise additions, and MAXTREES set to 10 000. Bootstrapping with 10 000 replications (as implemented in PAUP) was used to evaluate node support. ML analyses were based on the GTR + I + G model with empirical base frequencies using the heuristic search algorithm. A NJ tree (GTR distances) was used as the initial topology for branch swapping. Node support was evaluated by 100 bootstrap pseudo-replicates.
Bayesian analyses of the COI, cytb, and combined datasets were also based on the GTR + I + G model. Several short runs were first conducted using the default random tree option to determine when the log likelihood sum reached a stable value (by plotting the log likelihood scores of sample points against generation time). For all three datasets, the log likelihood values converged upon a stable state after approximately 50 000 generations. Metropolis-coupled Markov chain Monte Carlo simulations were then run with four chains using the default random tree option for 1 000 000 generations, and Markov chains were sampled at intervals of ten generations to obtain 100 000 sample points. The last 95 000 sampled trees with branch lengths (the first 5000 trees, equal to 50 000 generations, having been removed as ‘burn-in’) were used to generate a 50% majority rule consensus tree. The percentage of samples that recovered a specific clade on this topology represented the clade’s posterior probability; these were P-values and P ≥ 95% was considered evidence of significant support (Huelsenbeck & Ronquist, 2001).
Preliminary COI analyses with European Lithoglyphus naticoides (Pfeiffer, 1828) used as the root consistently depicted F. virens, which is the most morphologically divergent member of the genus (Hershler & Frest, 1996), as basally positioned relative to other congeners. Because we do not have cytb sequences for L. naticoides or representatives of other lithoglyphid genera, we used divergent F. virens as the root in our final analyses.
Pairwise sequence divergence and mean genetic distances were calculated using MEGA version 3.0 (Kumar, Tamura & Nei, 2004). In order to estimate divergence times of lineages, we calibrated a molecular clock for Fluminicola based on the divergence of F. virginius, which is endemic to a spring in the Virginia Mountains c. 800 m above Pyramid Lake (Hershler, 1999). Recent geological studies suggest that in this portion of Nevada, faulting that began approximately 3.0 Ma resulted in the production of the present-day basin and range landscape (Trexler et al., 2000; Henry & Perkins, 2001; Henry, Faulds & dePolo, 2006). Using this estimate of the inception of range uplift and the 5.78 ± 0.83% COI divergence of F. virginius relative to its sister species, F. dalli (see below), a clock rate of 1.93 ± 0.28% per million years is implied. This rate, which should be considered a maximum estimate as divergence of these two species could have taken place prior to range uplift, closely approximates the 1.83 ± 0.21% per million years COI divergence that was previously derived for European hydrobiid snails (Wilke, 2003).
Morphology and taxonomy
The methods used for the morphological study were generally those of Hershler (1998). Counts and measurements (in mm) of the following parameters were obtained for the holotype and up to 31 paratypes of each new species and are summarized in Tables 3–15: total shell whorls (WH), shell height (SH), shell width (SW), height of body whorl (HBW), width of body whorl (WBW), aperture height (AH), aperture width (AW). For paratypes, the parameters listed are the mean (above) and the range of values (below). Shell data were analysed using SYSTAT 10.2.01. t-tests were utilized in some instances to facilitate shell comparisons between closely related species and were based on paratype data (unless otherwise indicated) provided in the systematics section. The study of shells, opercula, and radulae using a scanning electron microscope was generally restricted to specimens from the type series of each new species. Radular tooth cusp counts were obtained from five specimens from each sample studied. For each specimen, cusp counts for each tooth type (on both sides of the tooth row) were obtained from single tooth rows near the anterior, middle, and posterior portion of the radular ribbon, thereby providing a consistent and relatively detailed survey of variation in cusp number. Snail anatomy was studied in dissection for five to ten specimens of each sex of each new species, again generally from the type series only. Although the focus was on reproductive characters that were previously shown to constitute the main source of anatomical variation within the genus (Hershler & Frest, 1996), other features were also examined. Morphological terminology follows that of Hershler & Frest (1996) and Hershler & Ponder (1998).
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.75 | 3.57 | 3.05 | 3.10 | 2.68 | 1.86 | 1.83 |
| Paratypes (N = 31) | 3.50 | 3.03 | 2.66 | 2.58 | 2.23 | 1.66 | 1.57 |
| *N = 26 | 3.25–3.75* | 2.70–3.98 | 2.37–3.23 | 2.31–3.27 | 1.99–2.77 | 1.46–2.03 | 1.39–2.00 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.75 | 3.43 | 2.87 | 2.91 | 2.37 | 1.93 | 1.70 |
| Paratypes (N = 30) | 3.50 | 3.67 | 3.08 | 3.14 | 2.52 | 2.02 | 1.85 |
| 3.25–3.75 | 3.35–4.44 | 2.71–3.47 | 2.86–3.80 | 2.29–2.91 | 1.81–2.31 | 1.65–2.20 | |
| F. turbiniformis | |||||||
| USNM 883470 (N = 30) | 3.43 | 2.97 | 2.44 | 2.62 | 2.08 | 1.60 | 1.54 |
| 3.00–3.75 | 2.67–3.40 | 2.21–2.71 | 2.35–2.98 | 1.90–2.28 | 1.42–1.86 | 1.39–1.77 | |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.75 | 3.96 | 3.38 | 3.55 | 2.82 | 2.26 | 2.14 |
| Paratypes (N = 28) | 3.55 | 3.41 | 2.92 | 3.00 | 2.33 | 2.00 | 1.81 |
| 3.25–4.0 | 2.95–4.48 | 2.59–3.57 | 2.63–3.86 | 2.00–2.88 | 1.73–2.54 | 1.58–2.30 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | * | 2.38 | 2.06 | 2.38 | 1.81 | 1.45 | 1.38 |
| Paratypes (N = 6) | 3.46 | 2.33 | 1.88 | 1.89 | 1.60 | 1.18 | 1.09 |
| *spire eroded | 3.25–3.5 | 2.22–2.47 | 1.76–1.99 | 1.82–1.98 | 1.54–1.69 | 1.09–1.25 | 1.03–1.18 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.5 | 3.13 | 2.63 | 2.68 | 2.09 | 1.74 | 1.59 |
| Paratypes (N = 19) | 3.37 | 2.98 | 2.51 | 2.56 | 2.03 | 1.66 | 1.43 |
| 3.0–3.75 | 2.52–3.36 | 2.25–2.69 | 2.25–2.84 | 1.77–2.33 | 1.54–1.82 | 1.26–1.59 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.5 | 3.32 | 3.08 | 2.87 | 2.39 | 2.04 | 1.85 |
| Paratypes (N = 30) | 3.51 | 3.40 | 2.92 | 2.88 | 2.30 | 1.97 | 1.78 |
| 3.25–4.0 | 2.96–4.35 | 2.65–3.48 | 2.46–3.48 | 2.00–2.90 | 1.71–2.25 | 1.56–2.05 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | * | 2.46 | 2.64 | 2.32 | 1.66 | 1.81 | 1.70 |
| Paratypes (N = 30) | 2.87 | 2.62 | 2.53 | 2.46 | 1.73 | 1.80 | 1.63 |
| *spire eroded | 2.25–3.25 | 2.28–3.02 | 2.32–2.91 | 2.13–2.72 | 1.47–1.99 | 1.63–1.98 | 1.49–1.85 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.5 | 3.27 | 3.11 | 2.82 | 2.29 | 2.04 | 1.83 |
| Paratypes (N = 21) | 3.38 | 2.87 | 2.68 | 2.43 | 2.09 | 1.72 | 1.62 |
| 3.25–3.50 | 2.53–3.18 | 2.48–2.96 | 2.18–2.68 | 1.87–2.27 | 1.53–1.96 | 1.50–1.83 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.5 | 3.32 | 2.94 | 2.89 | 2.54 | 1.88 | 1.81 |
| Paratypes (N = 30) | 3.78 | 4.46 | 3.79 | 3.88 | 3.14 | 2.40 | 2.19 |
| 3.5–4.25 | 3.67–5.41 | 3.39–4.20 | 3.27–4.58 | 2.67–3.73 | 2.11–2.67 | 1.87–2.47 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.5 | 3.08 | 2.60 | 2.72 | 2.09 | 1.74 | 1.66 |
| Paratypes (N = 30) | 3.37 | 2.90 | 2.57 | 2.54 | 2.04 | 1.65 | 1.54 |
| 3.25–3.5 | 2.75–3.22 | 2.40–2.76 | 2.42–2.78 | 1.88–2.18 | 1.41–1.80 | 1.43–1.63 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | 3.25 | 2.67 | 2.27 | 2.23 | 1.91 | 1.46 | 1.36 |
| Paratypes (N = 30) | 3.43 | 2.78 | 2.35 | 2.36 | 1.94 | 1.53 | 1.40 |
| 3.0–3.75 | 2.55–3.31 | 2.19–2.82 | 2.15–2.84 | 1.82–2.28 | 1.37–1.91 | 1.24–1.73 |
| WH | SH | SW | HBW | WBW | AH | AW | |
| Holotype | 3.5 | 2.78 | 2.52 | 2.37 | 2.11 | 1.52 | 1.40 |
| Paratypes (N = 18) | 3.60 | 2.83 | 2.49 | 2.42 | 2.09 | 1.52 | 1.40 |
| *N = 17 | 3.25–4.0* | 2.56–3.42 | 2.31–2.77 | 2.22–2.89 | 1.90–2.37 | 1.41–1.81 | 1.29–1.55 |
| WH | SH | SW | HBW | WBW | AH | AW | |
|---|---|---|---|---|---|---|---|
| Holotype | * | 3.5 | 3.04 | 3.21 | 2.53 | 2.07 | 2.00 |
| Paratypes (N = 15) | 3.52 | 3.55 | 2.99 | 3.05 | 2.41 | 2.01 | 1.89 |
| *spire eroded | 3.5–3.75 | 3.19–3.71 | 2.86–3.19 | 2.83–3.23 | 2.28–2.57 | 1.86–2.20 | 1.70–2.03 |
In order to resolve the taxonomy of these morphologically conservative snails, we used sequence data to identify populations or groups of populations that are phylogenetically independent and substantially divergent. Our methodology closely followed that utilized by García-París & Wake (2000). In those cases in which such lineages were also found to be diagnosable based on morphology, we describe them as new species. In other cases in which lineages appear to be morphologically cryptic, we chose not to describe these as new species as we believe that additional research is needed to confidently assess their evolutionary and taxonomic status. Nonetheless, we acknowledge that several of the pebblesnails described herein may ultimately merit subdivision into multiple taxonomic units that can be diagnosed on the basis of molecular data (fideBond & Sierwald, 2003). Common names are proposed for each new species. Most of the species treated herein have a lengthy record of mention (informally as ‘n. sp.’ or ‘new species’) in agency and consulting reports. A detailed compilation of these citations may be obtained from the third author (TJF). For the sake of brevity, herein we only cite (in the ‘Remarks’ sections) the upper Sacramento River field survey reports in which these snails were first noted.
RESULTS
The alignment of COI sequences yielded 658 bp, of which 224 sites were variable (34.0%) and 188 were parsimony informative (28.57%). Average base frequencies for COI were 27.5% A, 34.1% T, 19.7% C, and 18.7% G. Base frequencies were homogeneous across all sites (χ2 = 36.54, d.f. = 318, P = 1.0). For cytb, 361 bp were sequenced, of which 161 were variable (44.6%) and 132 were parsimony informative (35.6%). Average base frequencies for cytb were 30.0% A, 36.3% T, 20.4% C, and 14.3% G. Base frequencies were homogeneous across all sites (χ2 = 56.46, d.f. = 318, P = 1.0).
Pairwise sequence divergence (uncorrected p distance) among the currently recognized species of pebblesnails ranged from 4.1 to 18.8% (COI) and 4.4 to 25.7% (cytb). F. virens, which was previously shown to be morphologically divergent relative to other congeners (Hershler & Frest, 1996), had the highest levels of sequence divergence, ranging from 17.3 to 18.8% (COI) and 19.1 to 25.6% (cytb). The two previously described species that live in the upper Sacramento River basin, F. modoci and F. seminalis, were among the least divergent pairs of congeners (5.0–6.1% COI; 6.4–8.0% cytb).
Separate and combined NJ, MP, ML, and Bayesian analyses were highly concordant and thus only representative (Bayesian) trees for COI, cytb, and the combined dataset are illustrated (2-4, respectively). The resulting trees were well structured and delineated 13 genetically differentiated (sequence divergence > 2% for both genes in most cases) and morphologically distinctive upper Sacramento River lineages (2-4: 1–13) that do not correspond to previously described species. The mean pairwise sequence divergence within and among these lineages and bootstrap support values (for ML, MP, and NJ analyses) are summarized in Table 2. Lineages 10 and 13 had weak bootstrap support values of < 50% (Table 2) and a Bayesian posterior probability of < 73% in the COI analyses. Lineage 6 was not depicted as monophyletic in any of the cytb analyses (e.g. Fig. 3). Lineage 8 had weak support in the cytb NJ analysis, and did not form a monophyletic group in the other cytb analyses (e.g. Fig. 3). However, all 13 lineages were depicted as monophyletic groups with strong bootstrap support and posterior probability values in each of the combined analyses (Table 2, 2-4).
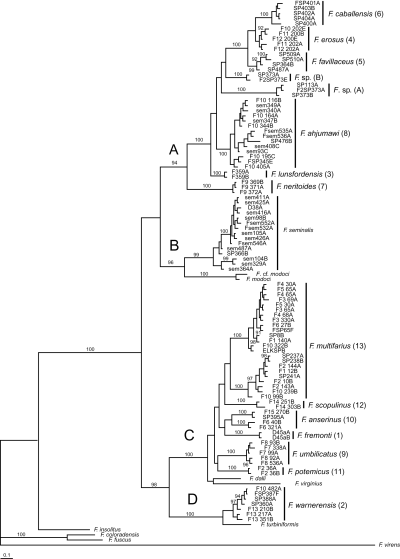
Bayesian phylogram for cytochrome c oxidase subunit I sequences. Upper Sacramento River basin haplotypes are distributed among clades A–D. Posterior probability values ≥ 90% are shown. Upper Sacramento River basin lineages newly discovered in this study are highlighted by the larger font. Specimen codes are from Table 1.
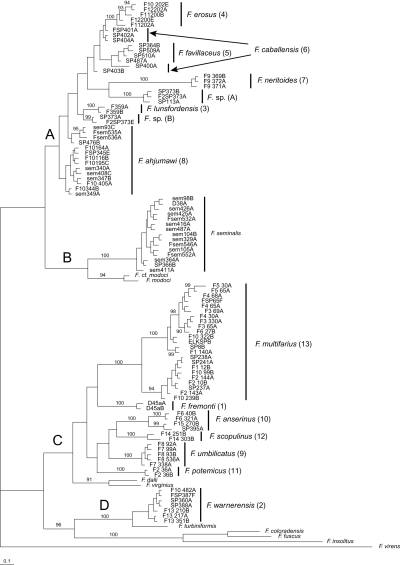
Bayesian phylogram for cytochrome b sequences. Upper Sacramento River basin haplotypes are distributed among clades A–D. Posterior probability values ≥ 90% are shown. Upper Sacramento River basin lineages newly discovered in this study are highlighted by the larger font. Specimen codes are from Table 1.
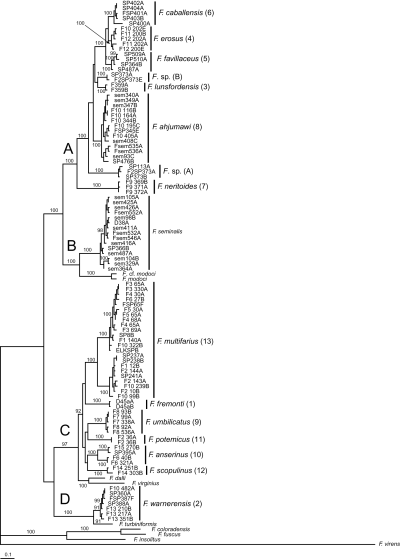
Bayesian phylogram for the combined dataset (cytochrome c oxidase subunit I and cytochrome b). Upper Sacramento River basin haplotypes are distributed among clades A–D. Posterior probability values ≥ 90% are shown. Upper Sacramento River basin lineages newly discovered in this study are highlighted by the larger font. Specimen codes are from Table 1.
| Species (lineage) | N | COI divergence | Support | cytb Divergence | Support | Combined Support |
|---|---|---|---|---|---|---|
| F. fremonti (1) | 2 | 3.7–17.0% (0.0 ± 0.0%) | 100/100/100 | 5.7–21.2% (0.3 ± 0.3%) | 99/100/100 | 100/100/100 |
| F. warnerensis (2) | 7 | 2.52–18.45% (0.3 ± 0.2%) | 55/91/88 | 3.7–21.1% (0.1 ± 0.1%) | */99/96 | 81/100/99 |
| F. lunsfordensis (3) | 2 | 2.2–17.0% (0.0 ± 0.0%) | 100/100/98 | 2.6–19.4% (0.3 ± 0.3%) | 93/97/95 | 100/100/100 |
| F. erosus (4) | 5 | 1.6–17.6% (0.3 ± 0.1%) | 57/92/65 | 1.8–21.1% (0.7 ± 0.3%) | 94/91/91 | 99/100/98 |
| F. favillaceus (5) | 4 | 1.4–17.1% (0.4 ± 0.2%) | 89/76/86 | 1.6–19.5% (0.3 ± 0.2%) | 57/61/* | 94/98/95 |
| F. caballensis (6) | 5 | 1.6–17.7% (0.3 ± 0.1%) | 97/90/98 | 1.6–20.1% (1.0 ± 0.3%) | ♯/♯/♯ | 92/84/87 |
| F. neritoides (7) | 3 | 4.9–17.6% (0.0 ± 0.0%) | 99/100/100 | 7.9–20.8% (0.0 ± 0.0%) | 100/100/100 | 100/100/100 |
| F. ahjumawi (8) | 14 | 2.2–16.9% (0.5 ± 0.2%) | 86/96/87 | 2.3–20.1% (0.5 ± 0.2%) | ♯/*/♯ | 93/98/90 |
| F. umbilicatus (9) | 5 | 3.6–16.7% (0.2 ± 0.1%) | 97/100/100 | 5.3–21.3% (0.0 ± 0.0%) | 96/100/100 | 98/100/100 |
| F. anserinus (10) | 4 | 3.4–17.6% (1.8 ± 0.5%) | */*/* | 5.7–21.1% (1.8 ± 0.4%) | 96/100/99 | 98/100/99 |
| F. potemicus (11) | 2 | 3.6–17.8% (0.0 ± 0.0%) | 99/100/100 | 5.5–21.6% (0.0 ± 0.0%) | 100/100/100 | 100/100/100 |
| F. scopulinus (12) | 2 | 4.0–17.6% (0.5 ± 0.3%) | 99/100/100 | 5.3–21.2% (1.1 ± 0.5%) | 90/100/98 | 93/100/100 |
| F. multifarius (13) | 23 | 3.4–17.4% (1.4 ± 0.3%) | */*/51 | 7.6–23.0% (2.4 ± 0.5%) | 95/88/92 | 93/94/97 |
| F. coloradensis | 1 | 4.1–17.8% | – | 5.9–25.7% | – | – |
| F. dalli | 1 | 3.8–17.5% | – | 4.4–20.6% | – | – |
| F. fuscus | 1 | 4.1–18.1% | – | 5.9–25.5% | – | – |
| F. insolitus | 1 | 9.1–17.5% | – | 13.3–23.3% | – | – |
| F. modoci | 21 | 5.6–17.6% (0.9 ± 0.4%) | 98/100/100 | 6.7–19.4% (1.1 ± 0.5%) | 84/100/99 | 99/100/100 |
| F. seminalis | 15 | 5.6–18.7% (0.6 ± 0.2%) | 70/100/100 | 6.8–21.1% (0.3 ± 0.1%) | 79/100/100 | 88/100/100 |
| F. turbiniformis | 1 | 2.5–17.5% | – | 3.7–21.9% | – | – |
| F. virginius | 1 | 5.5–18.2% | – | 4.4–18.8% | – | – |
| F. virens | 1 | 16.7–18.7% | – | 18.8–25.7% | – | – |
- COI, cytochrome c oxidase subunit I; cytb, cytochrome b.
- N = number of sequenced specimens used in the phylogenetic analyses.
- * < 50% support; ♯nonmonophyletic.
- 1Includes F. cf. modoci specimen.
Upper Sacramento River lineages were consistently depicted as a polyphyletic assemblage of four well-supported clades (A–D, 2-4). Clade A contains six lineages (3–8) that are distributed in the Pit River basin. Five of these lineages (3–7) share a uniquely elongate basal tongue of the central radular tooth. Clade A also contains an enigmatic, conchologically variable (Fig. 5) population living in the Rush Creek drainage (site 373), Pit River basin, which was studied in some detail. Among the 25 snails sequenced from this population, 33 variable sites were observed for COI, resulting in five haplotypes; 19 variable sites were observed for cytb, resulting in five haplotypes. These were distributed among two clades, one of which also contained haplotypes from a morphologically distinct population living along the Pit River (site 113). The two clades, Fluminicola sp. (A) and Fluminicola sp. (B) (2-4), were nonsister in all analyses and were substantially differentiated from one another by 4.7–5.0% (31–33 bp) for COI and 4.4–5.0% (16–18 bp) for cytb. Within each clade, haplotypes only differed by 0–0.3% (0–2 bp) for COI and 0–0.6% (0–2 bp) for cytb. (The two sequenced specimens from site 113 shared a COI haplotype with site 373 specimens, whereas their cytb sequence differed from one of the site 373 haplotypes by a single bp.) Although our data suggest that two evolutionarily distinct lineages are present at the Rush Creek site, we were unable to confidently sort these on the basis of their shells, nor have we been able to find other diagnostic morphological features. This confusing pattern may reflect incipient speciation, incomplete lineage sorting, or secondary contact and merits additional study.
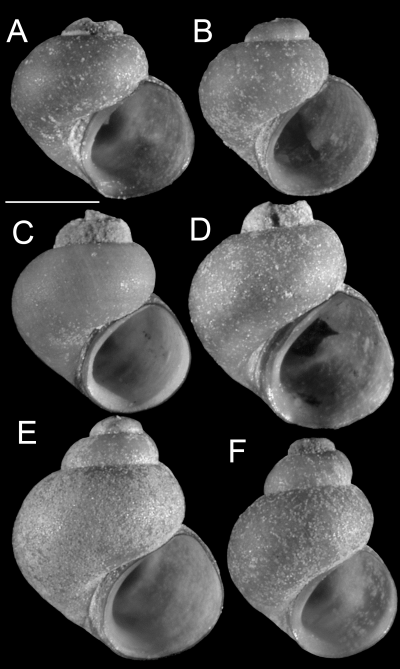
Shell variation within the Rush Creek population (Fluminicola sp., site 373). A, E, F, USNM 1020795. B, C, USNM 1020793. D, USNM 1020794. Scale = 1.0 mm.
Clade B contains populations of F. seminalis, which formed a well-supported (70–100%) monophyletic unit that was sister to a similarly well-supported (84–100%) subclade composed of F. modoci and an undescribed (and possibly conspecific) population from the Klamath Lake basin (F. cf. modoci). Clade C is composed of six lineages that were usually depicted as sister to a subclade composed of two Great Basin species (F. dalli, F. virginius). Two of these lineages (9, 12) share a large shell umbilicus that is unique within the genus. Lineages 10 and 13 are both composed of allopatric, genetically divergent (> 2% for both genes) subclades that are not morphologically distinguishable. Clade D is composed of lineage 2, which is distributed in the upper portion of the Pit River basin, and its sister, F. turbiniformis, a Great Basin species. In some analyses, clade D was depicted as sister to clade C, with the three remaining congeners (F. coloradensis, F. fuscus, F. insolitus) occupying a more basal position (2, 4), whereas in others clade D was sister to a clade composed of the latter three species (Fig. 3).
DISCUSSION
Taxonomic implications
Analysis of the mitochondrial DNA sequence data revealed 13 divergent and phylogenetically independent upper Sacramento River lineages that are morphologically diagnosable and do not correspond to previously described species. Although most of the lineages are distributed allopatrically, lineage 8 is sympatric with F. seminalis or with lineage 9 at one or more sites, and lineage 5 is sympatric with F. seminalis at another site. The sequence divergence for most of the 13 lineages was > 2% for both genes (Table 2). COI sequence divergence among these 13 lineages is within the range observed among congeners of other North American freshwater rissooidean gastropods (e.g. Tryonia, 1.3–14.8%, Hershler, Liu & Mulvey, 1999; Pyrgulopsis, 1.1–13.1%, Liu & Hershler, 2005), but is generally less than that of previously described pebblesnail species (Table 2). However, the latter are widely scattered within a much larger region than our study area (Hershler & Frest, 1996: figs 11, 14) and presumably have been long isolated from each other. Consequently, it may be expected that the sequence divergence between these species will be greater than among congeners inhabiting a single watershed, such as the upper Sacramento River drainage. Application of the COI clock for Fluminicola (1.93% per million years; see Methods section) suggests that the upper Sacramento River basin lineages have been minimally isolated for 0.72–2.90 million years. Based on our morphological and genetic evidence, we conclude that each of these 13 lineages merits recognition as a distinct species new to science using the evolutionary species concept that we advocate (see Methods section) as well as other currently utilized operative definitions of this taxonomic category (summarized in Mayden, 1997; Wheeler & Meier, 2000). Although it is possible that several of these lineages may be composed of multiple cryptic species (notably lineage 13), we chose not to describe additional new taxa at this time solely based on genetic evidence.
Our finding of a large number of new species within a single drainage basin suggests that other northwestern North American watersheds may also contain substantial undescribed diversity of this geologically ancient group of snails. Inasmuch as a reasonably complete taxonomic study of Fluminicola will not be available for many years, it may be appropriate in the meanwhile for land managers to provisionally treat populations or groups of populations that are isolated by dry land or by reaches of unsuitable aquatic habitat as distinct conservation units, especially when these snails are also shown to be morphologically distinctive.
Evolutionary considerations
Our findings provide some insight into the evolutionary development of the upper Sacramento River pebblesnails, although this topic should be revisited following similar studies of the largely undescribed faunas of adjacent regions (e.g. Frest & Johannes, 1995b). The highly endemic upper Sacramento River pebblesnail fauna does not form a monophyletic unit consistent with recognition as a single species flock (Fig. 4). In two major clades (C, D), upper Sacramento River species form monophyletic subunits that are sister to western Great Basin pebblesnails, which parallels previously hypothesized close relationships between the fish faunas of these areas (see Minckley, Hendrickson & Bond, 1986). Clade D is composed of two species whose closely proximal ranges (F. warnerensis, Fig. 9; F. turbiniformis, Hershler, 1989, Fig. 7) are separated by the Warner Mountains, which form the eastern flank of the South Fork Pit River Valley (Fig. 1). Our data indicate that these two species diverged approximately 1.27 Ma (COI sequence divergence, 2.45%), and we suggest that this vicariance may have resulted from the large amount of uplift and tilting of the Warner Mountains that occurred in the past 3 million years (Fosdick et al., 2005; Markman et al., 2005; Carmichael et al., 2006). Clade C contains subclades composed of two species from the Pyramid Lake basin in western Nevada (F. dalli, F. virginius) and six Sacramento River basin species that, with one exception (F. fremonti, Goose Lake basin), are distributed in the lower portion of this drainage (Fig. 20). The estimated 2.60 Ma divergence (COI sequence divergence, 5.0%) of these broadly disjunct subclades may have also resulted from tectonic development of the topographic boundary between the Modoc Plateau and northwestern Great Basin, which in some areas began as early as the late Miocene (Colgan, Dumitru & Miller, 2005).
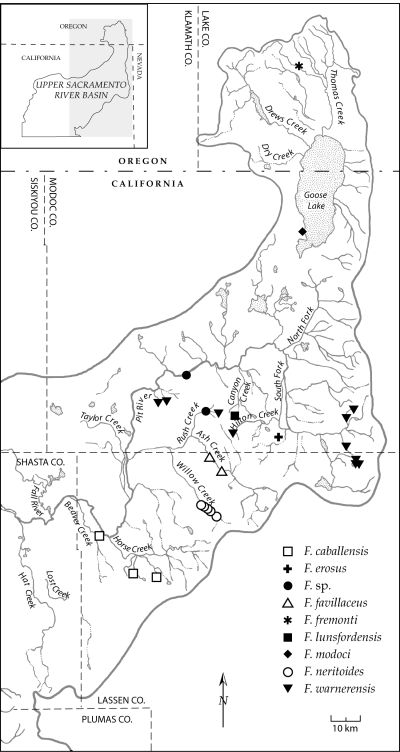
Map showing the distribution of eight species (plus Fluminicola sp.) in the Goose Lake and Pit River basins. Some symbols represent multiple, closely proximal localities.
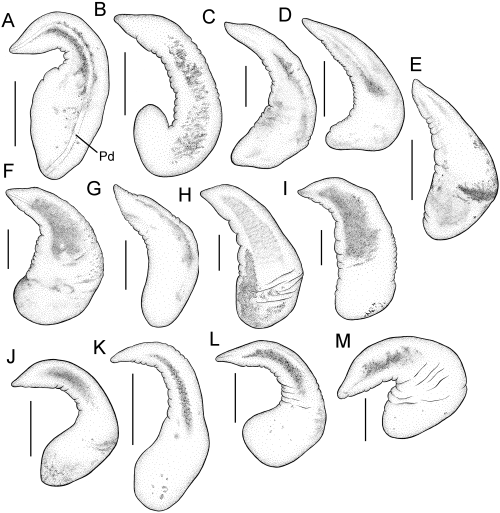
Penes (upper side) of the 13 Fluminicola species newly described herein. A, F. fremonti (USNM 1020661). B, F. warnerensis (USNM 1020653). C, F. lunsfordensis (USNM 1020689). D, F. erosus (USNM 1020664). E, F. favillaceus (USNM 1020670). F, F. caballensis (USNM 1020676). G, F. neritoides (USNM 883563). H, F. ahjumawi (USNM 1020699). I, F. umbilicatus (USNM 1020706). J, F. anserinus (USNM 1020728). K, F. potemicus (USNM 1020719). L, F. scopulinus (USNM 1020722). M, F. multifarius (USNM 883782). Scale = 0.25 mm. Pd, penial duct.
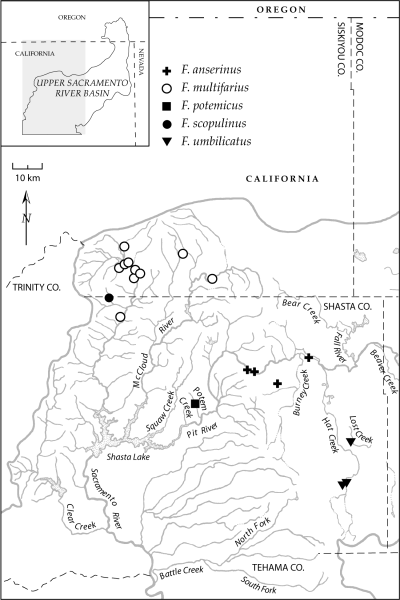
Map showing the distribution of five species in the lower Pit River and McCloud River basins and Sacramento River headwaters. Some symbols represent multiple, closely proximal localities.
A third clade (B, Fig. 4) shows a different pattern of extra-limital relationships in which a species from the Goose Lake basin (F. modoci), which had an occasional stream connection with the upper Pit River basin during the late Quaternary, is sister to an undescribed (and possibly conspecific) population from the Klamath River basin (F. cf. modoci), with an estimated 0.47 Ma (COI sequence divergence, 0.91%) age of divergence. Pease (1965) suggested on the basis of geological evidence that the upper portion of the Pit River basin (Alturas and Goose Lake basins) drained to the north-west (towards the Klamath River basin) during the late Pliocene and Pleistocene. He further speculated that this connection was blocked by the eruption of the Garden Basalt, which formed a broad plateau extending north of Alturas and west of the Goose Lake basin (Fig. 1), with the upper Pit River subsequently developing its modern westward course to the Sacramento River. Hubbs & Miller (1948; also see Robins & Miller, 1957) proposed a Pleistocene stream connection between the Goose and Klamath Lake basins based on ichthyological evidence that was contested by Minckley et al. (1986), who suggested that the former fauna was instead derived from that of the Pit River. The Garden Basalt is now known to be late Miocene to early Pliocene in age (e.g. McKee, Duffield & Stern, 1983), which seemingly precludes the possibility of westward drainage across this area from the Goose Lake basin during the Quaternary. Nonetheless, our finding, which is paralleled by recent molecular studies demonstrating 0.2 Ma divergence of the lamprey fish faunas of the Goose and Klamath lake basins (M. F. Docker, pers. comm.), provides compelling evidence of some form of geologically recent drainage integration or transfer between these areas.
The evolutionary origin of the pebblesnails of the Goose Lake basin is of additional interest because this area is considered to have had only a recent (late Quaternary), intermittent connection with the rest of the upper Sacramento River basin (Phillips & Van Denburgh, 1971). The substantial COI differentiation of both the F. modoci–F. cf. modoci clade and a second Goose Lake basin pebblesnail (F. fremonti) with respect to their upper Sacramento River basin sister taxa (F. seminalis, 5.39–5.88%, 2.79–3.05 Ma; F. multifarius, 4.23%, 2.19 Ma, respectively) nonetheless suggests a divergence event that preceded the recent integration of these areas.
Diversification of pebblesnails within the upper Sacramento River basin may be attributed to several aspects of regional geological history. The volcanic eruptions that formed the main axis of the southern High Cascades during the Pliocene and Quaternary (Christiansen, 1985) may have played a role in isolating ancestral fauna in the western part of the study area (McCloud and Sacramento Rivers). The two species that are endemic to this region minimally diverged in the late Pliocene or earliest Pleistocene (Table 2, F. multifarius, 3.4% COI divergence, 1.76 Ma; F. scopulinus, 4.0% COI divergence, 2.07 Ma), consistent with the age of this prominent topographical feature. Another defining barrier may have been introduced by the tectonic development of the Hat Creek and McArthur grabens, which are bounded by major normal faults that are down dropped to the west and have steep scarps that displace Pliocene to Pleistocene basalt (Wills, 1991). This fault zone has been interpreted as the northern portion of a 400 km long trough that extends from Lake Tahoe to the Oregon state line (Page et al., 1993; Page & Renne, 1994; Page, 1995). The distributions of clades A and C (9, 17) correspond in large part to the two regions separated by this fault zone, and their estimated 3.80–3.98 Ma age of divergence is consistent with its Pliocene inception. Additional opportunities for pebblesnail differentiation were surely created by the repeated interruption, ponding, and shifting of Modoc Plateau drainage that resulted from basaltic volcanism and extensional faulting in the late Cenozoic (Pease, 1965).
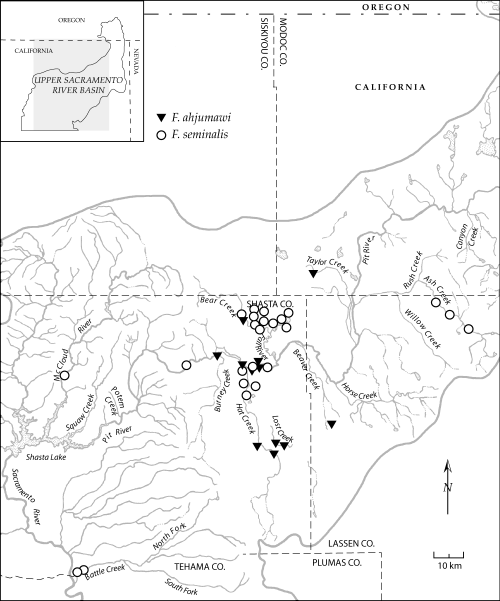
Map showing the distribution of Fluminicola ahjumawi and F. seminalis in the upper Sacramento River basin. Some symbols represent multiple, closely proximal localities.
The evolutionary diversification of this fauna has also been strongly influenced by pebblesnail ecology. Most of the upper Sacramento River basin species are restricted to spring influenced habitats and all but one (F. warnerensis) of these are narrowly distributed. This pattern of high diversity and local endemism in spring environments is shared by rissooidean gastropod faunas elsewhere in North America (e.g. Thompson, 1968; Hershler, 1998) and in other parts of the world (e.g. Ponder, Hershler & Jenkins, 1989; Haase & Bouchet, 1998). The frequent restriction of snail species to a single spring or spring complex can be attributed in part to the low physical connectivity of these habitats, whose outflows frequently disappear into the ground without connecting to other water bodies. This feature is especially pronounced in arid regions and is further enhanced in the Modoc Plateau owing to its highly permeable, volcanic landscape (Rose, Davisson & Criss, 1996). Even in situations where springs are connected either with each other or with other water bodies by stream outflows, genetic isolation of snail populations may still occur as a result of physiological specialization to unique, stable headspring environments and an associated inability to disperse and/or survive more than a short distance downflow (e.g. O’Brien & Blinn, 1999; Ponder & Colgan, 2002). In contrast with the typically narrow distributions of spring-dwelling pebblesnails of the upper Sacramento River basin, all three species that were collected from regional rivers or large streams (F. ahjumawi, F. multifarius, F. seminalis) have broad ranges that span multiple hydrographic units (17, 20). The relatively shallow divergence of populations within these species (e.g. F. seminalis, COI sequence divergence 0.6%, 0.31 Ma) suggests that their wide distributions may have resulted from geologically recent dispersal or contemporary gene flow within integrated streams and rivers.
SYSTEMATICS
Family Lithoglyphidae Troschel, 1857GenusFluminicola Carpenter, 1864
Fluminicola Carpenter, 1864: 676. Type species Paludina nuttalliana Lea, 1838, by original designation.
Fluminicola shares with other members of the family Lithoglyphidae (fideWilke et al., 2001) a distinctive combination of a flattened, blade-like penis lacking glands and a female capsule gland with an enclosed ventral channel (Radoman, 1983; Thompson, 1984; Hershler & Thompson, 1990). Hershler & Frest (1996) provided a morphology-based phylogenetic analysis that resolved a ‘Fluminicola clade’ that was more closely related to eastern North American Somatogyrus than to divergent F. virens. However, because the phylogenetic placement of the type species of Fluminicola (Paludina nuttalliana Lea, 1838) is unknown (this snail has never been anatomically studied and is probably extinct), the genus continues to be broadly envisaged as a paraphyletic unit consisting of F. virens and all other regional lithoglyphids (Hershler & Frest, 1996). A detailed description of Fluminicola was provided by Hershler & Frest (1996) and does not need to be repeated or emended here.
The fauna of the upper Sacramento River basin described below may be referred to as the ‘Fluminicola clade’ (see above) as they share the two synapomorphies of this unit – an elongate pedal commissure and a sickle-shaped or elongate penis that lacks an eversible, terminal papilla (Hershler & Frest, 1996). Variation in reproductive morphology, which has often been useful in taxonomic studies of freshwater rissooidean snails, proved to be relatively minor among the members of this fauna (6, 7) and most of the new species described below are instead diagnosed by shell or radular features. Species are treated in the text below in approximately the order of their distribution within the upper Sacramento River basin.

Female glandular oviduct and associated structures (viewed from the left side) for six species of Fluminicola. A, F. fremonti (USNM 1020661). B, F. warnerensis (USNM 1020653). C, F. erosus (USNM 1020664). D, F. favillaceus (USNM 1020670). E, F. neritoides (USNM 1020682). F, F. anserinus (USNM 1020728). Scales = 0.5 mm. Ag, albumen gland; Bu, bursa copulatrix; Cg, capsule gland; Dbu, bursal duct; Ga, genital aperture; Pc, posterior wall of pallial cavity; Rf, rectal furrow; Sr, seminal receptacle; Vc, ventral channel of capsule gland.
Fluminicola fremonti sp. nov. (Fremont pebblesnail)
Type material: Holotype (Fig. 8A), USNM 1020660, Hunters Spring on the north side of FS28, Fremont National Forest, Lake County, Oregon (704840 E, 4684990 N, 1604 m), 26 August 2000 TF, EJ. Paratypes, USNM 1020661, from same lot as holotype.
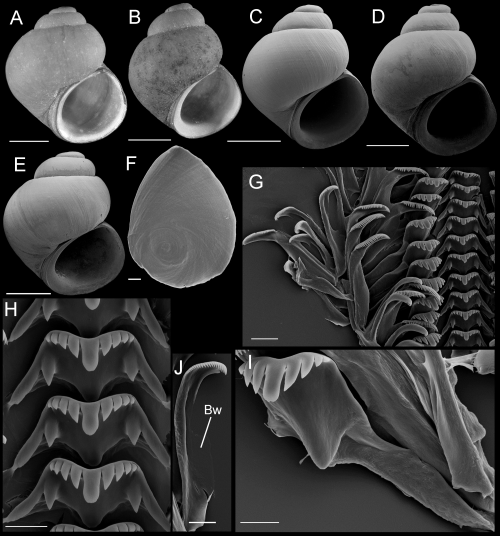
Shells, opercula, and radula of Fluminicola fremonti. A, holotype (USNM 1020660). B–E, USNM 1020661. Scales = 1 mm. F, outer side of operculum (USNM 1020661). Scale = 100 µm. G, portion of radular ribbon (USNM 1020661). Scale = 20 µm. H, central radular teeth (USNM 1020661). Scale = 10 µm. I, lateral radular tooth (USNM 1020661). Scale = 10 µm. J, outer marginal tooth, with basal, rectangular wing (Bw) on outer edge. Scale = 10 µm.
Referred material: USNM 1020662, topotypes, 30 October 2002, TF, EJ.
Diagnosis: Differs from closely similar F. turbiniformis in its smaller basal cusps on the central radular teeth, the typically pointed distal end of the penis, the presence of internal pigment in the penis, a more triangular-shaped bursa copulatrix, a longer bursa copulatrix duct, a larger seminal receptacle, and the absence of a seminal receptacle duct. Readily distinguished from geographically proximal F. modoci by its typically broader shell, narrower columellar lip, and lighter coloured periostracum.
Description: Shell (Fig. 8A–E; Table 3) usually subglobose, rarely ovate-conic, rarely having eroded spire; height, 2.32–3.98 mm; whorls, 3.25–4.0. Protoconch 1.3–1.4 whorls, diameter approximately 0.70 mm. Teleoconch whorls medium convex, narrowly shouldered. Aperture broad, angled above. Parietal lip complete, usually adnate, thin and curved across body whorl; occasionally slightly disjunct, thickened. Columellar lip narrow to medium width, often overlapping part of umbilical region. Outer lip usually thin, prosocline. Shell anomphalous or having narrow umbilicus, umbilical region narrowly excavated. Periostracum tan or light brown. Outer surface of operculum smooth (Fig. 8F). Central radular tooth approximately 37 µm wide, cutting edge convex, lateral cusps four to five; central cusp near parallel-sided, distal end rounded; basal cusps one; basal tongue broadly V- or U-shaped, equal to lateral margin (Fig. 8H). Lateral tooth face broadly rectangular; central cusp rounded; lateral cusps three (inner), four to five (outer); outer wing flexed, medium length (Fig. 8I). Inner marginal teeth (Fig. 8G) having 27–33 cusps. Outer marginal teeth having 28–36 cusps; basal wing rectangular (Fig. 8G, J). Head-foot dark brown, almost black. Ctenidium connected to pericardium by short, efferent branchial vessel (fideHershler & Ponder, 1998: fig. 18c); ctenidial filaments approximately 16, weakly pleated. Osphradium elongate, positioned opposite middle of ctenidium. Female reproductive anatomy shown in Figure 6A. Bursa copulatrix longitudinal, triangular, duct medium length. Seminal receptacle medium-sized, partly overlapped by albumen gland. Albumen gland having moderate rectal furrow. Capsule gland a little longer than albumen gland. Genital aperture a simple pore. Penis medium-sized, coiled, distal end usually pointed, rarely tapering or having papilla (Fig. 7A). Penis surface pale; internal black granules scattered along length of penial duct. Penial duct near centrally positioned, weakly undulating medially.
Distribution: Known only from the type locality, which is located in the Thomas Creek drainage, Goose Lake basin (Fig. 9).
Etymology: Named after John C. Frémont, intrepid early explorer of the American West. The single locality for this species is located in the Fremont National Forest.
Remarks: On the basis of sequence divergence data, F. fremonti is most similar to species living in the lower Pit River basin: F. anserinus (described below) (COI 3.5–3.8%) and F. scopulinus (described below) (cytb 5.5–5.8%).
Fluminicola warnerensis sp. nov. (warner pebblesnail)
Type material: Holotype (Fig. 10A), USNM 1020652, Parsnip Springs, south of South Warner Road (FS64, also designated as 39NO1) in wet, open meadow, Modoc National Forest, Lassen County, California (729680 E, 4559760 N, 1957 m), 18 September 2001, TF, EJ. Paratypes (from same lot), USNM 1020653.
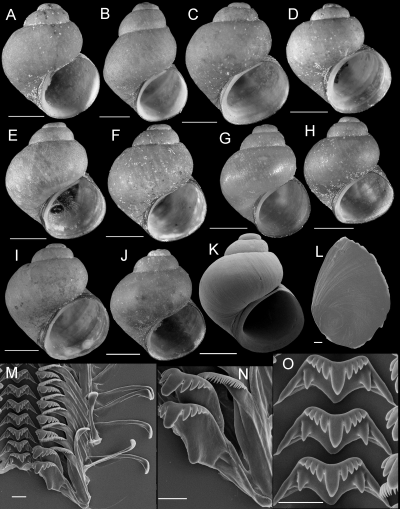
Shells, opercula, and radula of Fluminicola warnerensis. A, holotype (USNM 1020652). B, USNM 1020654. C, USNM 1020655. D, USNM 1020656. E, 1020657. F, USNM 1020658. G, USNM 883559. H, USNM 883561. I, USNM 883564. J, USNM 1020655. K, USNM 1020653. Scales = 1.0 mm. L, outer side of operculum (USNM 1020653). Scale = 100 µm. M, portion of radular ribbon (USNM 1020653). Scale = 20 µm. N, lateral and inner marginal radular teeth (USNM 1020653). Scale = 20 µm. O, central radular teeth (USNM 1020653). Scale = 20 µm.
Referred material: CALIFORNIA. Lassen County: USNM 1020655, spring brook in sedge meadow on east side of Blue Lake just east of Blue Lake Campground access road, c. 0.16 km east of lake, Modoc National Forest (728375 E, 4558240 N, 1853 m), 17 September 2001 TF, EJ.—USNM 1020656, spring creek to Blue Lake on west side of FS64, c. 0.81 km south-east of Blue Lake Campground, Modoc National Forest (729020 E, 4557760 N, 1894 m), 18 June 1994 TF, EJ. – USNM 883557, USNM 883561, spring tributary to Blue Lake (729770 E, 4557577 N, 1830 m), 2 June 1993 DS. – USNM 883559, spring tributary to large meadow, Harvey Creek drainage, Jess Valley (725112 E, 4563474 N), 2 June 1993 DS. – USNM 883564, stream tributary to south end of Blue Lake (728489 E, 4558041 N, 1830 m), 1 July 2003 DS. Modoc County: USNM 1020654, Soup Spring just north of Soup Spring Campground, Modoc National Forest (728000 E, 4576530 N, 2067 m), 17 September 2001 TF, EJ. – USNM 1020789, Soup Creek on north side of FS40N24 crossing, Modoc National Forest (726000 E, 4574760 N, 1702 m), 17 September 2001 TF, EJ. – USNM 1020657, springs south-east of Hilton on south side of Hilton Road, 0.48 km west of Hilton Creek (689280 E, 4567560 N, 1769 m), 18 September 2001, TF, EJ. – USNM 1020658, head spring of Rush Creek west of FS40N18, north-west end of Manzanita Ridge, Modoc National Forest (682590 E, 4573530 N, 1903 m), 20 July 2002 TF, EJ. – USNM 1020659, Miller Spring run, 0.32 km south of FS41N11, Miller Gulch, Modoc National Forest (664700 E, 4577150 N, 1586 m), 22 September 2001 TF, EJ. – USNM 1020790, springs in gulch east of Miller Gulch, 0.48 km on road off (south of) FS41N12, Modoc National Forest (667260 E, 4576920 N, 1513 m), 22 September 2001, TF, EJ.
Diagnosis: Differs from similar F. turbiniformis in its larger size (SH, SW, P = 0; data for latter given below), darker body pigment, less prominent sperm storage area in the coiled oviduct, complete overlap of the seminal receptacle by the albumen gland, broader bursa copulatrix, absence of a seminal receptacle duct, and frequent occurrence of a papilla-like capsule gland opening. Distinguished from geographically proximal F. erosus and F. lunsfordensis (both described below) by its more elongate shell, more convex shell whorls, single basal cusp of the central radular tooth, and enlarged large central cusp of the lateral radular tooth. Also differs from the former in its larger size and from the latter by its weakly angled adapical portion of the aperture.
Description: Shell (Fig. 10A–K; Table 4) turbiniform to ovate-conic, rarely having eroded spire; height, 2.40–4.68 mm; whorls, 3.5–4.0. Protoconch 1.4 whorls, diameter approximately 0.70 mm. Teleoconch whorls medium to weakly convex, sometimes wider below, narrowly shouldered. Last 0.125 whorl rarely disjunct. Aperture broad, angled above. Parietal lip complete, slightly disjunct and thickened in larger specimens. Columellar lip usually medium width and overlapping part of umbilical region, sometimes narrow. Outer lip usually thin, prosocline. Shell anomphalous or narrowly rimate, umbilical region sometimes excavated. Periostracum tan, light brown, or red. Last 0.5 whorl of operculum very weakly frilled (Fig. 10L). Central radular tooth approximately 53 µm wide, cutting edge convex, lateral cusps four (one tooth having three); central cusp pointed; basal cusps one; basal tongue V-shaped, equal to lateral margin (Fig. 10O). Lateral tooth face broadly rectangular; central cusp rounded, lateral cusps two (inner), three to five (outer); outer wing flexed, medium length (Fig. 10N). Inner marginal teeth having 20–26 cusps (Fig. 10M, N). Outer marginal teeth having 24–38 cusps; basal wing rectangular (Fig. 10M). Head-foot dark brown, almost black. Ctenidium connected to pericardium by short, efferent branchial vessel; ctenidial filaments approximately 17, without pleats. Osphradium elongate, positioned opposite middle of ctenidium. Female reproductive anatomy shown in Figure 6B. Bursa copulatrix horizontal, pyriform or triangular, duct short. Seminal receptacle medium-sized, overlapped by albumen gland. Albumen gland having pronounced rectal furrow. Capsule gland a little longer than albumen gland. Genital aperture a short slit, usually forming a small papilla. Penis medium-sized, narrow, coiled, distal end pointed (Fig. 7B). Base and medial section grey, pigment concentrated along penial duct. Penial duct near centrally positioned, weakly undulating in proximal section.
Distribution: Distributed within the drainage of the south fork of the Pit River, and along the Pit River just above Big Valley (upper Pit River basin) (Fig. 9).
Etymology: The species name refers to the type locality area in the Warner Mountains, north-eastern California.
Remarks: Populations allocated to F. warnerensis vary somewhat in shell form (Fig. 10A–K). Nonetheless they share diagnostic morphological features, are little divergent genetically (0–0.6% for COI, 0–0.3% for cytb), and were consistently depicted as a well-supported monophyletic group (2-4), justifying recognition as a single species. In all of the phylogenetic analyses, F. warnerensis was depicted as most closely related to F. turbiniformis, with moderate to strong bootstrap support (75–100%). Haplotypes of these two species differ by 2.3–2.6% (COI) and 3.6–3.9% (cytb).
This species was referred to as Fluminicola n. sp. 13 by Frest & Johannes (1995a).
Fluminicola lunsfordensis sp. nov. (Lunsford pebblesnail)
Type material: Holotype (Fig. 11A), USNM 1020688, Lunsford Spring above Lunsford Springs Road, source of Canyon Creek, Modoc County, California (688920 E, 4572300 N, 1678 m), 18 September 2001 TF, EJ. Paratypes, from same sample, USNM 1020689.
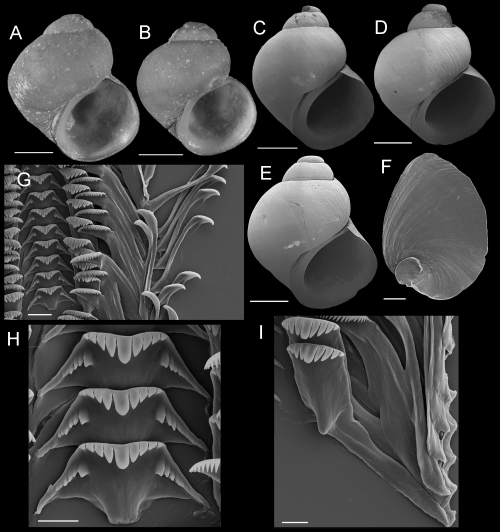
Shells, opercula, and radula of Fluminicola lunsfordensis. A, holotype (USNM 1020688). B–E, USNM 1020689. Scales = 1.0 mm. F, outer side of operculum (USNM 1020689). Scale = 200 µm. G, portion of radular ribbon (USNM 1020689). Scale = 20 µm. H, central radular teeth (USNM 1020689). Scale = 10 µm. I, lateral radular teeth (USNM 1020689). Scale = 10 µm.
Referred material: USNM 1020690, topotypes, 17 October 2003 TF, EJ.
Diagnosis: Differs from closely related F. caballensis and F. favillaceus in its less convex teleoconch whorls, absence of whorl shoulders, strongly angulate (adapically) aperture, and typically broader columellar lip. Differs from geographically proximal F. erosus (described below) by its larger size (SH, SW, P = 0), lower shell spire (mean SH/AH, 1.70, 1.98, respectively; P = 0), less convex teleoconch whorls, thinner parietal lip, and larger, more rounded central cusp of the central radula tooth. Distinguished from geographically proximal F. warnerensis above.
Description: Shell (Fig. 11A–E; Table 5) trochiform, often having eroded spire; height, 2.86–4.48 mm; whorls, 3.25–4.0. Protoconch 1.4 whorls, diameter approximately 0.77 mm. Teleoconch whorls weak to moderately convex, rarely having weak subsutural cord. Aperture strongly angled above. Parietal lip complete, adnate, often forming callus. Columellar lip rather broad, usually overlapping entire umbilical region. Outer lip thin, prosocline. Shell usually anomphalous, sometimes rimate or having small umbilicus. Periostracum tan. Last 0.5 whorl of operculum frilled. Central radular tooth 43 µm wide, cutting edge near horizontal, lateral cusps four to seven; central cusp rounded or weakly pointed; basal cusps three to six; basal tongue square, extending beyond lateral margins (Fig. 11H). Lateral tooth face narrowly rectangular; central cusp parallel-sided, rounded or weakly pointed; lateral cusps three to five (inner), four to seven (outer); outer wing flexed, elongate (Fig. 11I). Inner marginal teeth having 26–31 cusps (Fig. 11G). Outer marginals having 25–35 cusps; basal wing triangular, weakly developed (Fig. 11G). Head-foot dark brown. Ctenidium connected to pericardium by long, efferent branchial vessel (fideHershler & Ponder, 1998: fig. 18d); filaments approximately 15, without pleats. Osphradium elongate, positioned opposite middle of ctenidium. Bursa copulatrix horizontal, ovate or pyriform, duct short. Seminal receptacle small, overlapped by albumen gland. Albumen gland having deep rectal furrow. Capsule gland slightly longer than albumen gland. Genital aperture a simple pore or weak papilla. Penis medium-sized, narrow-elongate, curved to flexed, distal end tapered (Fig. 7C). Medial section of penis having black internal pigment core. Penial duct positioned near outer edge, weakly undulating medially.
Distribution: Endemic to the type locality in the Canyon Creek watershed, which empties into Warm Springs Valley (upper Pit River basin) (Fig. 9).
Etymology: Refers to the name of the type locality.
Remarks: This species is frequently depicted in the phylogenetic analyses as closely related to morphologically similar and geographically proximate F. caballensis, F. erosus, F. favillacea (all described below), and Fluminicola sp. (B), one of the Rush Creek lineages. On the basis of sequence divergences, F. lunsfordensis is most similar to F. ahjumawi (described below) (COI 1.9–2.7%) and Fluminicola sp. (B) (cytb 1.7–2.2%).
Fluminicola erosus sp. nov. (Smokey Charley pebblesnail)
Type material: Holotype (Fig. 12A), USNM 1020663, moderate-sized unnamed spring, c. 0.40 km south-east of Smokey Charley Spring and 0.32 km west of Modoc County 63, at source next to homestead cabin, Modoc County, California (705090 E, 4566300 N, 1366 m), 19 September 2001, TF, EJ. Paratypes (from same lot), USNM 1020664.

Shells, opercula, and radula of Fluminicola erosus. A, holotype (USNM 1020663). B, USNM 1020667. C, USNM 1020664. D, USNM 1020668. E, F, USNM 1020664. Scales = 1.0 mm. G, apical portion of shell showing spiral depressions (USNM 1020664). Scale = 100 µm. H, outer side of operculum (USNM 1020664). Scale = 100 µm. I, portion of radular ribbon (USNM 1020664). Scale = 10 µm. J, central radular teeth (USNM 1020664). Scale = 2 µm. K, lateral radular tooth (USNM 1020664). Scale = 10 µm.
Referred material: CALIFORNIA. Modoc County: USNM 1020665, topotypes, 20 October 1994, TF, EJ. – USNM 1020666, Smokey Charley Spring at source on hill side, c. 0.40 km west of Modoc County 63 (704900 E, 4560570 N, 1366 m), 20 October 1994 TF, EJ. – USNM 1020667, ibid., 19 September 2001, TF, EJ. – USNM 1020668, Smokey Charley Spring run, c. 0.24 km east of source (below where double channels from source combine), west of Modoc County 63 (705080 E, 4566600 N, 1360 m), 20 October 1994, TF, EJ.
Diagnosis: Differs from closely related F. caballensis and F. favillaceus (described below) by its smaller size (SH, SW, P = 0), narrow or absent whorl shoulders, thicker shell parietal lip, and square basal tongue of the central radular tooth. Differentiated from geographically proximal F. warnerensis above.
Description: Shell (Fig. 12A–F; Table 6) ovate-conic, usually having eroded spire, height approximately 2.1–2.5 mm; whorls approximately 3.5. Protoconch 1.4 whorls, diameter approximately 0.47 mm; microsculpture sometimes including series of weak spiral depressions spread over much of the width of the initial 0.6 whorl (Fig. 12H). Teleoconch whorls medium to highly convex, sometimes narrowly shouldered, last 0.5 whorl sometimes slightly disjunct. Aperture lunate, weakly angled above. Parietal lip complete, slightly thickened, slightly disjunct or adnate. Columellar lip usually medium width, often overlapping part of umbilical region; rarely narrow. Outer lip thin, orthocline or prosocline. Shell anomphalous or having narrow umbilicus; umbilical region usually excavated. Periostracum brown or green. Last 0.5 whorl of operculum frilled (Fig. 12G). Central radular tooth approximately 18 µm wide, cutting edge near horizontal, lateral cusps six to eight; central cusp small, pointed; basal cusps four to six, small; basal tongue square, extending beyond lateral margins (Fig. 12J). Lateral tooth face narrowly rectangular; central cusp small, pointed; lateral cusps six to eight (inner), five to eight (outer); outer wing flexed, medium length (Fig. 12K). Inner marginal teeth having 26–37 cusps (Fig. 12I). Outer marginal teeth having 25–32 cusps; basal wing rectangular, weakly developed (Fig. 12I). Head-foot grey or dark brown. Ctenidium connected to pericardium by short, efferent branchial vessel; ctenidial filaments approximately 13, small, narrow (almost finger-like), without pleats. Osphradium elongate, expanded anteriorly, opposite posterior part of ctenidium. Female reproductive anatomy shown in Figure 6C. Bursa copulatrix horizontal, ovate or pyriform, duct short. Seminal receptacle medium-sized, usually overlapped by albumen gland. Albumen gland entirely visceral, having weak rectal furrow. Capsule gland a little longer than albumen gland. Genital aperture a simple pore. Penis medium-sized, straight or weakly coiled, base narrowed, distal end pointed or forming papilla (Fig. 7D). Penis pale except for central patch of brown pigment on ventral surface and occasional dusting along edges of base. Penial duct positioned near outer edge, weakly undulating medially.
Distribution: Distributed among two closely proximal springs along the South Fork Pit River west-north-west of Likely (upper Pit River basin) (Fig. 9).
Etymology: From New Latin erosus, erosion, referring to the typically eroded aspect of the shell spire.
Remarks: Two morphs were observed in this species. One consists of large specimens that have an eroded spire, a relatively large aperture, a rather broad columellar lip, and a strongly prosocline outer lip (Fig. 12A, B, D, E). The other is smaller, has as many as three shell whorls, and has a smaller aperture, a narrower columellar lip, and an orthocline outer lip (Fig. 12C, F). The larger morph also has darker body pigment and a less attenuate distal section of the penis. These snails were indistinguishable in other anatomical details and exhibited more genetic variation within than among morphs. (Both morphs were collected at the type locality, whereas only the large morph was found at the other two localities.) This pattern may be linked to the utilization of separate microhabitats, as evidenced by the much heavier deposits on the shells of the larger morph, and could reflect incipient speciation or phenotypic plasticity.
In the phylogenetic analyses, this species was usually depicted as most closely related to morphologically similar and geographically proximal F. caballensis and F. favillaceus (described below). F. erosus differs from F. caballensis by 1.4–2.0% (COI) and 0.8–3.6% (cytb) and from F. favillaceus by 0.9–1.8% (COI) and 1.9–3.0% (cytb).
Populations of this species were referred to as Fluminicola n. sp. 11 and Fluminicola n. sp. 12 by Frest & Johannes (1995a).
Fluminicola favillaceus sp. nov. (Ash Valley pebblesnail)
Type material: Holotype (Fig. 13A), USNM 1020669, Ash Creek south culvert channel on west side of Ash Valley Road (Modoc County 527), Crown D Ranch, Lassen County, California (692060 E, 4551010 N, 1536 m), 23 July 2002 TF, EJ, SR. Paratypes (from same lot), USNM 1020670.
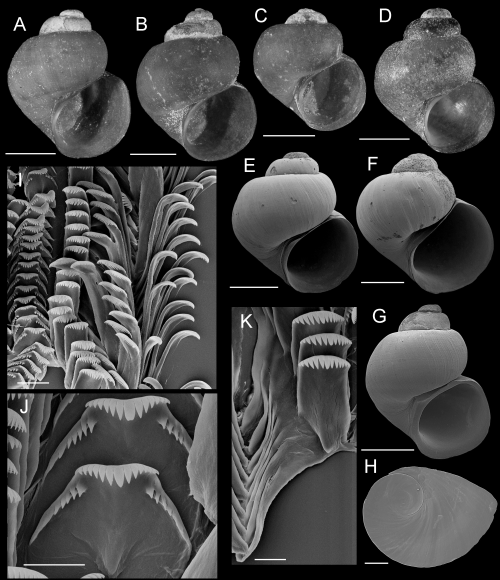
Shells, opercula, and radula of Fluminicola favillaceus. A, holotype (USNM 1020669). B, USNM 1020672. C, USNM 1020671. D, USNM 1020673. E, F, USNM 1020670. Scales = 1.0 mm. H, outer side of operculum (USNM 1020670). Scale = 200 µm. I, portion of radular ribbon (USNM 1020670). Scale = 20 µm. J, central radular teeth (USNM 1020670). Scale = 10 µm. K, lateral radular teeth (USNM 1020670). Scale = 10 µm.
Referred material: CALIFORNIA. Lassen County: USNM 1020671, Ash Creek at north culvert on west side of Ash Valley Road (Modoc County 527) (692080 E, 4551080 N, 1536 m), 19 September 2001 TF, EJ. – USNM 1020672, ibid., 23 July 2002 TF, EJ. – USNM 1020673, Ash Creek on south side of FS39N50 bridge, 1.6 km east of Hunsinger Draw, Modoc National Forest (685560 E, 4555260 N, 1498 m), 20 July 2002 TF, EJ. – USNM 1020674, Chisolm Spring on south side of Ash Creek, west side of Ash Valley Road (Modoc County 527), Crown D Ranch (692030 E, 4550990 N, 1537 m), 23 July 2002 TF, EJ.
Diagnosis: Differs from the similar F. caballensis (described below) in its less convex teleoconch whorls, more angulate whorl shoulders, larger umbilicus, pointed central cusp and V-shaped basal tongue of the central radular tooth, and larger central cusp and strongly curved outer wing of the lateral radular tooth. Readily distinguished from syntopic F. seminalis by its much smaller size and thicker parietal shell lip. Differentiated from F. erosus above.
Description: Shell (Fig. 13A–G; Table 7) globose to ovate-conic, spire usually eroded; height, 2.10–3.49 mm; whorls, 3.00–3.75. Protoconch 1.25 whorls, diameter approximately 0.59 mm. Teleoconch whorls medium convex, sometimes wider above, often having strongly angulate shoulders. Aperture ovate, angled above. Parietal lip complete, broadly adnate to slightly disjunct, slightly thickened. Columellar lip usually medium width, often overlapping part of umbilical region, only slightly thickened, often broader above than below. Outer lip thin, orthocline or slightly prosocline. Umbilicus rimate or perforate. Periostracum tan, brown or light green. Last 0.5 whorl of operculum frilled (Fig. 13H). Central radular tooth approximately 27 µm wide, cutting edge near horizontal, lateral cusps five to eight; central cusp pointed or weakly rounded; basal cusps three to eight, small; basal tongue V-shaped, extending beyond lateral margins (Fig. 13J). Lateral tooth face narrowly rectangular; central cusp small, pointed; lateral cusps five to nine (inner), five to eight (outer); outer wing flexed, elongate, strongly curved (Fig. 13K). Inner marginal teeth having 27–35 cusps (Fig. 13I). Outer marginal teeth having 26–32 cusps; basal wing narrowly rectangular (Fig. 13I). Head-foot dark brown. Ctenidium abutting pericardium; ctenidial filaments approximately 17, without pleats. Osphradium elongate, anterior end expanded, positioned opposite middle of ctenidium. Female reproductive anatomy shown in Figure 6D. Bursa copulatrix horizontal, ovate or pyriform, duct short. Seminal receptacle small, usually overlapped by albumen gland. Albumen gland entirely visceral or having very short pallial section. Capsule gland a little longer than albumen gland. Genital aperture a short slit. Penis medium-sized, often coiled, base somewhat narrowed, distal end tapered (Fig. 7E). Penis pale or lightly pigmented on base. Penial duct positioned near outer edge, straight throughout or weakly undulating proximally.
Distribution: Ash Creek (and an associated spring), a major tributary of the upper Pit River (Fig. 9).
Etymology: From New Latin favilla, meaning of ashes; referring to the distribution of the species in Ash Valley, California.
Remarks: On the basis of sequence divergences, this species is most similar to F. erosus (COI 0.9–1.8%) and F. caballensis (described below) (cytb 1.1–2.2%).
Fluminicola caballensis sp. nov. (Horse Creek pebblesnail)
Type material: Holotype (Fig. 14A), USNM 1020675, Davis Creek at crossing of road 0.32 km south of junction with FS22, Lassen National Forest, Lassen County, California (658660 E, 4521870 N, 1500 m), 16 October 2003 TF, EJ. Paratypes (from same lot), USNM 1020676.
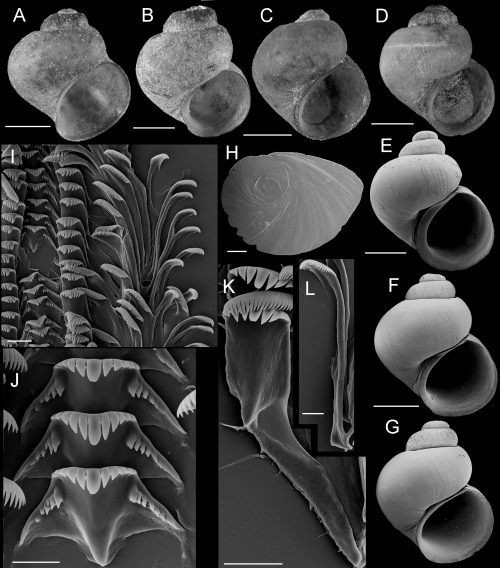
Shells, opercula, and radula of Fluminicola caballensis. A, holotype (USNM 1020675). B, USNM 1020679. C, USNM 1020678. D, USNM 1020680. E–G, USNM 1020676. Scales = 1.0 mm. H, outer side of operculum (USNM 1020676). Scale = 200 µm. I, portion of radular ribbon (USNM 1020676). Scale = 20 µm. J, central radular teeth (USNM 1020676). Scale = 10 µm. K, lateral and marginal radular teeth (USNM 1020676). Scale = 20 µm. L, outer marginal tooth; note absence of wing along outer edge. Scale = 10 µm.
Referred material: CALIFORNIA. Lassen County: USNM 1020677, topotypes, 24 September 2001 TF, EJ. – USNM 1020678, Bob Creek at crossing of Little Valley Road, Beaver Creek Ranch (647090 E, 4534080 N, 1135 m), 24 September 2001 TF, EJ. – USNM 1020679, Russell Dairy Spring on south side of road, 0.48 km south of FS22 junction, inholding in Fremont National Forest (666740 E, 4521130 N, 1545 m), 24 September 2001 TF, EJ. – USNM 1020680, spring run on south side of FS22, west of Russell Dairy Spring run, inholding in Lassen National Forest (666390 E, 4521240 N, 1515 m), 24 September 2001 TF, EJ. – USNM 1020681, spring on north side of FS22, west of Russell Dairy Spring, inholding in Lassen National Forest (666010 E, 4521420 N), 24 September 2001 TF, EJ.
Diagnosis: Differentiated from similar species above (F. erosus, F. favillaceus, F. lunsfordensis) on the basis of shell and anatomical features (see above).
Description: Shell (Fig. 14A–G; Table 8) subglobose or ovate-conic, spire usually slightly eroded; height, 2.42–4.35 mm; whorls, 3.25–4.0. Protoconch 1.3 whorls, diameter approximately 0.70 mm. Teleoconch whorls medium to highly convex, often having wide shoulders, evenly rounded. Aperture broad, angled above. Parietal lip complete, thin, usually broadly adnate and curved across body whorl. Columellar lip medium width, often overlapping part of umbilical region. Outer lip thin, prosocline. Shell anomphalous or rimate. Periostracum usually tan or brown, rarely light green. Last 0.75 whorl of operculum frilled (Fig. 14H). Central radular tooth approximately 36 µm wide, cutting edge near horizontal, lateral cusps four to six; central cusp near parallel-sided, rounded or weakly pointed; basal cusps four to six; basal tongue V-shaped, extending beyond lateral margins (Fig. 14J). Lateral tooth face narrowly rectangular; central cusp parallel-sided and rounded, or spoon-shaped; lateral cusps four to five (inner and outer); outer wing flexed, elongate (Fig. 14K). Inner marginal teeth having 25–33 cusps (Fig. 14I). Outer marginal teeth having 25–34 cusps; basal wing absent (Fig. 14I, L). Head-foot dark brown. Ctenidium connected to pericardium by short, efferent branchial vessel; filaments approximately 18, without pleats. Osphradium elongate, positioned opposite middle of ctenidium. Bursa copulatrix horizontal; ovate, pyriform, or circular; duct short (absent in one specimen). Seminal receptacle small, overlapped by albumen gland. Albumen gland having weak rectal furrow. Capsule gland longer than albumen gland. Genital aperture a short slit, usually forming a weak papilla. Penis medium-sized, rather thickened, distal end pointed or papillate (Fig. 7F). Penis having dense core of black pigment along much of length or only in medial section, dorsal surface light to darkly pigmented with brown melanin and also covered with white pigment granules. Penial duct positioned near outer edge, weakly undulating in basal and medial sections.
Distribution: Horse Creek and Bob Creek drainages, lower Pit River basin (Fig. 9).
Etymology: From New Latin caballus, meaning horse, and referring to the distribution of this species in the Horse Creek drainage.
Fluminicola neritoides sp. nov. (Willow Creek pebblesnail)
Type material: Holotype (Fig. 15A), USNM 883563, springs at Willow Creek Campground along CA 139, Lassen County, California (682701 E, 4542277 N, 1507 m), 2 June 1993 DS. Paratypes (from same lot), USNM 1020682.
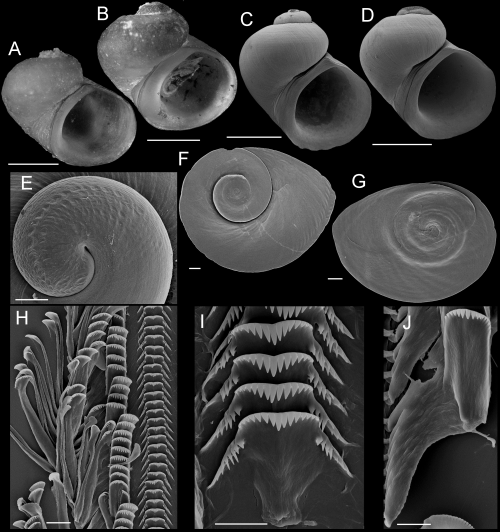
Shells, opercula, and radula of Fluminicola neritoides. A, holotype (USNM 883563). B, USNM 1020684. C, D, USNM 1020682. Scales = 1.0 mm. E, protoconch, showing spiral depressions (USNM 1020682). Scale = 100 µm. F, outer side of operculum (USNM 1020682). G, inner side of operculum (USNM 1020682). Scales = 100 µm. H, portion of radular ribbon (USNM 1020682). Scale = 20 µm. I, central radular teeth (USNM 1020682). Scale = 10 µm. J, marginal radular tooth (USNM 1020682). Scale = 10 µm.
Referred material: CALIFORNIA. Lassen County: USNM 883562, Willow Creek, along CA 139 (682583 E, 4542682 N), 2 June 1993, DS. – USNM 1020683, seventh spring run from the east in Lower McBride Springs on north-east side of Willow Creek, c. 0.32 km north of CA 139 and 0.805 km west of Willow Creek Campground, Modoc National Forest (682140 E, 4542620 N, 1522 m), 10 September 1993 TF, EJ. – USNM 883756, fourth spring run from the east in Lower McBride Springs on north-east side of Willow Creek, c. 0.32 km north of CA 139 and 0.805 km west of Willow Creek Campground, Modoc National Forest (682210 E, 4542620 N, 1522 m), 10 September 1993 TF, EJ. – USNM 1020684, east-most spring run in Lower McBride Springs on north-east side of Willow Creek, c. 0.32 km north of CA 139 and 0.805 km west of Willow Creek Campground, Modoc National Forest (682260 E, 4542570 N, 1525 m), 10 September 1993 TF, EJ. – USNM 1020685, Willow Creek just upstream of Hayden Hill Road (Lassen County Road 534, FS37N42) junction, along west side of CA 139, mouth of Hayden Canyon, Modoc National Forest (680680 E, 4542990 N, 1488 m), 20 September 2001 TF, EJ. – USNM 1020686, Willow Creek at lower end of Lower McBride Springs, north-east side of CA 139, c. 0.64 km east of Hayden Hill Cut Off Road junction, Modoc National Forest (682030 E, 4542680 N, 1504 m), 20 September 2001 TF, EJ. – USNM 1020687, Willow Creek on both sides of wooden foot bridge at a picnic area just downstream of Willow Creek Campground, north side of CA 139, c. 0.16 km west of Hayden Hill Cut Off Road junction, Modoc National Forest (682500 E, 4542280 N, 1522 m), 20 September 2001 TF, EJ.
Diagnosis: Readily distinguished from all other regional congeners by its neritiform shell shape, consistently broad and thickened columellar lip, and strongly frilled operculum whorls.
Description: Shell (Fig. 15A–D; Table 9) neritiform, often having slightly eroded spire; height, 2.06–3.16 mm; whorls, 2.5–3.25. Protoconch 1.25 whorls, diameter approximately 0.66 mm; microsculpture includes a spiral series of flame-shaped depressions spread over most of the width of the initial 0.7 whorl (Fig. 15E). Teleoconch whorls highly convex, without shoulders. Terminal portion of body whorl often slightly disjunct in larger specimens. Aperture broad, D-shaped. Parietal lip complete, adnate or slightly disjunct, often thickened and straight in larger specimens. Columellar lip broad, thickened, often continuous with parietal callus. Outer lip thin or slightly thickened, strongly prosocline, sometimes weakly sinuate adapically. Shell anomphalous or rimate; umbilical region often excavated. Periostracum tan or brown. Last whorl of operculum strongly frilled (Fig. 15F, G). Central radular tooth approximately 28 µm wide, cutting edge near horizontal, lateral cusps six to eight; central cusp small, pointed; basal cusps four to seven; basal tongue square, extending beyond lateral margins (Fig. 15I) Lateral tooth face narrowly rectangular; central cusp small, pointed; lateral cusps six to eight (inner), six to seven (outer); outer wing flexed, curved, elongate (Fig. 15J). Inner marginal teeth having 20–28 cusps (Fig. 15H). Outer marginal teeth having 19–28 cusps; basal wing absent (Fig. 15H). Head-foot dark brown, almost black. Ctenidium connected to pericardium by long, efferent branchial vessel; ctenidial filaments approximately 11, small, finger-like, without pleats. Osphradium elongate, positioned opposite middle of ctenidium. Female reproductive anatomy shown in Figure 6E. Oviduct coils rather broad. Bursa copulatrix horizontal, ovate or pyriform, duct short. Seminal receptacle medium-sized, usually overlapped by albumen gland. Albumen gland having weak rectal furrow. Capsule gland longer than albumen gland, anterior section not obviously distinct from posterior section in dissection. Genital aperture a simple pore. Penis small, weakly coiled; distal end tapered or papillate (Fig. 7G). Penis pale or having small, distal pigment patch. Penial duct centrally positioned, nearly straight.
Distribution: Willow Creek and associated warm springs, which flow into Big Valley (upper Pit River basin) (Fig. 9).
Etymology: Referring to the neritiform shape of the shell.
Remarks: This unusual species was frequently depicted in our phylogenetic trees as the basal member of clade A. F. neritoides differs from other members of this clade by > 4.0% for both genes, and is most similar to F. lunsfordensis (COI 4.9%) and F. ahjumawi (cytb 7.5–8.3%).
This species was referred to as Fluminicola n. sp. 9 by Frest & Johannes (1994).
Fluminicola ahjumawi sp. nov. (Ahjumawi pebblesnail)
Type material: Holotype (Fig. 16A), USNM 1020691, spring run north of Sam Wolfin Spring, south of Pit River and powerlines, 1.93 km south-west of Pit 1 Powerhouse, Pacific Gas and Electric land, Shasta County, California (625140 E, 4537060 N, 860 m), 31 August 2001 TF, EJ. Paratypes, USNM 1020699, from same lot.
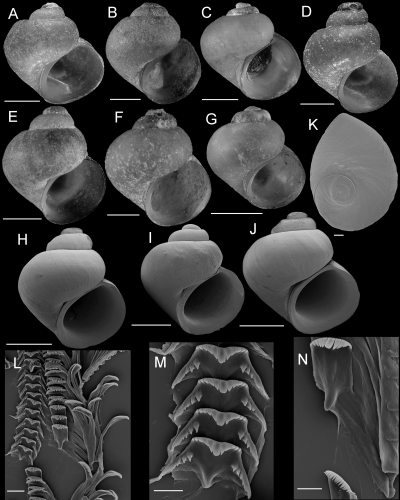
Shells, opercula, and radula of Fluminicola ahjumawi. A, holotype (USNM 1020691). B, USNM 1020693. C, USNM 1020699. D, USNM 1020700. E, USNM 1020701. F, USNM 1020702. G, USNM 1020703. H–J, USNM 1020699. Scales = 1.0 mm. K, outer side of operculum (USNM 1020699). Scale = 100 µm. L, portion of radular ribbon (USNM 1020699). Scale = 20 µm. M, central radular teeth (USNM 1020699). Scale = 10 µm. N, lateral radular tooth (USNM 1020699). Scale = 10 µm.
Referred material: CALIFORNIA. Modoc County: USNM 1020703, Jimmerson Spring on south side of Modoc County 93, 3.38 km north-west of Jimmerson Mountain (642800E, 4567230 N, 1318 m), 19 July 2002 TF, EJ. Shasta County: USNM 1020692, spring on west side of run of Thousand Spring, 0.32 km south-west of source of Thousand Springs, south of Mares Meadow, Thousand Springs Ranch (621560 E, 4552530 N, 1011 m), 1 September 2001 TF, EJ. – USNM 1020693, Hat Creek at Bridge Picnic Area across from Bridge Campground, Lassen National Forest (632000 E, 4510000 N, 1262 m), 29 August 2001 TF, EJ. – USNM 1020694, three unnamed springs on point opposite large island in Pit River (north-east side), c. 0.31 km north-west of CA 299 bridge across Pit River (621680 E, 4537580 N, 841 m), 12 September 1993 TF, EJ. – USNM 1020695, ibid., 3 August 2001 TF, EJ. – USNM 1020696, lower part of unnamed spring run, north-east side of Lions Club Picnic Grounds, near access road and just above (west of) Pit River, near Pit River Hatchery (625090 E, 4538340 N, 869 m), 31 August 2001 TF, EJ. – USNM 1020697, ibid., 16 August 2000, TF, EJ. – USNM 1020698, Honn Creek at Honn Creek Campground, just east of CA 89, c. 2.9 km south of Hat Creek Forest Service Station, Lassen National Forest (626450 E, 4515120 N, 1034 m), 29 August 2001 TF, EJ. – USNM 1020700, west spring source of Mallard Creek, west of source of Thousand Springs, Thousand Springs Ranch (621120 E, 4552740 N, 1011 m), 1 September 2001 TF, EJ. – USNM 1020701, Beaver Creek just over 0.16 km downstream (west of) Beaver Spring, 0.16 km upstream of FS34N10 crossing, Lassen National Forest (649540 E, 4520640 N, 479 m), 24 September 2001 TF, EJ. – USNM 1020702, Burney Creek at Falls Trail bridge, c. 0.08 km upstream of Burney Falls, McArthur–Burney Falls Memorial State Park (613420 E, 4540640 N, 881 m), 25 September 2001 TF, EJ. – USNM 1020704, Lost Creek (upper site) in lava field on north side of Wilcox Road, west of Hat Creek Rim, Lassen National Forest (632560 E, 4513140 N, 1141 m), 29 August 2001 TF, EJ. – USNM 1020797, Lost Creek above intake for upper penstock (of two), c. 0.32 km above mouth of canyon, Hat Creek Rim, Lassen National Forest (634450 E, 4513060 N, 1241 m), 3 November 2002 TF, EJ. – USNM 1020798, Lost Creek (upper most site) in lava field on north side of Wilcox Road, west of Hat Creek Rim, inside and west of Lassen National Forest boundary (632730 E, 4513100 N, 1141 m), 3 November 2002 TF, EJ. – USNM 1020791, upper end of Sucker Springs Creek channel, north-east of California Fish and Game Pit River Hatchery on west side of access road and Pit River, 0.97 km south-west of Pit 1 Powerhouse (Pacific Gas and Electric) (625320 E, 4538180 N, 875 m), 31 August 2001 TF, EJ.
Diagnosis: Readily differentiated from geographically proximal and occasionally syntopic F. seminalis by its smaller size, broader shell, more convex and narrowly shouldered teleoconch whorls, broader columellar lip, weaker collabral growth lines, and thinner, lighter coloured periostracum. Differs from other congeners of the Pit River basin by the combination of fairly large size, globose or broadly conical shell, ovate or weakly angled aperture, absent or very narrow umbilicus, and well-developed columellar lip.
Description: Shell (Fig. 16A–J; Table 10) usually subglobose to trochoidal, rarely ovate-conic, usually having eroded spire; height, 2.42–4.53 mm; whorls, 3.25–3.75. Protoconch 1.4 whorls, diameter approximately 0.70 mm. Teleoconch whorls medium to high convexity, often having broad, prominent shoulders, rarely having subsutural thickening or weak cord. Aperture ovate or weakly angled adapically, sometimes having subsutural angulation. Parietal lip complete, adnate, sometimes thickened across parietal wall and forming fairly broad callus. Columellar lip medium width, often covering umbilical region. Outer lip usually thin, rarely thickened, prosocline, sometimes weakly sinuate. Shell usually anomphalous, sometimes having rimate umbilicus; umbilical region sometimes weakly excavated. Periostracum tan or brown. Last 0.5 whorl of operculum weakly frilled (Fig. 16K). Central radular tooth approximately 37 µm wide, cutting edge almost horizontal (very slightly indented), lateral cusps three to six; central cusp parallel-sided, rounded; basal cusps two to four; basal tongue V-shaped, even with lateral margin (Fig. 16M). Lateral tooth face rectangular; central cusp rounded; lateral cusps four to five (inner), four to five (outer); outer wing flexed, elongate (Fig. 16N). Inner marginal teeth having 24–32 cusps (Fig. 16L). Outer marginal teeth having 25–37 cusps; basal wing narrowly rectangular or absent (Fig. 16L). Head-foot dark brown or black. Ctenidium abutting pericardium; ctenidial filaments 18, broadly triangular, lacking pleats. Osphradium elongate, positioned opposite centre of ctenidium. Bursa copulatrix as long as wide, pyriform; duct short. Seminal receptacle medium-sized, completely overlapped by albumen gland. Rectal furrow well developed on albumen gland. Capsule gland a little longer than albumen gland. Genital aperture a simple pore. Penis medium-sized, thick, distal end tapering or pointed (Fig. 7H). Penis having black internal pigment concentrated in distal two-thirds of length; brown melanin cover often present on base. Penial duct near centrally positioned, weakly undulating medially.
Distribution: Broadly ranging within the lower Pit River basin, including drainages of Hat Creek, Lost Creek, and Fall River. Also found at one locality in the upper Pit River basin (Jimmerson Spring).
Etymology: Referring to the Native American tribe (also known as the Achumawi) that lived in the vicinity of the Fall and Pit Rivers.
Remarks: In the phylogenetic trees, this species often occupied a near basal position within clade A. On the basis of sequence divergence, F. ahjumawi is most similar to F. lunsfordensis (COI 1.9–2.7%) and Fluminicola sp. (B) (cytb 1.4–2.5%).
This species was referred to as F. turbiniformis by Frest & Johannes (1993) and as Fluminicola n. sp. 10 by Frest & Johannes (1995a).
Fluminicola seminalis (Hinds, 1842)
Paludina seminalis Hinds, 1842: 83–84 (type locality, Rio Sacramento, California) [see Hershler & Frest (1996) for a detailed synonymy of this species].
Referred material: CALIFORNIA. Lassen County: USNM 1020741, Ash Creek on west side of Ash Valley Road (Modoc County 527), Ash Valley (692080 E, 4551080 N, 1536 m), 23 July 2002 TF, EJ. – USNM 1020742, Ash Creek north-west of Ash Creek Campground, below (west of) FS22 and a spring, Modoc National Forest (682130 E, 4558830 N, 1462 m), 19 September 2001 TF, EJ. – USNM 1020748, Ash Creek on south side of FS39N50 bridge, 1.6 km east of Hunsinger Draw, Modoc National Forest (685560 E, 4555260 N, 1498 m), 20 July 2002 TF, EJ. Shasta County: USNM 883742, Big Lake at Rat Farm Public Fishing Access, 0.8 km north of Rat Farm site, Pacific Gas and Electric land (635100 E, 4551300 N, 1008 m), 25 October 1992 TF, EJ. – USNM 1020736, ibid., 30 August 2001 TF, EJ. – USNM 1020737, Baum Lake (impoundment of Hat Creek) just offshore from a boat ramp, north of parking lot of Baum Lake Public Fishing Access (Pacific Gas and Electric), off Hat Creek Powerhouse Road, north-west of Crystal Lake State Hatchery, north of Cassel (622400 E, 4532260 N, 908 m), 30 August 2001 TF, EJ. – USNM 1020738, Fall River at Caltrout Public Fishing Access Area just east of Island Road bridge, south of The Island, north of Glenburn (626700 E, 4549500 N, 1008 m), 30 August 2001 TF, EJ. – USNM 1020739, Baum Lake west of a boat ramp, north-west of parking lot of Baum Lake Public Fishing Access (Pacific Gas and Electric), off Hat Creek Powerhouse Road, north-west of Crystal Lake State Hatchery, north of Cassel (622360 E, 4532260 N, 908 m), 30 August 2001 TF, EJ. – USNM 1020743, Crystal Springs, Ahjumawi Lava Springs State Park (630328 E, 4552200 N, 1008 m), 26 September 2001 TF, EJ. – USNM 1020744, 1020745, Big Lake Springs west spring pool, north end of Big Lake, Ahjumawi Lava Springs State Park (633640 E, 4554560 N, 1008 m), 27 September 2001 TF, EJ. – USNM 1020746, Pit River on south-east side of CA 299 bridge near (upstream of) confluence of Hat Creek, Pacific Gas and Electric public fishing access (622340 E, 4537400 N, 840 m), 29 September 2001 TF, EJ. – USNM 883739, Pit River on south side, c. 0.64 km above footbridge, upstream of Pit 1 Powerhouse (627240 E, 4538620 N, 894 m), 12 September 1993 TF, EJ. – USNM 1020747, Spring Creek on south side of Spring Creek Road (624470 E, 4550920 N, 1008 m), 29 September 2001 TF, EJ. – USNM 1020749, Lava Creek at boathouse on Hanna Estate (626800 E, 4552380 N, 1008 m), 2 November 2002 TF, EJ. –USNM 1020750, spring source and pool of Lava Creek tributary north of boathouse on Hanna Estate (626660 E, 4552570 N, 1008 m), 2 November 2002 TF, EJ. – USNM 883180, Lava Creek at and west of Island Road bridge near mouth to Eastman Lake, Lava Creek Ranch, north side of The Island, c. 8.4 km north of Glenburn 102 (626720 E, 4551820 N, 1008 m), 18 August 1991 TF, EJ, JJ. – USNM 1020751, spring on west side of Spring Creek, flowing out under east side of road, c. 1.38 km north-west of junction with Spring Creek Road, Spring Creek Ranch (625020 E, 4552070 N, 1009 m), 6 November 2002 TF, EJ. – USNM 1020752, Lava Creek source spring pool, Spring Creek Ranch (626050 E, 4553040 N, 1008 m), 6 November 2002 TF, EJ. – USNM 1020804, spring on west side of run of Thousand Spring, 0.32 km south-west of source of Thousand Springs, south of Mares Meadow, Thousand Springs Ranch (621560 E, 4552530 N, 1011 m), 1 September 2001 TF, EJ. – USNM 1020805, west spring source of Mallard Creek, west of source of Thousand Springs, Thousand Springs Ranch (621120 E, 4552740 N, 1011 m), 1 September 2001 TF, EJ. – USNM 1020806, Pit River, 0.81 km south-west of Pit 4 Dam, 0.48 km north-east of Ruling Creek mouth, south-east side of Chalk Mountain off FS50, Shasta National Forest (603010 E, 4537620 N, 721 m), 19 October 1992 TF, EJ. – USNM 1020807, below dam diverting Hat Creek into a canal for Hat 1 Powerhouse, north side of Cassel Road bridge, Cassel (622040 E, 4530600 N, 970 m) 24 October 1992 TF, EJ. – USNM 1020808, Crystal Lake at south-west end off Hat Creek Powerhouse Road, c. 0.97 km west of Crystal Lake State Fish Hatchery (621140 E, 4532360 N, 911 m), 17 August 1991 TF, EJ, JJ. – USNM 1020809, Pit River, c. 0.2 km east of Pit River Fish Hatchery, below Lions Club Picnic area spring channels (east end) (625360 E, 4538080 N, 868 m), 20 October 1994 TF, EJ. – USNM 1020810, Ja She Creek on south-east side of bridge of Lava Springs Rim Trail, Ahjumawi Lava Springs State Park (629680 E, 4552170 N, 1008 m), 26 September 2001 TF, EJ. – USNM 883188, west side of McCloud River south of the McCloud Bridge (Gilman Road, FS27), north of the McCloud Bridge Campground, Whiskeytown–Shasta–Trinity National Recreation Area (563620 E, 4532200 N, 317 m), 15 October 1992 TF, EJ. – USNM 1020740, Battle Creek on east side of Coleman Fish Hatchery Road, 1.29 km east of Coleman Fish Hatchery, near (upsteam of) former site of pedestrian suspension bridge (573950 E, 4472540 N, 134 m), 28 August 2001 TF, EJ. – USNM 883465, Battle Creek near county park off Jellys Ferry Road, c. 0.16 km east of road on north side of creek near Tehama/Shasta County line (569860 E, 4471400 N, 111 m), 13 September 1993 TF, EJ.
Diagnosis: Readily differentiated from other regional pebblesnails by its large size (up to 8.0 mm shell height), very thin shell parietal lip, thick periostracum, very large central cusps of the central and lateral radular teeth, and smooth, rather elongate, penis.
Description: Hershler & Frest (1996) provided a detailed description of this species. A few aspects of this description are emended or expanded below, and some additional details are also provided, based on our study of a larger amount of new material.
Shell (Fig. 18) subglobose to ovate-conic. Shell body whorl sometimes having a distinct, subsutural angulation. Outer lip orthocline or weakly prosocline. Shell periostracum thickened. Last 0.5–0.75 whorl of operculum frilled. Cutting edge of central radular tooth near horizontal or slightly convex, lateral cusps two to eight; basal cusps one to three; basal tongue U-shaped, even with lateral margin. Inner marginal teeth having 13–23 cusps. Outer marginal teeth having 18–33 cusps; basal wing rectangular. Osphradium elongate, positioned opposite middle of ctenidium. Bursa copulatrix globose or pyriform (horizontal). Seminal receptacle small. Penis medium or large.
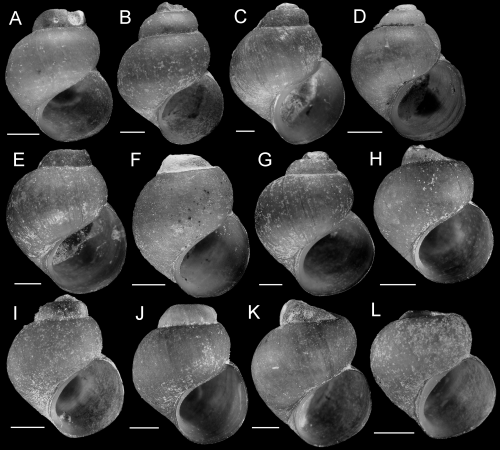
Shells of Fluminicola seminalis. A, USNM 1020737. B, USNM 1020738. C, USNM 1020740. D, USNM 1020742. E, USNM 1020743. F, USNM 1020744. G, USNM 1020746. H, USNM 1020747. I, USNM 1020748. J, USNM 1020750. K, USNM 1020804. L, USNM 1020805. Scales = 1.0 mm.
Distribution: The new records provided above extend the distribution of this species within the Pit River basin (Fig. 17).
Remarks: The populations in lower Ash Valley (Fig. 17) are distinguished by their small and frequently subglobose-shaped shells (Fig. 18D), but otherwise are identical with this species in all morphological details. Mitochondrial DNA sequences differed among lower Ash Valley populations by 0.2–0.6% (COI) and 0% (cytb), and varied among other populations of F. seminalis included in this study by 0–1.4% (COI) and 0–0.6% (cytb). The mean genetic distances between Ash Valley and other F. seminalis haplotypes were 0.91% (COI) and 0.18% (cytb).
In all of the phylogenetic analyses, F. seminalis was depicted (often with strong bootstrap support) as sister to F. modoci, with these two species forming clade B. On the basis of sequence divergence, these species differ by 5.0–6.1% (COI) and 6.4–8.0% (cytb).
Fluminicola umbilicatus sp. nov. (Hat Creek pebblesnail)
Type material: Holotype (Fig. 19A), USNM 1020705, Big Spring, tributary of Hat Creek, south of Old Station (PO) at the south end of Hat Creek Hill, flowing from beneath CA 89/44 on the south side, Lassen National Forest, Shasta County, California (629540 E, 4500140 N, 1403 m), 24 October 1992 TF, EJ. Paratypes (from same lot), USNM 1020706.
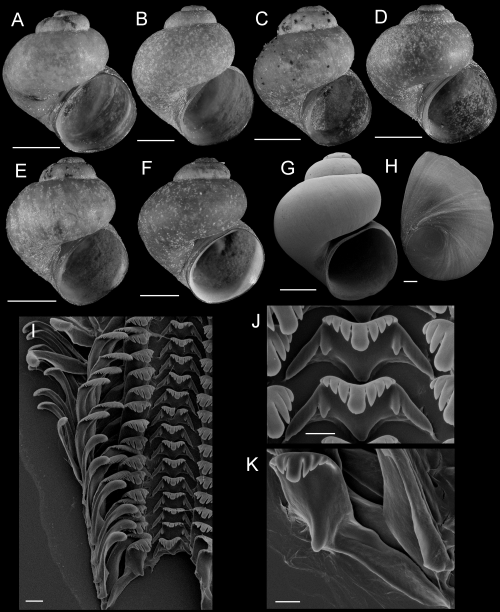
Shells, opercula, and radula of Fluminicola umbilicatus. A, holotype (USNM 1020705). B, USNM 1020706. C, USNM 1020709. D, USNM 1020710. E, USNM 1020711. F, USNM 1020716. G, USNM 1020706. Scales = 1.0 mm. H, outer side of operculum (USNM 1020706). Scale = 200 µm. I, portion of radular ribbon (USNM 1020706). Scale = 20 µm. J, central radular teeth (USNM 1020706). Scale = 10 µm. K, lateral radular tooth (USNM 1020706). Scale = 10 µm.
Referred material: CALIFORNIA. Shasta County: USNM 1020707, topotypes, 28 August 2001 TF, EJ. – USNM 1020708, Lost Creek (lower site) in lava field on north side of Wilcox Road, west of Hat Creek Rim, Lassen National Forest (632120 E, 4513600 N, 1141 m), 17 August 1991 TF, EJ, JJ. – USNM 1020709, ibid., 24 October 1992 TF, EJ. – USNM 1020710, ibid., 29 August 2001. TF, EJ. – USNM 1020711, Lost Creek (upper site) in lava field on north side of Wilcox Road, west of Hat Creek Rim, Lassen National Forest (632560 E, 4513140 N, 1141 m), 29 August 2001 TF, EJ. – USNM 1020799, Lost Creek (upper most site) in lava field on north side of Wilcox Road, west of Hat Creek Rim, inside and west of Lassen National Forest boundary (632730 E, 4513100 N, 1141 m), 3 November 2002 TF, EJ. – USNM 1020716, Hat Creek on upstream (east) side of CA 89 bridge, both sides of creek at Hat Creek Resort, east of Hat Creek Hill, Old Station Post Office (630240 E, 4500940 N), 28 August 2001 TF, EJ. – USNM 1020717, ibid., 29 August 2001 TF, EJ.
Diagnosis: Differs from similar congeners living elsewhere in the lower Pit River basin and Sacramento River headwaters (F. anserinus, F. potemicus, both described below) in its larger size, thinner inner shell lip, larger umbilicus, and longitudinally orientated bursa copulatrix. Readily distinguished from other pebblesnails living in the Lost Creek and Hat Creek drainages (F. ahjumawi, F. seminalis) by its smaller size and larger shell umbilicus.
Description: Shell (Fig. 19A–G; Table 11) subglobose to ovate-conic, spire usually entire; height, 2.10–5.41 mm; whorls, 3.25–4.50. Protoconch 1.4–1.6 whorls, diameter approximately 0.78 mm. Teleoconch whorls medium to highly convex, often having prominent shoulder. Last 0.5–0.25 whorl often disjunct in larger specimens. Aperture ovate. Parietal lip complete, usually narrowly adnate, thin, straight. Columellar lip narrow, thin. Outer lip thin, prosocline, weakly sinuate. Shell perforate, umbilicus usually broad and deep. Periostracum tan, brown or light green. Outer surface of operculum smooth (Fig. 19H). Central radular tooth approximately 56 µm wide, cutting edge convex, lateral cusps three to four; central cusp spoon-shaped; basal cusps usually one (rarely two); basal tongue V-shaped, even with lateral margin (Fig. 19J). Lateral tooth face broadly rectangular, central cusp weakly rounded or truncate; lateral cusps three to five (inner and outer); outer wing flexed, medium length (Fig. 19K). Inner marginal teeth having 22–27 cusps (Fig. 19I). Outer marginal teeth having 28–36 cusps; basal wing rectangular (Fig. 19I). Head-foot dark brown, almost black in some specimens. Ctenidium connected to pericardium by short, efferent branchial vessel; filaments approximately 20, well developed but without pleats. Osphradium elongate, positioned opposite middle of ctenidium. Bursa copulatrix longitudinal; ovate, pyriform, or triangular; duct short. Seminal receptacle medium-sized, often only partly overlapped by albumen gland. Capsule gland about as long as albumen. Genital aperture a small papilla. Penis large, thick, tightly coiled; rectangular along most of length; distal end pointed or papillate (Fig. 7I). Penis having dense black core extending to distal section; external pigment absent or consisting of a small brown patch near base. Penial duct positioned near outer edge, weakly undulating medially.
Distribution: Lost Creek and upper section of the Hat Creek drainage (lower Pit River basin) (Fig. 20).
Etymology: Referring to the large shell umbilicus of this species.
Remarks: This species was frequently depicted as sister to F. potemicus (described below) in the phylogenetic analyses. On the basis of sequence divergence, F. umbilicatus differs from this species by 3.6–3.8% (COI) and 5.5% (cytb) and from other upper Sacramento River basin species in clade C by 3.3–4.9% (COI) and 5.0–9.4% (cytb).
Populations of this species were referred to as Fluminicola n. sp. 7 and Fluminicola n. sp. 8 by Frest & Johannes (1993).
Fluminicola anserinus sp. nov. (Goose Valley pebblesnail)
Type material: Holotype (Fig. 21A), USNM 1020727, unnamed spring on west side of Goose Valley Road and Goose Valley, north of Goose Creek c. 0.8 km, Shasta County, California (607350 E, 4531460 N, 985 m), 30 September 1996 TF, EJ. Paratypes (from same lot), USNM 1020728.
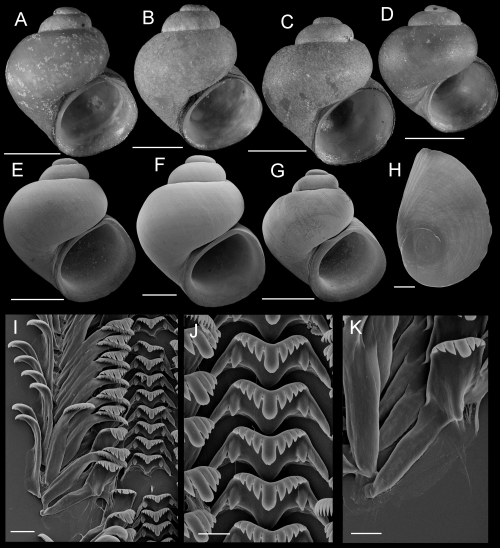
Shells, opercula, and radula of Fluminicola anserinus. A, holotype (USNM 1020727). B, USNM 1020730. C, USNM 1020732. D, USNM 1020734. E, G, USNM 1020728. F, USNM 1020732. Scales = 1.0 mm. H, outer side of operculum (USNM 1020728). Scale = 200 µm. I, portion of radular ribbon (USNM 1020728). Scale = 20 µm. J, central radular teeth (USNM 1020728). Scale = 10 µm. K, lateral radular tooth (USNM 1020728). Scale = 10 µm.
Referred material: CALIFORNIA. Shasta County: USNM 1020729, topotypes, 28 September 2001 TF, EJ. – USNM 1020730, Rim of the Lake Spring both sides of trail 0.4 km off Pacific Crest National Scenic Trail, south side of Lake Britton (Pit River) (616535 E, 4540800 N, 878 m), 23 September 2001 TF, EJ. – USNM 1020732, Blackberry Creek, north side of FS50, 5.87 km east of Pit 5 Dam, above (north of) Oak Flat and the Pit River, south side of Chalk Mountain, Shasta National Forest (598580 E, 4535740 N, 732 m), 28 September 1996 TF, EJ, JL. – USNM 1020733, ibid., 28 September 2001 TF, EJ, JL. – USNM 1020734, spring creek, north side of FS50, 7.68 km east of Pit 5 Dam, above (north side of) the Pit River, south side of Chalk Mountain, Shasta National Forest (600220 E, 4535460 N, 750 m), 19 October 1992 TF, EJ. – USNM 1020735, ibid., 28 September 2001 TF, EJ.
Diagnosis: Differs from similar and geographically proximal F. potemicus (described below) in its less evenly teleoconch whorls, larger aperture, broader columellar lip, thicker and consistently prosocline outer shell lip, smaller umbilicus, and shorter duct of the bursa copulatrix.
Description: Shell (Fig. 21A–G; Table 12) subglobose to ovate-conic; height, 1.99–3.69 mm; whorls, 3.25–3.75. Protoconch 1.3 whorls, diameter approximately 0.68 mm. Teleoconch whorls medium to highly convex, often wider below, often having well-developed shoulders. Last 0.25 of body whorl sometimes slightly loosened. Aperture ovate, slightly angled above. Parietal lip complete, usually narrowly adnate, rarely slightly disjunct. Columellar lip medium width, covering portion of umbilical region. Outer lip often somewhat thickened, often expanded, prosocline, sometimes weakly sinuate. Shell umbilicus rimate or perforate. Periostracum tan or light green. Last 0.5 whorl of operculum weakly frilled (Fig. 21H). Central radular tooth approximately 40 µm wide, cutting edge convex, lateral cusps four to five; central cusp parallel-sided, rounded, basal cusps one to two, basal tongue V- or U-shaped, even with lateral margin (Fig. 21J). Lateral tooth face broadly rectangular; central cusp truncate, lateral cusps two to three (inner), three to four (outer); outer wing flexed, medium length (Fig. 21K). Inner marginal teeth 22–28 cusps (Fig. 21I). Outer marginal teeth 24–38 cusps, basal wing rectangular (Fig. 21I). Head-foot dark brown. Ctenidium connected to pericardium by short, efferent branchial vessel; ctenidial filaments approximately 16, rather short, without pleats. Osphradium elongate, positioned opposite middle of ctenidium. Female reproductive anatomy shown in Figure 6F. Bursa copulatrix horizontal; ovate, pyriform or triangular; duct short. Seminal receptacle medium-sized, variably overlapped by albumen gland. Albumen gland having shallow rectal furrow. Capsule gland longer than albumen gland. Genital aperture a simple pore or small slit. Penis medium-sized, fairly thick, variably coiled, distal section pointed or papillate (Fig. 7J). Medial section of penis having dark melanin core, surface pale. Penial duct positioned near outer edge, almost straight.
Distribution: Short section of the lower Pit River drainage from Lake Britton (including Burney Creek drainage) to the vicinity of Chalk Mountain (Fig. 20).
Etymology: Referring to the type locality area, Goose Valley.
Remarks: This species was most frequently depicted in the phylogenetic analyses as sister to F. scopulinus (described below), from which it differs by 3.8–4.4% (COI) and 5.5–6.7% (cytb). It differs from other species of clade C described herein by 2.6–4.1% (COI) and 5.5–10.0% (cytb).
This species was referred to as Fluminicola n. sp. 6 by Frest & Johannes (1993).
Fluminicola potemicus sp. nov. (Potem Creek pebblesnail)
Type material: Holotype (Fig. 22A), USNM 1020718, unnamed spring pool on the west side of FS27 (Fenders Ferry Road), c. 10.8 rd km north of CA 299 junction, 4.18 km north of FS27 bridge over Potem Creek, west side of Potem Creek, inholding in Shasta National Forest, Shasta County, California (580660 E, 4524820 N, 439 m), 18 October 1992 TF, EJ. Paratypes (from same lot), USNM 1020719.
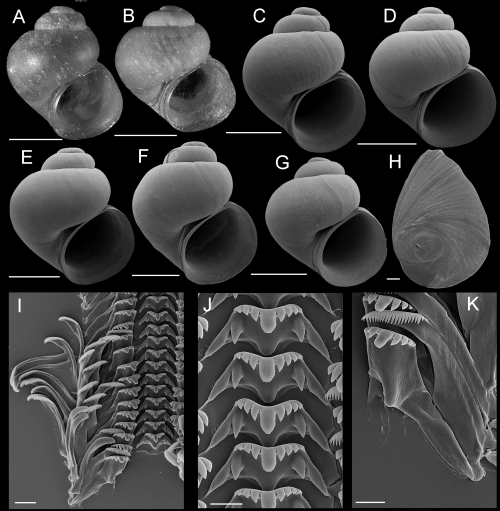
Shells, opercula, and radula of Fluminicola potemicus. A, holotype (USNM 1020718). B, USNM 1020719. C–G, USNM 1020719. Scales = 1.0 mm. H, outer side of operculum (USNM 1020719). Scale = 100 µm. I, portion of radular ribbon (USNM 1020719). Scale = 20 µm. J, central radular teeth (USNM 1020719). Scale = 10 µm. K, lateral and inner marginal radular teeth (USNM 1020719). Scale = 10 µm.
Referred material: USNM 1020720, topotypes, 28 September 2001 TF, EJ.
Diagnosis: Differentiated from similar species also living in the lower Pit River basin (e.g. F. anserinus) above. Differs from F. scopulinus (described below), from the Sacramento River headwaters, in its narrower shell (mean SH/SW, 1.182, 1.136, respectively, P = 0.008), smaller shell umbilicus, and more posteriorly positioned ctenidium.
Description: Shell (Fig. 22A–G; Table 13) broadly or ovate-conic, rarely having eroded spire; height, 2.55–3.31 mm; whorls, 3.0–3.75. Protoconch approximately 1.4 whorls, diameter approximately 0.51 mm. Teleoconch whorls medium convex, shoulders well developed. Aperture ovate, angled above. Parietal lip complete, narrowly adnate. Columellar lip narrow. Outer lip thin, orthocline or weakly prosocline. Shell perforate, umbilical region not excavated. Periostracum tan or light green. Outer surface of operculum smooth (Fig. 22H). Central radular tooth approximately 35 µm wide, cutting edge convex, lateral cusps three to four; central cusp parallel sided, rounded or weakly pointed; basal cusps one (two seen in one specimen); basal tongue U-shaped, even with lateral margin (Fig. 22J). Lateral tooth face broadly rectangular; central cusp rounded, lateral cusps two to three (inner), three to four (outer); outer wing weakly flexed, medium length (Fig. 22K). Inner marginal teeth having 22–28 cusps (Fig. 22I). Outer marginal teeth having 21–36 cusps; basal wing absent (Fig. 22I). Head-foot near pale, brown or grey. Ctenidium abutting pericardium; ctenidial filaments 14, without pleats. Osphradium elongate, positioned opposite middle of ctenidium. Bursa copulatrix horizontal, ovate or triangular, duct medium length. Seminal receptacle medium-sized, overlapped by albumen gland. Capsule gland a little longer than albumen gland. Genital aperture a simple pore. Penis medium-sized, usually tightly coiled, distal end tapering or pointed (Fig. 7K). Penis having medial patch of dark, internal pigment. Penial duct positioned near outer edge, nearly straight.
Distribution: Restricted to the type locality, which is situated in the lower Pit River basin, a short distance above Shasta Lake (Fig. 20).
Etymology: The specific epithet is based on the name of the type locality, which may have been derived from a Native American word meaning ‘mountain lion’ (Gudde, 1998).
Remarks: Fluminicola potemicus differs from other species of clade C described herein by 3.2–5.0% (COI) and 5.5–8.0% (cytb).
This species was referred to as Fluminicola n. sp. 2 by Frest & Johannes (1993).
Fluminicola scopulinus sp. nov. (Castle Creek pebblesnail)
Type material: Holotype (Fig. 23A), USNM 1020721, northern-most of three springs and runs south-west of Popcorn Spring, west side of North Fork Castle Creek, 0.35 km from FS25 along FS38N35Y (west side), Shasta National Forest, Shasta County, California (552540 E, 4557500 N, 808 m), 26 September 1996 TF, EJ, JL. Paratypes (from same lot), USNM 1020722.
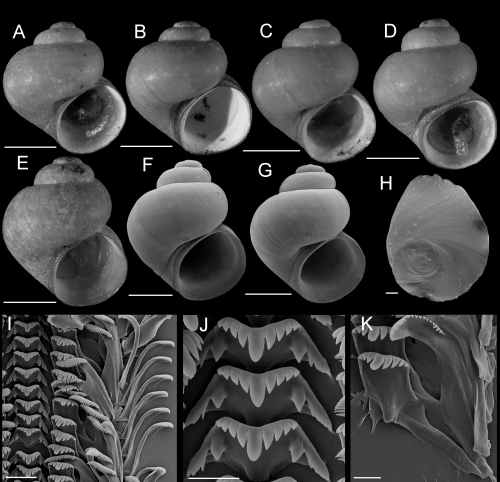
Shells, opercula, and radula of Fluminicola scopulinus. A, holotype (USNM 1020721). B, F, G, USNM 1020722. C, D, USNM 1020724. E, USNM 1020725. Scales = 1.0 mm. H, outer side of operculum (USNM 1020722). Scale = 100 µm. I, portion of radular ribbon (USNM 1020722). Scale = 20 µm. J, central radular teeth (USNM 1020722). Scale = 10 µm. K, lateral and marginal radular teeth (USNM 1020722). Scale = 10 µm.
Referred material: CALIFORNIA. Shasta County: USNM 1020723, topotypes, 25 August 2001 TF, EJ. – USNM 1020724, middle of three springs and runs south-west of Popcorn Spring, west side of North Fork Castle Creek, 0.27 km from FS25 along FS38N35Y (west side), Shasta National Forest (552560 E, 4557420 N, 805 m), 26 September 1996 TF, EJ, JL. – USNM 1020725, northern-most spring west of Popcorn Spring, west side of North Fork Castle Creek, 0.97 km from FS25 where run crosses FS38N35Y, Shasta National Forest (552540 E, 4558040 N, 842 m), 4 October 1996 TF, EJ. – USNM 1020726, ibid., 25 August 2001, TF, EJ.
Diagnosis: Differentiated from similar species living in the lower Pit River drainage (F. potemicus, F. umbilicatus) above. Also differs from these congeners in having a consistently triangular-shaped bursa copulatrix.
Description: Shell (Fig. 23A–G; Table 14) subglobose to ovate-conic, rarely having eroded spire; height, 2.06–3.49 mm; whorls, 3.25–4.0. Protoconch 1.3 mm, diameter approximately 0.58 mm. Teleoconch whorls medium to highly convex, often strongly shouldered. Last 0.25 whorl sometimes disjunct. Parietal lip complete, narrowly adnate or slightly disjunct. Columellar lip narrow. Outer lip rarely thickened, prosocline, often sinuate. Shell having rather wide, open umbilicus. Periostracum tan or light green. Outer surface of operculum smooth (Fig. 23H). Central radular tooth approximately 32 µm wide, cutting edge convex, lateral cusps three to four; central cusp parallel-sided, weakly pointed; basal cusps one to three; basal tongue V- or U-shaped, even with lateral margin (Fig. 23J). Lateral tooth face broadly rectangular; central cusp rounded, lateral cusps three to four (inner), three to five (outer); outer wing weakly flexed, medium length (Fig. 23K). Inner marginal teeth having 23–28 cusps (Fig. 23I). Outer marginal teeth having 25–35 cusps; basal wing broadly rectangular (Fig. 23I). Head-foot nearly pale or light grey. Ctenidium connected to pericardium by short, efferent branchial vessel; ctenidial filaments approximately 15, without pleats. Osphradium elongate, positioned opposite posterior part of ctenidium. Dorsal section of coiled oviduct sometimes having discrete, sperm-filled sac (secondary seminal receptacle). Bursa copulatrix horizontal, triangular, duct medium length. Seminal receptacle medium-sized, often exposed or only partly overlapped by albumen gland. Capsule gland longer than albumen gland, having rectal furrow. Genital aperture a short slit. Penis medium-sized, narrow, nearly straight to tightly coiled, distal end tapering or pointed (Fig. 7L). Penis having medial patch of black internal pigment, surface pale. Penial duct positioned near outer edge, undulating medially.
Distribution: Restricted to three closely proximal springs in the Castle Creek drainage, Sacramento River headwaters (Fig. 20).
Etymology: From New Latin scopulus, meaning crag, and referring to the distribution of this species in the vicinity of Castle Crags.
Remarks: Fluminicola scopulinus differs from other species of clade C described herein by 3.5–5.0% (COI) and 5.0–8.6% (cytb).
This species was referred to as Fluminicola n. sp. 14 by Frest & Johannes (1997).
Fluminicola multifarius sp. nov. (Shasta pebblesnail)
Type material: Holotype (Fig. 24A), USNM 883782, Big Springs (source) at Big Springs City Park north-west of the city of Mount Shasta, south of Spring Hill, Siskiyou County, California (556400 E, 4575265 N, 1092 m), 25 May 1991 TF, EJ. Paratypes (from same lot), USNM 1020753.
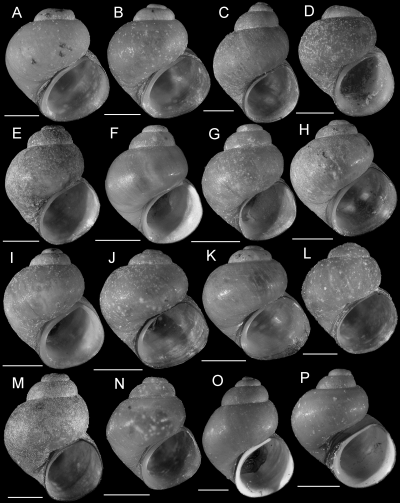
Shells of Fluminicola multifarius. A, holotype (USNM 883782). B, USNM 1020754. C, USNM 1020756. D, USNM 1020757. E, USNM 1020758. F, USNM 1020762. G, USNM 1020764. H, USNM 1020765. I, USNM 1020766. J, USNM 1020768. K, USNM 1020769. L, USNM 1020771. M, USNM 1020772. N, USNM 1020774. O, USNM 1020775. P, USNM 1020776. Scales = 1.0 mm.
Referred material: CALIFORNIA. Siskiyou County: USNM 1020754, topotypes, 12 October 1992 TF, EJ. – USNM 1020755, topotypes, 23 August 2001 TF, EJ. – USNM 1020756, Ney Springs on the north side of Ney Springs Road, north tributary of Ney Springs Creek, east of Faery Falls, south-west of the city of Mount Shasta (556120 E, 4568040 N, 964 m), 12 October 1992 TF, EJ. – USNM 1020757, ibid., 23 August 2001 TF, EJ. – USNM 1020758, Sacramento River on the north side, just upstream and opposite from the mouth of Stink Creek, at former site of USGS gauging station, just west (upstream) of the site of Cantara (Cantara Bend), near the end of Cantara Road, Cantara/Ney Springs Wildlife Area (California Fish and Game) (557860 E, 4568220 N, 915 m), 13 October 1992 TF, EJ. – USNM 1020759, ibid., 21 September 1996 TF, EJ. – USNM 1020760, ibid., 17 August 2000 TF, EJ. – USNM 1020761, unnamed spring above north side of Sacramento River, ditched on north side of UPRR track, tributary (west side) to first permanent creek west of Big Canyon Creek, east of Cantara Bend (Cantara town site) (559160 E, 4568405 N, 885 m), 6 September 1993 TF, EJ. – USNM 1020762, ibid., 1 October 2001 TF, EJ. – USNM 1020763, unnamed spring above sharp bend in Sacramento River (north side), collected on north side of UPRR track, east of Cantara Bend (Cantara town site) (558820 E, 4568560 N, 878 m), 6 September 1993 TF, EJ. – USNM 1020764, ibid., 1 October 2001 TF, EJ. – USNM 1020765, Big Springs three middle runs upstream of wooden bridges of access trail, c. 31 m west of eastern-most spring run, Big Springs City Park north-west of the city of Mount Shasta, south of Spring Hill (556370 E, 4575265 N, 1092 m), 23 August 2001 TF, EJ. –USNM 1020766, Big Springs western-most run upstream of wooden bridge of access trail, c. 61 m west of eastern-most spring run and 31 m west of three middle spring runs, south of Spring Hill on west edge of Big Springs City Park north-west of the city of Mount Shasta (556330 E, 4575280 N, 1092 m), 23 September 1996 TF, EJ, JL. – USNM 1020767, ibid., TF, EJ, JL. – USNM 1020768, Big Springs east spring run downstream of wooden bridge of access trail, c. 31 m south of source springs, Big Springs City Park north-west of the city of Mount Shasta, south of Spring Hill (556390 E, 4575240 N, 1092 m), 23 August 2001 TF, EJ. – USNM 1020769, west spring run of Big Springs on west side of Big Springs City Park, collected approximately 61 m below spring sources, north-west of the city of Mount Shasta, south of Spring Hill (556350 E, 4575220 N, 1089 m), 23 August 2001 TF, EJ. – USNM 1020771, Bundoora Spring c. 0.16 km west of Tom Cabin Spring and ruins of Tom’s Cabin, west of access road off FS40N44 c. 0.48 km west of Camp 4, Shasta National Forest (584400 E, 4564900 N, 1083 m), 30 September 2001 TF, EJ. – USNM 1020801, Lower Elk Spring, at spring house (577223 E, 4572303 N, 1179 m), 4 November 2004 AH. – USNM 1020802, ibid., 21 December 2004 JC. – USNM 1020803, Elk Spring, outflow channel (577316 E, 4572190 N, 1172 m), 21 December 2004 JC. – USNM 1020772, spring flowing under UPRR tracks (source on west side of tracks), collected on both sides of tracks, west of Sacramento River, opposite mouth of Sweetbrier Creek, near the former site of Conant (556720 E, 4551100 N, 561 m), 25 August 2001 TF, EJ. – USNM 1020773, Crystal Spring near its source (which is now a covered well) on west-facing slope above the Sacramento River, above bridge of Zig Zag Trail, Shasta Springs, St. Germain Foundation (561580 E, 4566360 N, 854 m), 17 October 1992 TF, EJ. – USNM 1020774, ibid., 25 August 2001 TF, EJ. – USNM 1020775, 1020776, Rock Spring above (east of) UPRR tracks, above Sacramento River (east side), Shasta Springs (561580 E, 4566200 N, 769 m), 17 October 1992 TF, EJ. – USNM 1020777, 1020778, ibid., 24 August 2001 TF, EJ. – USNM 1020779, fourth spring from the north at the lower (downstream) end of Shasta Springs complex, east side of the Sacramento River above UPRR track (561560 E, 4566000 N, 756 m), 21 October 1992 TF, EJ. – USNM 1020780, 1020781, 1020782, 1020792, ibid., 24 August 2001 TF, EJ. – USNM 1020800, springs north of Mossbrae Falls on a west-facing slope, east side of the Sacramento River above UPRR track near the north end of railroad bridge (561520 E, 4565800 N, 756 m), 21 October 1992 TF, EJ. – USNM 1020783, ibid., 24 August 2001 TF, EJ. – USNM 1020784, spring runs north of Mossbrae Falls on a west-facing slope, east side of the Sacramento River, near the east side of UPRR bridge (561560 E, 4565720 N, 769 m), 21 October 1992 TF, EJ. – USNM 1020785, ibid., 24 August 2001 TF, EJ. – USNM 1020786, east side of the Sacramento River at Cave Springs below Cave Springs Resort and Motel, Dunsmuir; 30 m transect north (upriver) from Cave Springs run (560710 E, 4564080 N, 724 m), 24 August 2001 TF, EJ. – USNM 1020787, ibid., 14 October 2003 TF, EJ. – USNM 1020788, spring run north of Crystal Spring (Glacier Spring) run, north side of northern most switch back of Zig Zag Trail, Shasta Springs, Saint Germain Foundation (561520 E, 4566310 N), 25 August 2001 TF, EJ.
Diagnosis: Readily distinguished from F. scopulinus, which also lives in the Sacramento River headwaters, by its smaller shell umbilicus and broader columellar lip. Distinguished from F. seminalis, which also lives in the McCloud River drainage, by its smaller size, more convex shell whorls, well-developed shell parietal lip, more numerous radular tooth cusps, and squatter penis.
Description: Shell (24, 25; Table 15) usually subglobose to narrow conical; height, 2.30–4.64 mm; whorls, 3.25–4.50. Protoconch 1.4 whorls, diameter approximately 0.82 mm; spiral microsculpture often wavy or wrinkled near apex. Teleoconch whorls weakly to highly convex, often having weak subsutural angulation; shoulders usually absent or narrow, rarely broad. In some conical shelled populations, specimens had last 1.0 whorl loosened from body whorl, producing an almost scalariform appearance (24, 25). Aperture broadly ovate, often angled above. Parietal lip complete, thin or slightly thickened, usually adnate, sometimes slightly disjunct, lip usually forming callus. Columellar lip medium width, often covering all or most of umbilical region. Outer lip thin or slightly thickened, sometimes markedly so, prosocline, weakly sinuate. Shell usually anomphalous, sometimes narrowly rimate, rarely perforate. Periostracum tan, brown, or light green. Last 0.5 operculum whorl frilled (Fig. 25L). Central radular tooth approximately 41 µm wide, cutting edge convex, lateral cusps three to four; central cusp parallel-sided, rounded or weakly pointed; basal cusps one to two, basal tongue V- or U-shaped, even with lateral margin (Fig. 25N). Lateral tooth face broadly rectangular; central cusp rounded, lateral cusps three (inner), three to four (outer); outer wing flexed, medium length (Fig. 25O). Inner marginal teeth having 22–29 cusps (Fig. 25M). Outer marginal teeth having 23–33 cusps; basal wing rectangular (Fig. 25M). Head-foot dark brown, almost black. Ctenidium connected to pericardium by short, efferent branchial vessel; ctenidial filaments approximately 17, without pleats. Osphradium elongate, positioned opposite middle of ctenidium. Bursa copulatrix horizontal; ovate, pyriform or triangular; duct medium length. Seminal receptacle small or medium-sized, sometimes only partly overlapped by albumen gland. Glandular oviduct having well-developed rectal furrow. Capsule gland about as long as albumen gland. Genital aperture a simple pore or weak papilla. Penis medium-sized, fairly broad, tightly coiled, distal end pointed or papillate (Fig. 7M). Penis having dark brown or black patch of internal pigment concentrated distally, surface pigment very light. Penial duct positioned near outer edge, straight except for a few distal undulations.
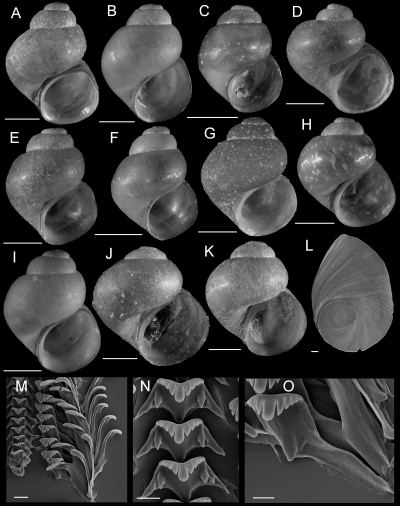
Shells, opercula, and radula of Fluminicola multifarius. A, USNM 1020779. B, USNM 1020780. C, USNM 1020781. D, USNM 1020782. E, USNM 1020783. F, USNM 1020784. G, USNM 1020786. H, USNM 1020788. I, USNM 1020792. J, K, USNM 1020801. Scales = 1.0 mm. L, outer side of operculum (USNM 1020753). Scale = 100 µm. M, portion of radular ribbon (USNM 1020753). Scale = 20 µm. N, central radular teeth (USNM 1020753). Scale = 10 µm. O, lateral radular teeth (USNM 1020753). Scale = 10 µm.
Distribution: Sacramento River headwater region (as far downflow as Conant), and a few sites in the upper reaches of the McCloud River drainage (Fig. 20).
Etymology: From New Latin multifarius, meaning in many places, or in various manners, and referring to the broad range of shell shape observed in this species.
Remarks: As noted in the Results section, this species is composed of two distinct subclades that differ by 2.1–3.0% (COI) and 3.3–4.7% (cytb) and are allopatrically distributed, with one ranging from Shasta Springs to Conant along the Sacramento River and the other distributed in the Sacramento headwater region from Cantara Bend to Big Springs (north of the city of Mount Shasta) as well as in the upper McCloud River drainage. Marked shell variation, with shape ranging from subglobose to narrowly conic and the entire body whorl sometimes completely loosened from the coiling axis, was observed within both subclades and sometimes within individual populations. Distinct shell morphs (e.g. at site 65) collected in sympatry did not differ in sequences of either gene and also did not differ anatomically. On the basis of these observations and the morphological overlap described above, we chose to treat the two subclades as a single species at this time, although the evolutionary and taxonomic significance of the remarkable shell variation within this taxon merits additional study. F. multifarius was variably positioned relative to other species of clade C described herein in the phylogenetic analyses and differs from these by 2.6–4.6% (COI) and 6.4–10.3% (cytb).
Populations of this species were referred to as Fluminicola n. sp. 1, Fluminicola n. sp. 3, Fluminicola n. sp. 4, and Fluminicola n. sp. 5 by Frest & Johannes (1993).
ACKNOWLEDGEMENTS
We thank California State Parks for issuing a collecting permit. We thank Susan Groves, Andrew Scotti, and Andrew Urlie (MacArthur–Burney Falls State Park, California); Leslie Steidl (California State Parks), and Stewart Reid (United States Fish and Wildlife Service, Klamath Falls, Oregon) for field assistance. We also thank many private landowners for permission to collect on their property. Richard Lis, Mark Stopher, and Stephen L. Turek (California Department of Fish and Game, Redding) assisted with various stages of this project. Tom Quinn (University of Denver) and Sara Oyler-McCance (United States Geological Survey) generously shared bench space and equipment in the Rocky Mountain Center for Conservation Genetics and Systematics. Karie Darrow prepared shell and anatomical drawings, Molly Ryan assisted with preparation of the distributional maps, and Yolanda Villacampa collected shell and radular data and prepared scanning electron micrographs. Chris Henry and Rebecca Lange provided copies of manuscripts in press and Margaret Docker shared the results of her unpublished molecular studies of Goose Lake lampreys. Fiscal support for this project was provided by California Department of Fish and Game (contract FG2016R1) and the Cantara Trustee Council (grants C0010010, C0110013).




