The ‘egg sandwich’: a method for linking spatially resolved salmonid hatching rates with habitat variables in stream ecosystems
Abstract
This paper describes the development of the ‘egg sandwich’, a system for assessing stream substratum quality by linking measurements of depth-specific salmonid egg hatching success and physico-chemical water variables from the same sites within the interstitial zone.
Biodiversity in freshwater ecosystems is critically threatened globally (Ricciardi & Rasmussen, 1999; Jenkins, 2003) with stream ecosystems being most heavily affected (Stein & Flack, 1997; Pimm et al., 2001; Gleick, 2003). Increasing evidence suggests that the properties of the stream substratum have a strong effect on the overall health of stream ecosystems (Palmer et al., 1997; Geist & Auerswald, 2007). Conservation efforts in salmonid habitats have traditionally focused on stream substratum and spawning site restoration (Grost et al., 1991; Acornley & Sear, 1999; Milan et al., 2000; Soulsby et al., 2001). In light of the strong interest in restoration and assessment of stream substrata quality and salmonid spawning grounds, there is a need to provide tools for integratively assessing physico-chemical and biological indicators.
Here, a method for assessing stream substratum quality by measuring depth-specific salmonid egg hatching success, and physico-chemical water variables from adjacent sites within the interstitial zone is presented. The applicability of this ‘egg sandwich’ (ES) was successfully tested in the laboratory and in natural and artificially constructed spawning sites of brown trout Salmo trutta L. and grayling Thymallus thymallus L.
The ES is composed of two principal subunits: an egg exposure unit and a unit for extracting interstitial water samples from the same substratum depth layers in which the eggs are exposed (Fig. 1). The egg exposure unit consists of an aluminium grid and two perforated aluminium plates on the outside, creating 10 × 13 dice-like chambers. Each chamber has a volume of 3·375 cm3, providing sufficient space for the hatched fry. In the test, one fertilized egg per chamber was exposed, resulting in 10–13 replicates distributed over different depth horizons, and a total of 112 exposed eggs per box. Five chambers are penetrated by stainless steel socket-head screws for fixing both units and thus cannot house eggs. The upper horizontal row serves as a visual indicator for monitoring the exposure depth of the box.
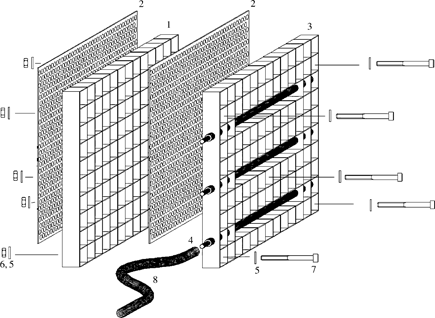
Construction scheme of the ‘egg sandwich’. 1, aluminium grid [naturally anodized, length (L): 195 mm, width (W): 150 mm, depth (D): 15 mm, material thickness (MT): 0·5 mm, 130 chambers, L: 15 mm, W: 15 mm and D: 15 mm]; 2, perforated aluminium plate (L: 198 mm, W: 140 mm and MT: 1 mm, perforation bore diameter: 2 mm, partition: 3·5 mm diagonally lined); 3, aluminium grid (like 1, bore diameter for PVC tubes 8 mm); 4, perforated PVC tube [L: 220 mm, inner diameter (ID): 5·5 mm, outer diameter (OD) 7·5 mm, one end with sliding socket OD: 5 mm and ID: 3·5 mm, other end sealed]; 5, flat washer (stainless steel, M4, OD: 19·5 mm and MT: 1·2 mm); 6, socket-head screw (stainless steel, M4, L: 45 mm, length of thread: 20 mm, thread lead: 1·5); 7, hexagonal nut or wing nut (stainless steel, M4, thread lead: 1·5); 8, PVC flexible hose (OD: 8 mm, MT: 1·5 mm and L: 1200 mm).
A second unit for extracting interstitial water is attached to the egg exposure unit. Its construction resembles that of the egg exposure unit, but the grid is penetrated by three perforated PVC tubes for sampling interstitial water at pre-defined depth horizons. One end of the tubes is equipped with sliding sockets to which flexible hoses with a length of 1·2 m are attached. The other end is closed with an elastic joint seal. Hoses can be sealed and individually marked with different colour codes to ensure correct depth assignments of water samples extracted through the hoses. In practical tests, sampling at 20, 70 and 115 mm proved successful, but this system can be easily adapted for sampling in even more horizons or in different depths. ES size, the size of the chambers and the number of eggs exposed in each chamber can be varied according to the research question addressed.
The ES is loaded with fertilized eggs by placing it into a shallow water tank. Ideally, the water level in the tank barely covers the grid. Individual chambers can be filled with fertilized eggs using a large core pipette or a turkey baster. After closing the lid, boxes should permanently stay immersed in cool, oxygenated water to prevent damage to the eggs. For the assessment of substratum conditions in a typical field setting, the egg-filled ‘sandwich box’ is vertically inserted into the stream substratum of the study site. For comparisons of conditions between free-flowing water and different substratum depths, it is recommended that the box is buried at a depth, which ensures that the upper box surface layer stays exposed to the free-flowing water conditions above the stream bed level. To ensure minimal disturbance of the native stream bed characteristics, a spade is used to create a small gap within the substratum into which the ES can be inserted (Fig. 2). Reference exposure of eggs in the free-flowing water (e.g. within a swimming box) allows for determination of specific development stages and hatching dates, which vary depending on the temperature day degree sum at the study sites.
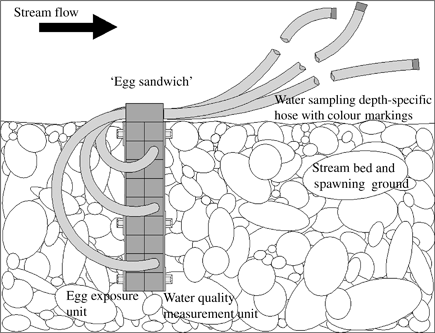
Schematic side view of the exposed ‘egg sandwich’ in the stream bed; note that the egg exposure unit is situated upstream of the water quality measurement unit.
During egg exposure, water samples are extracted by attaching a mobile 100 ml syringe to the hoses and by creating a vacuum, similarly to the procedure of sampling interstitial water in the stream substratum as described in Geist & Auerswald (2007). In a first step, the water volume entrapped inside the hose needs to be sampled and discarded before interstitial water from the defined substratum depths can be collected. The water volume has to be calculated or measured by the length and inner diameter of the hose. In the present exposures, water samples were analysed for pH, electric conductivity, temperature, dissolved oxygen, nitrite, nitrate, ammonium and redox potential.
After hatching, the ES can be excavated and re-opened. Hatching success can be assessed according to the following criteria: (a) living fry, indicating favourable substratum conditions, (b) dead fry, indicating favourable substratum conditions during egg development but unfavourable conditions in the final exposure stage, (c) dead egg, indicating non-fertilized eggs or unfavourable conditions during early development and (d) missing egg due to predation, decomposition or erroneous loading of the chamber. An example evaluation is shown in Fig. 3. Further variables, such as siltation or clogging of chambers, can also be assessed at this stage, e.g. by taking photographs of the chambers and using computer-based image processing applications.
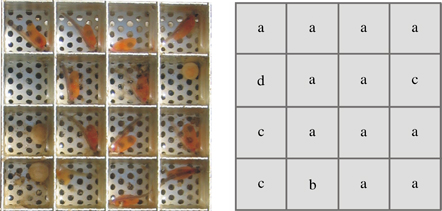
Proposed evaluation key for the ‘egg sandwich’; assessment of salmonid egg development (a, living fry; b, dead fry; c, undeveloped egg and d, chamber empty).
Applicability of the ES was tested by field and laboratory tests, including the following aspects: (1) mean hatching rates (HR) were compared between ‘egg sandwich’ exposure and reference egg exposure in regular upflow incubation trays (as typically used in salmonid hatcheries) under otherwise identical conditions (2) HR in the ES exposure were compared to the most commonly used field egg-exposure system, the modified Whitlock-Vibert boxes (WV-box; Whitlock, 1979; Mackenzie & Moring, 1988) at the same sites in the River Moosach (38°23′39″ N; 11°43′26″ E), (3) hatching rates of (1) and (2) were compared to ES substratum exposed in a laboratory flume. Detailed descriptions of numbers of replicates are provided in Fig. 4. One-way ANOVA and Tukey post hoc tests with SPSS 11 (SPSS Inc., Chicago, IL, U.S.A.) were used to compare treatments. The spatial resolution of the ES exposure was resolved by testing pair-wise differences in HR between three different depth layers of stream substratum (20, 70 and 115 mm) exposed ES boxes in the River Moosach. All investigations were carried out in winter 2007 to 2008.
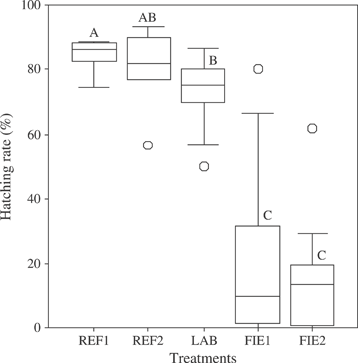
Comparison between different egg exposure treatments: REF1, reference exposure in upflow incubation trays under regular hatchery conditions (n = 4 replicates, each with 1000 eggs); REF2, reference exposure in the ‘egg sandwich’ under the same conditions as REF1 (n = 6 replicates, each with 30 eggs); LAB, ‘egg sandwich’ exposure in substratum within a laboratory flume (n = 18 replicates, each with 3 × 30 eggs at depths 1, 2 and 3); FIE1, ‘egg sandwich’ exposure to natural stream substrata in the River Moosach (n = 25 replicates, each with 3 × 30 eggs at depths 2·0, 7·0 and 11·5 cm); FIE2, Whitlock-Vibert box exposure (encased with 1 mm gauze to avoid the escape of fry) at the same sites like FIE1 (n = 25 replicates, each with 200 eggs). Different upper case letters indicate significant differences at P < 0·05; box plots show the median and the interquartile range; 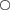 , outliers.
, outliers.
The results of these comparisons (Fig. 4) revealed that HR of eggs exposed in the ES (mean ±s.d. = 80 ± 13%) did not differ (Tukey HSD, P > 0·05) from those in the upflow incubation trays (84 ± 5%), suggesting no systematic correction factor must be applied when using an ES exposure instead of direct egg exposure. Hatching periods in the ES closely (± 2 days) matched the hatching periods of the reference samples. Mean HR did not differ (P > 0·05) between ES and WV-box in the field exposure, indicating that an assessment of stream substratum conditions will deliver similar results in both cases. Variability in HR was higher in the ES compared to the WV-box, however, since HR and physico-chemical water variables differed markedly in different depth horizons within ES boxes (Fig. 5). In the field test set, concentrations of dissolved oxygen, nitrite and nitrate, redox potential and pH value were the most determining factors for egg survival, whereas temperature, concentration of ammonium and electric conductivity explained little, or no variation in hatching success.
Pair-wise differences in hatching rates (▵HR) between three different depth layers of stream substratum exposed ‘egg sandwich’ boxes in the River Moosach (n = 25 for each depth layer); box plots show the median and the interquartile range;  , outliers; note that Δ is greatest between the most distant depth layers 1 and 3, although overall differences are not significant (ANOVA, P > 0·05).
, outliers; note that Δ is greatest between the most distant depth layers 1 and 3, although overall differences are not significant (ANOVA, P > 0·05).
Considering the significant sampling site effect (P < 0·001) on HR in ANOVA with interactions, the relation between exposure depth and hatching success became significant (P < 0·001). This indicates that an assessment of habitat quality at a high spatial resolution on a microhabitat scale is advantageous.
Hatching rates in the laboratory flume resembled those of the reference exposures in the upflow incubation trays but were significantly lower in both field exposures. This result can most probably be explained by the adverse effects of high fine sediment loads and low oxygen values in the stream substratum of the River Moosach compared to the flume exposure in coarse substratum with high oxygen saturation. Thus, the salmonid egg development in the ES unit is not significantly different from that under natural conditions if water quality is sufficient, which proves the suitability of the ES for assessing stream substratum quality.
Different alternative systems for hatching salmonid eggs in streams have been previously described (Vibert, 1949; Whitlock, 1979). Most of these systems, however, were primarily designed for salmonid propagation with the purpose of directly releasing hatched fishes into the stream. These systems are of limited use for assessing HR and for linking these with stream substratum quality variables, although some authors describe the use of modified Whitlock-Vibert boxes suitable for assessing hatching success (Mackenzie & Moring, 1988). Systems specifically designed for the assessment of HR under natural conditions both during exposure to the free-flowing water (Rubin, 1995; Donaghy & Verspoor, 2000) and in the stream substratum (Harris, 1973; MacCrimmon et al., 1989; Pauwels & Haines, 1994; Rubin, 1995; Donaghy & Verspoor, 2000; Bernier-Bourgault et al., 2005; Dumas & Marty, 2006) have been developed. Most of these methods, however, have not been designed to allow an assessment of spatial variation at different substratum depths (Harris, 1973; Pauwels & Haines, 1994; Rubin, 1995; Bernier-Bourgault et al., 2005), which appears to be crucial at least in the stream investigated in this study. As far as is known, however, none of these systems is coupled with a measurement unit, which allows linking the biological effect of hatching success with adjacent water variables. Also, exposure of single eggs in separate chambers is more difficult with other systems compared to the ES, which can be a crucial factor if infection and transmission of fungi is a major problem. Due to the compact slight design of the ES and the planting technique of creating a small gap in the riverbed substratum, the disruption of the interstitial zone is marginal compared to the planting of other systems (Donaghy & Verspoor, 2000).
In conclusion, practical experience with the use of the ES suggests that this technique provides an easy tool with high operational reliability for assessing stream substratum quality by linking spatially resolved salmonid egg survival and physico-chemical water variables from the same sites within the interstitial zone. This system may also be used for incubation of other species, such as juvenile freshwater bivalves, for which assessment of stream substratum quality is of great importance (Buddensiek et al., 1990; Geist & Auerswald, 2007).




