Behaviour and performance of juvenile shortnose sturgeon Acipenser brevirostrum at different water velocities
Abstract
Critical swimming speeds (mean ±s.e.) for juvenile shortnose sturgeon Acipenser brevirostrum were 34·4 cm s−1± 1·7 (2·18 ± 0·09 body lengths, BL s−1). Swimming challenges at 10, 20 and 30 cm s−1 revealed that juvenile A. brevirostrum are relatively poor swimmers, and that the fish did not significantly modify their swimming behaviour, although they spent more time substratum skimming (i.e. contact with flume floor) at 30 cm s−1 relative to 10 cm s−1. When present, these behavioural responses are probably related to morphological features, such as flattened rostrum, large pectoral fins, flattened body shape and heterocercal tail, and may be important to reduce the costs of swimming.
Levels of swimming performance in fishes are defined in terms of duration of swimming (Beamish, 1978). The range of swimming speeds over which a fish can function has been categorized into three broad categories: sustained, prolonged and burst-type (Beamish, 1978; Hammer, 1995; Plaut, 2001). Most swimming performance studies have been conducted on freshwater salmonids (Hammer, 1995; McDonald et al., 1998; McFarlane & McDonald, 2002). Many of these studies have focused on the physiological aspects of swimming, such as metabolic costs (e.g. oxygen consumption), time to fatigue and muscle and blood physiology (Milligan, 1996; Kieffer, 2000). Swimming ability among fishes can vary with differences in anatomy (e.g. position of fins and type of tail), physiology and behaviour (Webb, 1993; Peake & Farrell, 2006). While a relatively large amount of performance data has been collected for various salmonid species, comparatively little information exists for migratory non-salmonids, such as Acipenseridae (sturgeons) (Peake, 2004).
Sturgeons have retained many ancestral body characteristics that distinguish them as a ‘primitive’ fishes. Among these primitive characteristics are the protective armour or scutes, a largely cartilaginous skeleton (Kynard, 1997), the presence of a notochord, large and flattened rostrum, rigid pectoral fins and the shark-like heterocercal tail (Scott & Crossman, 1973). These anatomical features can influence the swimming ability and the behaviour of sturgeons (Webb, 1986; Wilga & Lauder, 1999; Liao & Lauder, 2000; Peake, 2004). For example, sturgeons generate more drag per unit surface area probably because of the presence of bony plates (scutes) and roughened skin (Webb, 1986). Bottom holding, via the large pectoral fins, is a behaviour that many sturgeons employ to hold station in a current (Peake, 2004). These behaviour patterns appear to be important for reducing the overall energetic costs associated with swimming in sturgeons (Peake, 2004).
Studies have examined the physiological response to exhaustive (burst) exercise stress in various acipenserids (Barton et al., 2000; Belanger et al., 2001; Kieffer et al., 2001; Lankford et al., 2003; Baker et al., 2005). These studies have demonstrated that juvenile sturgeons exhibit both behavioural and physiological responses to exhaustive exercise, but the anaerobic components and physiological responses (i.e. lactate and cortisol dynamics) are considerably reduced when compared to teleosts (Kieffer et al., 2001). It therefore appears that burst exercise may not appear to play a large role in the swimming performance of juvenile sturgeons. These results further suggest that aerobic swimming may be more important at this life-history stage. The swimming performance of shortnose sturgeon Acipenser brevirostrum Lesueur is not known, but Peake et al. (1995) found that lake sturgeon Acipenser fulvescens Rafinesque were poor swimmers in all categories (e.g. endurance and burst swimming) relative to salmonid species. Adams et al. (1999) also noted that pallid sturgeon Scaphirhynchus albus (Forbes & Richardson) demonstrated a reduced swimming performance compared with other fishes.
To address the swimming performance of juvenile A. brevirostrum, the (1) critical swimming capacity (UCrit), (2) ventilation frequency and (3) behavioural aspects of swimming (e.g. time swimming and time station holding) of A. brevirostrum swimming at various speeds were assessed. Station holding is a behavioural strategy used both by freshwater (Arnold et al., 1991; Graham et al., 1996; Gerstner, 2007) and marine species (Webb, 1989; Gerstner & Webb, 1998) to potentially reduce the cost of swimming in high-flow environments. It was hypothesized that juvenile A. brevirostrum, like other sturgeons (Peake, 2004) would have low UCrit relative to other fish species and would modify their swimming behaviour (e.g. increase the station holding performance) in response to increased water velocities. Because A. brevirostrum live in large tidal rivers (Scott & Crossman, 1973), which probably vary in water velocities, these potential behavioural changes could be important in reducing the metabolic costs of swimming in variable water flow environments.
Acipenser brevirostrum were raised from eggs originating from the St John River (New Brunswick, Canada) stock. Juvenile fish were fed daily to satiation, but were not fed on the day before the start of experiments. Individual fish [mean total length (LT) = 160 mm, range 140–180 mm; n = 8) were removed from their respective tanks and held overnight, at very low flows (c. 3 cm s−1), in a 10 l swim flume (Loligo Instruments, Tjele, Denmark). These flows did not induce swimming. The UCrit tests [temperature (T) = 15° C] were performed on individual fish by increasing the water velocity by increments of 10 cm s−1 every 60 min until the fish became exhausted (Kieffer et al., 1998). Water flow to the respirometers was continuous throughout the UCrit test. The UCrit was determined for fish using the equation: UCrit=Vf + (Tit−1 × dV) (Brett, 1964), where UCrit is in cm s−1, Vf is the speed in cm s−1 of the last completed swimming period, dV is the velocity increment in 10 cm s−1, t is the time swum at each velocity (60 min) and Ti (min) is the time swum at the final velocity before exhaustion. After the UCrit test, the fish were lightly anaesthetized (neutralized MS-222 final concentration 0·10 g l−1), patted dry and the fork length (LF, to the nearest mm) was measured. All UCrit values were expressed as both cm s−1 and body lengths per s (BL s−1).
In a second experiment, individual fish (T = 15° C; LF = 160 mm, range 140–180 mm; n = 8; note: these fish were not used for UCrit determination) were removed from their respective tanks and held overnight in the flume under the same conditions noted for the UCrit tests. Juvenile fish were fed daily to satiation but were not fed on the day before the start of experiments. The following morning, the water speed of the flume was increased to 10 cm s−1. Each fish (n = 8) was video-taped (Sony digital 8 recorder; Tokyo, Japan) for the various swim challenges. Following the completion of this 60 min 10 cm s−1 swim trial, the same individual was then challenged to swim at 20 cm s−1 for 60 min. This process was repeated for 30 and 40 cm s−1. All eight fish were able to swim for the 10, 20 and 30 cm s−1 swim speeds for the respective hour, but only six of these fish were able to complete part or all of the 1 h 40 cm s−1 swim challenge. As a result, only data for speeds up to and including 30 cm s−1 (c. 87% of UCrit) were analysed. From the video-tapes, the following were assessed: (1) ventilation frequency (number of opercular beats per min), (2) % station holding [the use of the broad pectoral fins and flattened ventral body surface to anchor the body to the floor of the tunnel (fish held this position without propulsion generated by body and caudal fin undulation)], (3) number of station holds per minute, (4) average duration of a station hold (in s) and (5) % time swimming. From the video-tapes the following were also assessed: (1) % total swimming, (2) % substratum skimming and (3) % free swimming (Adams et al., 2003). Substratum skimming is a behaviour fish use where the ventral body surface is in contact with the swim tunnel bottom, but propulsion continues to be generated by body and caudal fin movements, and free swimming is swimming within the water column without contact with the substratum (Adams et al., 2003).
Prior to analyses, data were tested for normality (Shapiro–Wilk test; PROC UNIVARIATE; SAS Institute, Cary, NC, U.S.A.) and homogeneity of variance using an F-max test (Sokal & Rohlf, 1981). Per cent data were arc-sine square-root transformed prior to analysis (Zar, 1999). Data were normal and variance homogeneous. Linear regression (Sigma stat, version 3.0) was used to test the effect of LF on the UCrit. Mixed repeated measures model of variance (PROC MIXED; SAS Institute), with water velocity as the fixed effect and fish as the random effect, was used to determine effect of water velocity on: ventilation frequency, % station holding, number of station holds per min, average duration of a station hold, % total swimming, % substratum skimming and % free swimming. An autoregressive function was used as the basis for the covariance structure since it proved to be the best fit for these data; this was based on SAS Fit Statistics for Proc Mixed. The d.f. were estimated using the Kenward–Roger function, and least-square means and their s.e. were generated. A posteriori analyses of means were conducted using a Tukey adjustment for multiple comparisons. α in all cases was 5%.
There was no relationship between UCrit and LF for the size range of the fish used in this study. There was no effect of water velocity on % time spent swimming [P > 0·05; Fig. 1(a)]. Critical swimming speeds (mean ±S.E.) for A. brevirostrum were 34 cm s−1 (2·18 ± 0·09 BL s−1). Time spent substratum skimming did increase with water velocity [P < 0·01; Fig. 1(b)]. There was no significant relationship between swimming speed and % time free swimming [P > 0·05; Fig. 1(c)], % time station holding [P > 0·05; Fig. 2(a)], number of station holds [P > 0·05; Fig. 2(b)] or average station hold [P > 0·05; Fig. 2(c)]. The average station hold was c. 15 s across all water velocities [Fig. 2(c)]. There was a clear and consistent increase in the metabolic cost of swimming, measured as ventilation frequency (gill opercular beats min−1), at higher speeds [P < 0·01; Fig. 3). The ventilation rate increased from c. 80 opercular beats min−1 at 10 cm s−1 to c. 100 opercular beats min−1 at 30 cm s−1.
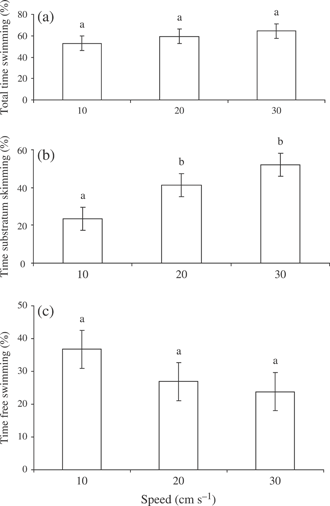
Least-square mean ±s.e. (a) % total time swimming (b) % time substratum swimming and (c) % time free swimming and swimming speed for juvenile Acipenser brevirostrum at 15° C (n = 8 at each speed). Values with different lower case letters are significantly different (P < 0·05). Note that data are presented as arc-sine square-root transformations.
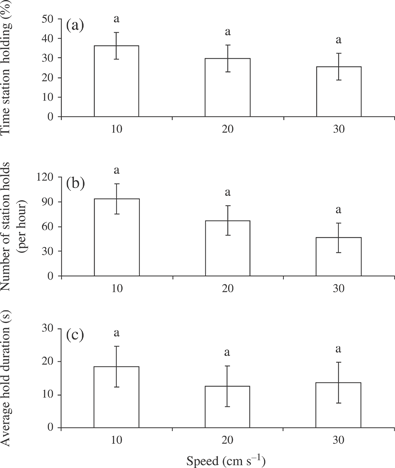
Least-square mean ±s.e. (a) % time station holding, (b) number of station holds and (c) average hold duration and swimming speed for juvenile Acipenser brevirostrum at 15° C (n = 8 at each speed). Values with different lower-case letters are significantly different (P < 0·05). (a) Note that data are presented as arc-sine square-root transformations.

Least-square mean ±s.e. gill ventilation frequency (opercular beats per minute) at increasing swimming speeds in juvenile Acipenser brevirostrum at 15° C (n = 8 at each speed). Values with different lower case letters are significantly different (P < 0·05).
This is the first study to examine the swimming performance and the behaviour in juvenile A. brevirostrum. It also corroborates what has been seen in other acipsenserids. For example, UCrit values for sturgeons are low relative to other species of fishes (Peake, 2004; McKenzie et al., 2007). Poorer performance of A. brevirostrum may be related to body morphology of the fish (Adams et al., 1997, 2003; Peake, 2004; Allen et al., 2006). For instance, the large pectoral fins, flat ventral surface and large flattened rostrum suggest that sturgeons compensate for low swimming performance by modifying their swimming patterns (Webb, 1986; Adams et al., 2003; Peake, 2004). Like other sturgeons (Wilga & Lauder, 1999), A. brevirostrum use a burst–glide behaviour at higher speeds when challenged to increasing water velocities. They also swim in close contact with the floor of the flume [Fig. 1(b)], a behaviour that was also shown for S. albus (Adams et al., 2003). This substratum appression has been suggested to be an important mechanism for sturgeons maintaining position against water current (Adams et al., 2003). Maintaining position in high flow may also be partly achieved by fishes alternating between substratum skimming and station holding. In the present study, it was observed that during station holding, fish normally press their abdomens to the flume floor and angle their fins in a direction that allows the fish to press itself against the floor of the flume. As water speed increased, station holding became more difficult [Fig. 2(a), (b)], and fish began to slide backwards in the flume. At this point, fish began to use their caudal fin for propulsion. This holding–gliding swimming behaviour is suggestive of an energetic cost-saving measure, as it was noticed in some cases that ventilation frequency would decrease slightly during the holding period (per. obs.). Once water speeds reached 30 cm s−1 (and in most cases at 40 cm s−1), some individuals swam on their sides, with one pectoral fin pushed to the floor of the flume. In other cases, fish alternated between burst–glide swimming and then attempted to station hold. It should be noted that this latter behaviour may have been a reflection of the simplicity (i.e. smooth plexiglass floor) of the experimental flume, as water velocity affects substrate preference in 0+ year Gulf sturgeon Acipenser oxyrinchus desotoi Vladykov (Chan et al., 1997).
The relationship between the swimming speed and the net cost of locomotion (i.e. measured as oxygen consumption) has not been fully studied in sturgeons (Khakimullin, 1988; McKenzie et al., 2001). An attempt was made to measure oxygen consumption rates of individuals in the present study; this proved difficult because of the large number of station holds that fish attempted. In the present study, metabolic cost of swimming was assessed by recording the changes in opercular beat frequency with increased swimming speeds. The relationship between swim speed and ventilation frequency (Fig. 3) was similar to those recorded for Adriatic sturgeon Acipenser naccarii Bonaparte (McKenzie et al., 2001) and green sturgeon Acipenser medirostris Ayres (Allen et al., 2006), but values for ventilation frequency are consistently lower in the present study compared with values for juvenile Siberian sturgeon Acipenser baerii Brandt (Khakimullin, 1988) and A. medirostris (Allen et al., 2006).
In conclusion, the findings of this study showed that Ucrit are low and are similar to the values noted for other acipenserids. Because these experiments were conducted at only 15° C, it would be interesting to determine whether temperature affects the patterns that were found in the present study. For example, Graham et al. (1996) showed that the holding performance of juvenile Atlantic salmon Salmo salar L. decreased with decreases in temperature. Also, fish size in the current study was very similar (140–180 mm). How body size (ontogeny) affects the swimming performance of sturgeons is also not entirely understood (Allen et al., 2006). Examination of these types of questions would enable researchers to begin to model the energy expenditure and fuel use patterns in sturgeons. From an applied perspective, the findings from the present study are important from a fish way and culvert design perspective. As noted by Peake (2004), stringent regulatory requirement concerning fish passage around migratory obstacles have created a need for information on the swimming capacity of sturgeons. Thus, understanding the aerobic swimming capacity (present study), and the anaerobic swimming capacity (Kieffer et al., 2001; Baker et al., 2005) of juvenile and adult sturgeons might assist in the design of compatible passage structures.
Acknowledgments
This research was supported by a Natural Sciences and Engineering Research Council of Canada (N.S.E.R.C.) Discovery Grants to J.D.K. and M.K.L., an NB Wildlife Trust Fun Grant to M.K.L. and an N.S.E.R.C. Undergraduate Student Research Awards fellowship to L.M.A. We also thank K. Herrington for assistance. The handling of fish was conducted with the approval of the UNB Animal Care Committee, meeting guidelines established by the Canadian Council for Animal Care.




