Species delimitation in Lippia section Goniostachyum (Verbenaceae) using the phylogenetic species concept
Abstract
Lippia section Goniostachyum comprises plants distinguished by their numerous axillary florescences (three to six, sometimes up to nine) and tetrastichous floral bracts. Species of section Goniostachyum occur in the Neotropics, from Mexico to northern Argentina. Delimitation of the species grouped under Goniostachyum has remained unclear. Forty-one names exist under this section, but only c. eight to ten names have been used frequently. To resolve the taxonomy of this group, a modified population aggregation analysis, based on the phylogenetic species concept, was employed. As a result, Goniostachyum is here circumscribed to only four species: L. grata, L. origanoides, L. sericea and L. stachyoides. These species are supported by different combinations of three characters of the 13 qualitative attributes analysed: canescent sericeous pubescence, frondose or frondose-bracteose inflorescences and free or fused florescence apical bracts. Two varieties based on significant differences among quantitative characters are recognized: L. stachyoides var. stachyoides and L. stachyoides var. martiana comb. nov. Fifteen lectotypifications and four neotypifications are proposed. © 2012 The Linnean Society of London, Botanical Journal of the Linnean Society, 2012, 170, 197–219.
Introduction
Lippia L. is one of the largest genera of Verbenaceae, with nearly 100 species in the Neotropics and Africa. The genus is placed in tribe Lantaneae Endler, the largest of the eight tribes of Verbenaceae, following the most recent circumscription of the family (Marx et al., 2010). Lippia is characterized by its capituliform, usually axillary, florescences, mostly bilobed calyx and dry fruit, separating into two units, referred to as nutlets.
The most recent treatment of Lippia (Troncoso, 1974) recognized seven sections. Many of these sections have recently been shown to be nonmonophyletic, but section Goniostachyum Schauer is monophyletic based on molecular data (Marx et al., 2010; Lu-Irving & Olmstead, 2011). Morphologically, Goniostachyum constitutes a homogeneous group, traditionally based on the unique inflorescence architecture and floral bract phyllotaxis. In addition, Small (1903) considered this section as a separate genus, Goniostachyum (Schauer) Small. This was followed by Sanders (2001: 348), who also suggested that this group is morphologically supported by its decussate floral bracts, tubular, puberulent or hirtellous calyx, up to four florescences at each node and conspicuous glands producing aromatic oils. Most species of section Goniostachyum are distributed in South America, from Venezuela to northern Argentina, with areas of high diversity in central and eastern Brazil; a few species grow in Mexico and Costa Rica.
Although section Goniostachyum appears to be a clearly delimited group, boundaries between species in the section have remained unresolved. Lippia section Goniostachyum was founded by Schauer (1847) with 18 species; subsequent authors, including Briquet (1904), Herzog (1916) and Moldenke (1940, 1970, 1975, 1981), described new taxa (at the specific and infraspecific level). As a consequence, 41 taxa are recognized at present in section Goniostachyum. Although many of these taxa were not formerly placed under Goniostachyum, they all bear the characters that define this section: numerous axillary florescences and imbricate decussate, tetrastichous floral bracts (Schauer, 1847). Several regional floras or taxonomic treatments include Goniostachyum taxa, e.g. from Argentina (Troncoso, 1952, 1974; Múlgura, 2003), Brazil (Salimena & Giulietti, 1998; Salimena & Santos Silva, 2009), Venezuela (Lopez Palacios, 1977), the Guianas (Jansen-Jacobs, 1988; Aymard, 2005), Nicaragua (Pool, 2001), Guatemala (Gibson, 1970) and Mexico and Baja California (Standley, 1924; Shreve & Wiggins, 1964; Wiggins, 1980; Nash & Nee, 1984; Willmann et al., 2000), but a rigorous approach to species delimitation has not been attempted. Some authors (Troncoso, 1952; Salimena & Giulietti, 1998; Salimena & Santos Silva, 2009) mentioned specimens with intermediate characters or simultaneously cited a single specimen for more than one species, highlighting the difficulties in trying to distinguish taxa in Goniostachyum.
Delimitation of Lippia taxa, traditional approaches
Morphological characters varying among Lippia spp. and traditionally used to distinguish species are those of inflorescence architecture, disposition of floral parts and pubescence on vegetative and reproductive organs. In Lippia, floral bract phyllotaxis, form, size and colour are attributes often used to define sections of the genus (Múlgura, Martínez & Suyama, 1998). The presence of fused florescence apical bracts is a character found only in species of section Goniostachyum and is absent in all other Lippia taxa, even though basal florescence bracts can frequently be fused; this has no taxonomic relevance. Inflorescence architecture also has important taxonomic value. Recent studies have helped us to understand and correctly interpret inflorescence morphology and structure for Verbenaceae (Martínez, Botta & Múlgura, 1996; Martínez & Múlgura, 1997; Múlgura et al., 1998, 2002; Drewes & Martínez, 1999), suggesting a more accurate and precise terminology. Lippia section Dipterocalyx (Cham.) Schauer is differentiated because of its heterothetic pleiobotrya (with terminal florescences), whereas all the other sections of Lippia have homothetic pleiobotrya (only axillary florescences), following the terminology of Múlgura et al. (1998). Most Lippia spp. have frondose inflorescences, except sections Corymbosae Schauer and Paniculatae Schauer and some species of sections Goniostachyum and Dioicolippia Tronc., in which inflorescences are frondose-bracteose. Species of section Goniostachyum have a unique inflorescence structure within Lippia, bearing numerous florescences in each leaf axil and tetrastichous floral bracts. The rest of the species of Lippia have just one florescence in each leaf axil and serially arranged floral bracts. Another useful attribute is pubescence, which is very variable in Verbenaceae, and Lippia spp. are no exception. Frequently old structures, such as mature leaves or stems, are almost glabrous to slightly pubescent. The presence of canescent, sericeous pubescence on mature structures is infrequent, having only been found in Verbena hirta Spreng. (O'Leary, Múlgura & Morrone, 2007) and in some taxa of Goniostachyum. Species delimitation using traditional characters has failed to resolve boundaries between taxa of Goniostachyum, and therefore a more rigorous approach is needed to clarify the taxonomy of this section and to identify characters that can help to delimit species.
Phylogenetic species concept, a new approach to the delimitation of Goniostachyum species
In order to delimit the species of Lippia section Goniostachyum, a particular species concept and methodology were pursued, following Henderson (2004, 2005). Nixon & Wheeler (1990) define the phylogenetic species concept (PSC) as ‘the smallest aggregation of population (sexual) or lineage (asexual) diagnosable by a unique combination of character states in comparable individuals’. Population aggregation analysis (PAA) is the method for the identification of phylogenetic species (Davis & Nixon, 1992); it searches for fixed differences among local populations, followed by successive rounds of aggregation of population (or previously aggregated population) groups that are not distinct from each other. The PSC-PAA approach distinguishes between characters and traits. A character is an attribute that is invariant in a terminal lineage; its constancy in populations provides evidence for fixation and a lack of reticulation. A trait is an attribute that occurs in some, but not all, representatives of a terminal lineage, and provides evidence of intersection and lack of hierarchy (Luckow, 1995). Phylogenetic species are distinguished by characters or character states, not by traits. Populations are the fundamental units of analysis in the PSC-PAA method, and the method requires an a priori placement of specimens in a population. Henderson (2004, 2005, 2011) proposed the scoring of specimens (instead of populations) for all attributes (traits and characters) and the use of successive cluster analyses (CAs) to distinguish traits from characters at the same time as groups of specimens are delimited. Henderson's modification allows the use of herbarium material in PAA. Finally, groups of specimens with unique combinations of character states are found and considered as phylogenetic species. Luckow (1995) discussed the extension of the PSC to the infraspecific level and stated that ‘groups of populations that can be distinguished by differences in mean value would be recognized as subspecies or varieties’.
In this contribution, PSC, by means of a modified PAA, is used for species delimitation, and Luckow (1995) is followed to recognize varieties. The 41 taxa of Lippia section Goniostachyum are treated. Morphological descriptions, geographical distribution, habitat, synonymy and other relevant notes are also included.
Material and Methods
Specimens
Herbaria holding important collections of Verbenaceae, especially of Lippia section Goniostachyum, were consulted. Statistical analysis was based on specimens from BHCB, CEN, CEPEC, CESJ, ESA, HUEFS, IBGE, MBM, NY, SI, SPF, TEX and VIC. Herbaria acronyms follow Thiers’ (http://sweetgum.nybg.org/ih/) Index Herbariorum. Morphological observations were conducted using a Leica ocular lens. Type specimens from worldwide herbaria and digital images (http://plants.jstor.org/) were studied. Geographical and ecological data were obtained from specimen labels. Representative specimens are presented for each taxon, including one specimen from each main political division of each country.
Sampling
Over 250 specimens of the 41 taxa of Goniostachyum were studied; 193 of these specimens were selected for statistical analysis (Appendix) as similar specimens from the same geographical area were not included. Among these specimens, types of 20 names are included (Fig. 1); the type specimens of the remaining 21 names were only available as images and were not included in the matrix. These images, together with the original descriptions, were used to complete taxon delimitation and synonymy, after the phylogenetic species were established. Two taxa, L. graveolens f. microphylla Moldenke and L. graveolens f. looseneriana Moldenke, were only studied from the protologue descriptions as no original material or images were found.
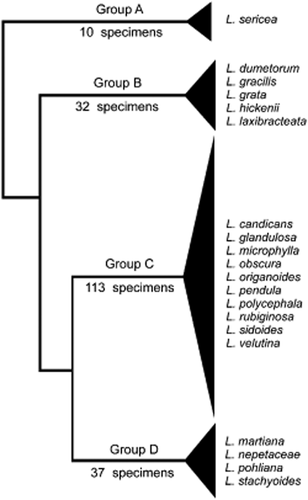
Dendogram obtained by cluster analysis of qualitative attributes showing the four groups found. Type specimens included in the analysis are shown in the cluster they joined.
Data matrix and analysis
Qualitative and quantitative attributes were explored from the entire plant. Twenty attributes, 13 qualitative and seven quantitative, were scored with no missing values for the 193 included specimens. All qualitative characters were binary coded, except for characters 7 and 12 which were multistate. No quantitative attributes showed gaps that could allow the delimitation of discrete character states. All attributes used in the analysis are listed and explained in Table 1. The specimens employed in the analysis are listed in the Appendix.
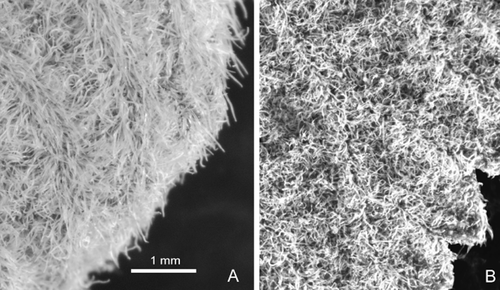
Photographs of different types of pubescence: A, canescent sericeous, from Lippia sericea, Mendoza 2592 (SI); B, sericeous noncanescent, from Lippia grata, Debeaux s.n. (SI). Same scale applies to (A) and (B).
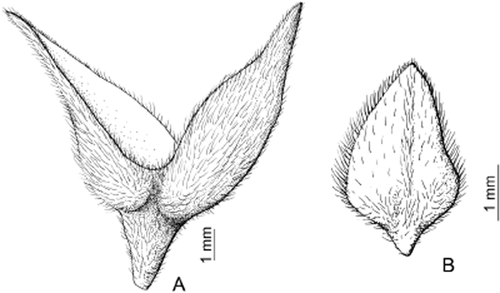
Florescence apical bracts: A, fused bracts, from Lippia grata, Schinini 19563 (CTES); B, free bract, from Lippia origanoides, Schinini 1947 (SI).
|
Multivariate methods of analysis were performed using the programs NTSYSpc 2.02h (Rohlf, 1986–1998) and InfoStat (Di Rienzo et al., 2009). Cluster Analyses (CA) with square-root-transformed qualitative variables were carried out based on similarity matrices obtained with the simple matching coefficient and using the unweighted pair group method with arithmetic average (UPGMA) clustering algorithm. Standardized quantitative variables (for a subgroup of specimens; see below) were examined with principal component analysis (PCA) using the product-moment correlation coefficient to compute the correlation matrix from which eigenvectors were extracted. Discriminant analysis (DA) was also performed for the same subgroup of specimens as for PCA. Characters were assumed to be normal multivariate because both procedures are relatively insensitive to deviations from normality; nevertheless, results are viewed as approximate. To avoid logically correlated characters in the PCA and DA, only five quantitative attributes were used (including attribute 6, excluding attributes 4 and 5, Table 1). To evaluate significant differences, we used the nonparametric Mann–Whitney U-test at P < 0.01.
Delimitation of specimen groups and taxonomic status
Qualitative attributes were recognized as characters or as traits using successive rounds of CA. All attributes were used in the first CA; then, suspect traits (highly polymorphic attributes in a cluster) were removed and not used in the following rounds of CA. In each round, a profile of each cluster was made indicating constant and nonconstant attributes in the specimens. Clusters with nondistinct profiles were aggregated in successive CAs until clusters of specimens with a unique combination of character states were found. Each group of specimens with a unique combination of constant character states was considered as a species under PSC.
Luckow's (1995) taxonomic concept for the recognition of infraspecific taxa considering groups of specimens instead of populations was followed here. Within recognized species, quantitative characters were analysed by PCA in order to detect internal consistency or the presence of specimen subgroups that could be considered as varieties. The DA of preclassified subgroups of specimens (those obtained by PCA) was used to analyse the most discriminative characters and the percentage of classification success. When subgroups could be distinguished by one or more significantly different character values, they were recognized as varieties (Luckow, 1995).
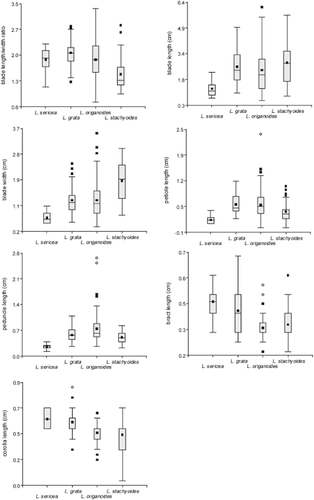
In order to identify the correct names and to construct synonymies, the diagnosable characters found for each finally accepted taxon were contrasted with protologues and type specimens. Traditional descriptions are given in the ‘Taxonomic conclusions’ section, including new taxon circumscriptions, synonymy and distributional data. Ranges (minimum–maximum) are given for plant structures, and character statistics are shown by box plots.
Results
Three of the 13 qualitative attributes were detected as characters by successive rounds of CA. The resulting dendrogram (Fig. 1) established four major clusters, groups A–D (Fig. 1), each characterized by a unique combination of the three qualitative characters (Table 2, Figs 2-4) and considered a species under PSC. Quantitative characters for species are shown in Figure 6.
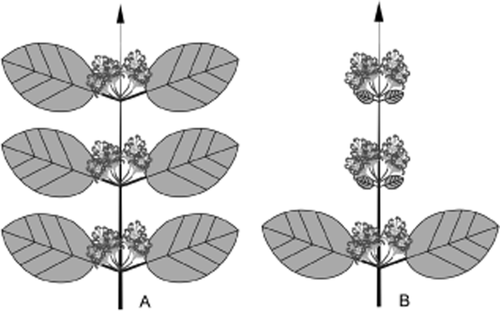
Schematic representation of different types of inflorescence: A, frondose inflorescences; B, frondose-bracteose inflorescences.
| Plants canescent sericeous: absence (0), presence (1) (attribute 1) | Inflorescence frondose (0), frondose-bracteose (1) (attribute 13) | Florescence apical bracts: free (0), fused (1) (attribute 15) | |
|---|---|---|---|
| Group A (L. sericea) | 1 | 0 | 0 |
| Group B (L. grata) | 0 | 0 | 1 |
| Group C (L. originoides) | 0 | 0 | 0 |
| Group D (L. stachyoides) | 0 | 1 | 0 |
When each group was examined for internal consistency by PCA, only group D could be further subdivided on the basis of quantitative characters (Fig. 5). Eigenvalues for the three first components represent 82% of the total variation. Among the characters that best explain the variation in the first component are corolla length (eigenvector 0.53) and petiole length (0.50). Characters that best explain the variation in the second and third components are peduncle length (0.82) and floral bract length (0.74), respectively. The PCA plot (Fig. 5) indicates two subgroups D1 and D2, considered as varieties, defined by components 1 and 2. D1 includes specimens with shorter corollas and petioles and rounded blades (smaller length/width ratio); D2 includes specimens with longer corollas and petioles and elliptic blades (greater length/width ratio). No significant resolution could be observed along the third axis (not shown). Discrimination analysis separates these two subgroups with 100% success, the blade length/width ratio being the most discriminating character. The Mann–Whitney U-test indicates significant differences between the two subgroups D1 and D2 in five of the seven quantitative character values: pedicel length (P = 0.0009), leaf blade length (P = 0.0014), leaf blade length/width ratio (P = 0.0002), floral bract length (P = 0.0012) and corolla length (P = 0.0022). Groups and subgroups were compared with type material and original descriptions in order to assign a name (Table 3).
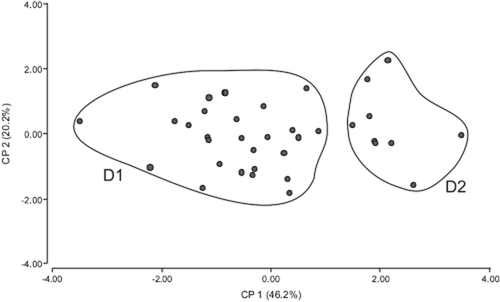
Scatter plots of the first two components from principal component analysis (PCA) based on five quantitative characters and 37 specimens from Lippia stachyoides (group D). D1 and D2 represent the two subgroups indicated by the PCA plot.
| Group | Taxa accepted in this study | Synonymy |
|---|---|---|
| A | Lippia sericea Cham. | |
| B | Lippia grata Schauer |
Lippia dumetorum Herzog Lippia gracilis Schauer Lippia hickenii Tronc. Lippia laxibracteata Herzog |
| C | Lippia origanoides Kunth |
Lantana origanoides Martens & Galeotti Lippia affinis Schauer Lippia berlandieri Schauer Lippia berteri Spreng. Lippia candicans Hayek Lippia elegans Cham. Lippia elegans var. macrophylla Moldenke Lippia elegans var. obtusifolia Moldenke Lippia glandulosa Schauer Lippia graveolens Kunth Lippia graveolens f. macrophylla Moldenke Lippia graveolens f. microphylla Moldenke Lippia graveolens f. loeseneriana Moldenke Lippia palmeri S. Watson Lippia palmeri var. spicata Rose Lippia mattogrossensis Moldenke Lippia microphylla Cham. Lippia obscura Briq. Lippia pendula Rusby Lippia polycephala Briq. Lippia polycephala var. aemilii Briq. Lippia rigida Schauer Lippia rubiginosa Schauer Lippia rubiginosa f. pauper Schauer Lippia salviifolia Cham. Lippia schomburgkiana Schauer Lippia sidoides Cham. Lippia velutina Schauer |
| D | Lippia stachyoides Cham. var. stachyoides | |
| Lippia stachyoides var. martiana (Schauer) Salimena & Múlgura |
Lippia martiana f. campestris Moldenke Lippia nepetacea Schauer Lippia pohliana Schauer Lippia pohliana var. longibracteolata Moldenke |
Discussion
Group A
This group corresponds to Lippia sericea Cham. In our analysis, this group of specimens is distinguished by the combination of canescent, sericeous pubescence, frondose inflorescences and free florescence apical bracts. Quantitative characters (Fig. 6) show that this species can also be distinguished by its short peduncles and petioles, longer corolla tubes and smaller leaf blades.
Box plots of quantitative characters of the four species in Lippia section Goniostachyum. Boxes incorporate 50% of values; horizontal line in box indicates median value; dark points within vertical line indicate mean value; dark points outside vertical line indicate outside values; open circles indicate far outside values.
Canescent, sericeous pubescence (Fig. 2A) is found many times in Lippia spp. on young structures, such as buds and small young leaves, but, in L. sericea, this pubescence is also found in old structures, such as mature leaves or stems. This is infrequent in Verbeneaceae.
Group B
This group corresponds to Lippia grata Schauer. In our analysis, this group of specimens is distinguished by the combination of strigose (not canescent sericeous) pubescence, fused florescence apical bracts and frondose inflorescences. This species is difficult to distinguish from the remaining taxa of Goniostachyum by quantitative characters (Fig. 6).
This group includes type specimens of five names (Fig. 1). Among them, L. grata Schauer and L. gracilis Schauer, both first treated as a single taxon here, are the oldest and both have the same priority; the name L. grata was chosen because it has been most frequently used. Type material of L. dumetorum Herzog and L. hickenii Tronc. does not differ at all from specimens of this group, whereas the type of L. gracilis has smaller leaves, and that of L. laxibracteata Herzog has larger leaves, but, in both cases, the leaf size is considered within range variation for this taxon. Troncoso (1952) mentioned the similarity between L. hickenii and L. dumetorum and stated that these taxa differ from L. sidoides Cham. (L. origanoides Kunth., group C) because the latter lacks typical fused florescence apical bracts. Later, Múlgura (2003) differentiated L. salviifolia Cham. (a synonym of L. origanoides) and L. laxibracteata (a synonym of L. grata). The author distinguished them because the latter has fused florescence apical bracts. This shows that this attribute has already been recognized as an important character.
Group C
This group corresponds to L. origanoides Kunth. In our analysis, this group of specimens is distinguished by the combination of strigose or hispid (not canescent, sericeous) pubescence, frondose inflorescences and free florescence apical bracts. This species is difficult to distinguish from the other taxa of Goniostachyum by quantitative characters (Fig. 6). This is a variable species, with the widest distribution, from Guyana to northern Argentina in South America, and is also found in Mesoamerica, which results in many different names being used for this species.
Lippia graveolens Kunth and L. origanoides are here treated as a single taxon, and the names have equal priority. The name L. graveolens has been frequently used for Mesoamerican specimens, whereas the name L. origanoides has been frequently used in South American floras. The latter name reflects its typical aromatic nature (it is a plant that smells like oregano or lavender), and this name is chosen to designate the taxon.
Calpouzos (1954) studied the condiment name ‘origanum’ and found that it does not refer to a particular species, but to a flavour from plants of different genera in different parts of the world. In Mexico, ‘origanum’ comes from L. origanoides, also sometimes determined as L. palmeri S.Watson, L. graveolens Kunth or L. berlandieri Schauer, all taxa considered here as synonyms of L. origanoides. Lopez Palacios (1977) differentiated L. schomburgkiana Schauer from L. origanoides because the former smells like oregano and the latter like lavender. However, these taxa cannot be considered as different species because of this subjective character, and the type specimens are morphologically indistinguishable.
Troncoso (1952) mentioned L. sidoides for Argentina and the need for a detailed study in this group, stating its similarity to L. salviifolia, L. velutina Schauer, L. rubiginosa Schauer, L. obscura Briq. and L. origanoides, all of which are considered here as synonyms of the latter.
Salimena & Giulietti (1998) differentiated L. origanoides (sub. nom. L. microphylla Cham. and L. salviifolia) from L. stachyoides var. martiana (Schauer) Salimena & Múlgura (sub. nom. L. martiana Schauer), group D, by the cordate leaf base of the latter. From our analysis, L. origanoides can have cordate or acute leaf bases, which shows that this is not a good attribute to distinguish between these two taxa, and should be considered a trait. Salimena & Giulietti (1998) stated that L. stachyoides Cham. is distinguished by its sericeous pubescence, whereas L. origanoides has strigose pubescence. From our analysis, both species can have strigose or sericeous leaf pubescence and, consequently, pubescence has also proved to be a trait, not a character.
Standley (1924) distinguished L. palmeri, L. graveolens and L. berlandieri by their peduncle and leaf lengths. All three taxa are considered here as synonyms of L. origanoides, given that these taxa have variable peduncle and leaf lengths, and Standley's taxa fall within the range of variation of L. origanoides.
Group D
This group corresponds to L. stachyoides Cham. In our analysis, this group of specimens is distinguished by the combination of strigose or hispid (not canescent sericeous) pubescence, frondose-bracteose inflorescences and free florescence apical bracts. Quantitative characters (Fig. 6) show that this species can also be distinguished by its smaller corolla tubes and wider leaf blades, which results in a smaller blade length/width ratio. In section Goniostachyum, L. stachyoides is the only species with frondose-bracteose inflorescences.
Lippia nepetacea Schauer, L. pohliana Schauer and L. martiana Schauer, all first treated as a single taxon here, have the same priority; L. martiana was chosen as a basionym to the variety, given that the latter had most frequently been used to refer to this taxon. Schauer differentiated L. martiana by its larger leaves, whereas L. nepetacea and L. pohliana have smaller leaves. The type specimens for these names are collections from the inflorescence of the plants, where leaves are smaller. Furthermore, the type specimen of L. pohliana is poor, with few florescences, which makes it look different. However, all three turn out to be synonyms, because they all have frondose-bracteose inflorescences and the differences in leaf size all fall within the range of variation for the species.
Taxonomic Conclusions
Lippia L. section Goniostachyum Schauer Prodr. 11: 574 (1847). Goniostachyum (Schauer) Small, Flora of the Southeastern United States 1012 (1903).
Type species
Lippia rubiginosa Schauer, designated by N. S. Troncoso, Darwiniana 18: 387 (1974).
Shrubs or shrubby plants, mostly aromatic, with strigose, hispid, sericeous or canescent, sericeous pubescence along the plant. Leaves opposite or ternate, sessile to petiolate, blades entire, elliptic or ovate, margins crenate. Inflorescences frondose or frondose-bracteose, flowers white or yellowish, disposed in axillary florescences, more than two per leaf axil, generally three to six, but up to nine. Floral bracts in tetrastichous disposition, triangular concave, external surface carinate, fused to each other or not. Fruit dividing into two small nutlets.
Notes
Múlgura et al. (1998) defined the inflorescence of Lippia section Goniostachyum as a homothetic pleiobotrya, with three to nine buds per leaf axil, in which the distal bud is the first to develop, followed basitonically by the others, each bud giving rise to a floral spike (florescence). In some specimens, the distal bud originates a floriferous stem with several fertile nodes and the subsequent buds develop into florescences (Múlgura et al., 1998: fig. 4A, B). This type of inflorescence should not be confused with that found in Lippia section Rhodolippia Schauer subsection Mexicana Moldenke (Múlgura et al., 1998), in which young nodes seem to have numerous florescences, but then the distal bud develops into a bracteose paraclade of umbellate aspect.
Distribution and habitat
Section Goniostachyum is principally distributed in South America, from Venezuela to northern Argentina; the greatest specific diversity is found in central and eastern Brazil. In Mesoamerica, it is also found in Mexico and Costa Rica. It grows from 200 to 1600 m.a.s.l.
- 1a. Plants with frondose-bracteose inflorescences2
- 1b. Plants with frondose inflorescences3
- 2a. Petioles (0.2–)0.4–1.0 cm long, blades 1.6–5.6 × 0.7–2.7 cm (length/width ratio, 2.06), base cuneate sometimes round, apex acute or obtuse; bracts (0.3–)0.4–0.6 cm long, corolla (0.4–)0.5–0.7 cm long 4a. L. stachyoides var. stachyoides
- 2b. Petioles 0.1–0.4(–0.8) cm long, blades 0.8–4.8 × 0.8–3 cm (length/width ratio, 1.28), base round, apex obtuse rarely acute; bracts 0.2–0.4 cm long, corolla 0.25–0.5(–0.6) cm long4b. L. stachyoides var. martiana
- 3a. Plants canescent sericeous on stems, leaves and floral bracts; leaves elliptic, usually ternate, sometimes opposite 3. L. sericea
- 3b. Plants strigose, hispid or sericeous, never canescent sericeous on stems, leaves and floral bracts; leaves elliptic or ovate, usually opposite, sometimes ternate4
- 4a. Apical bracts of the florescence fused1. L. grata
- 4b. Apical bracts of the florescence free2. L. origanoides
1. Lippia grata Schauer (Fig. 7)
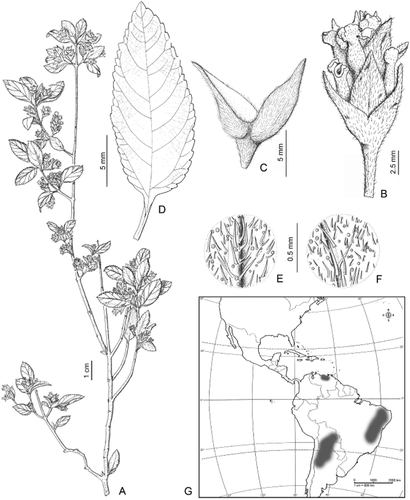
Lippia grata: A, plant general aspect; B, detail of florescence; C, fused florescence apical bracts; D, leaf, adaxial surface; E, leaf pubescence, abaxial surface; F, leaf pubescence, adaxial surface; G, geographical distribution. From Schinini 19563 (CTES).
Prodr. 11: 576. (1847). Type: Brazil. Bahia, Villa Nova da Rainha, C.F.P. Martius s.n. (holotype: M!; isotypes: TEX!, SI 3518!, photograph Field Museum neg. 20327!).
Lippia gracilis Schauer, Prodr. 11: 577 (1847), syn. nov. Type: Brazil. Bahia & Pernambuco, habitat in camporum sepibus ad Joazeiro, C.F.P. Martius s.n. (holotype: M!; isotypes: TEX!, SI 3517!, photograph Field Museum neg. 20325!).
Lippia dumetorum Herzog, Medded. Rijks-Herb. 29: 45 (1916), syn. nov. Type: Bolivia. Quebrada de Chajravuaitu, c. 1600 m, Abr 1911, T. Herzog 1851 (holotype: L!; isotypes: G!, NY 137755!, SI 3550!, SI 3501!, Z 29604!, photograph Field Museum neg. 26649!).
Lippia laxibracteata Herzog, Meded. Rijks-Herb. 29: 45 (1916), syn. nov. Type: Bolivia. Strauch im Kamp und Bush bei Villa Montes am Rio Pilcomayo, xi.1910, T. Herzog 1108 (holotype: M!, photograph Field Museum neg. 20330!; isotypes: G!, NY 137776, SI 3533! W!, Z 29606!).
Lippia hickenii Tronc., Darwiniana 10(1): 69 (1952), syn. nov. Type: Argentina. La Rioja, Dique Los Sauces, 16.ii.1944, L. R. Parodi 14864 (holotype: SI 003522!).
Shrubs 0.5–1.5(–2.5) m high, stems with strigose pubescence, long stems prostrate, internodes 1–5(–6) cm long. Leaves opposite, sometimes ternate, petioles 0.2–1.2 cm long, strigose, blades 1–5 × 0.5–2.5 cm, ovate to elliptic, base cuneate to round, apex acute to obtuse, margin crenate, venation pinnate, sometimes imperfect acrodromous, adaxial surface strigose, abaxial surface sericeous or strigose. Inflorescences frondose, four florescences per axil, sometimes three, peduncles 0.2–1.0 cm long, strigose, florescence 0.9–1.2(–1.4) cm long, bracts 0.2–0.7 cm long, apex curved or straight, apical bracts fused, abaxial surface slightly strigose all over the surface or only on the base and over the central vein, adaxial surface mostly all strigose. Calyx 0.2–0.25 cm long, external surface hispid or strigose. Corolla 0.6–0.9 cm long, external surface slightly strigose. Nutlets 0.12–0.15 cm long.
Notes
Flowers are perfumed and leaves are opposite, except in Pereira 2672 (SI), which has ternate leaves. Some specimens of L. grata have imperfect acrodromous venation (Hickey, 1974): three principal veins developed, where both lateral veins reach only two-thirds of the leaf blade length ( Fig. 8C).
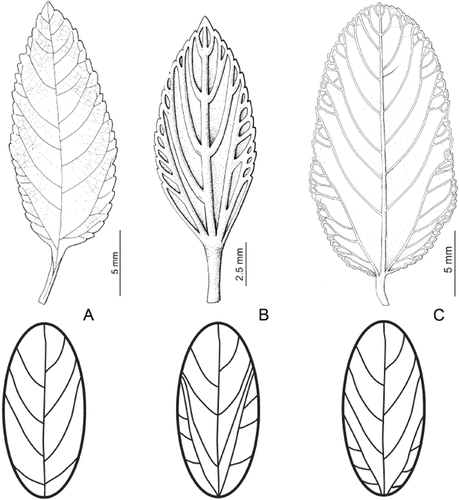
Leaf venation. A, Pinnate: a single central vein, with secondary veins originating from it; drawing from Lippia grata, Schinini 19563 (CTES). B, Perfect acrodromous: two or more principal veins extending for at least two-thirds of the blade length; drawing from L. sericea, Duarte 9916 (SI). C, Imperfect acrodromous: two or more principal veins extending for less than two-thirds of the blade length; drawing from L. grata, Krapovickas 30848 (SI).
Synonymy
Type material of all new synonyms for this species was included in the data matrix, and it appeared to be included within the same group in the CA.
Distribution and habitat
Lippia grata grows in eastern Brazil, Bolivia and north-western Argentina. There is a collection from Vargas province in Venezuela. It is found on sandy, calcareous or rocky soils at 350–1600 m elevation. (Fig. 7).
Representative specimens examined
ARGENTINA. Catamarca: Sierra del Alto, 1.ix.1952, Biloni 6251 (SI). La Rioja: Gobernador Gordillo, ruta prov. 4, km 46 entre Salina La Antigua y Esperanza de los Cerrillos, 15.vii.1976, Biurrun 596 (SI). Salta: Rivadavia, Cnel. Juan Solá, 18.ii.2005, Suárez 142 (SI). Santiago del Estero: Guasayán, Sierra de Guasayán, ruta 64 km 79, 17.xi.1994, Krapovickas 46215 (SI). BOLIVIA. Chuquisaca: Luis Calvo, El Salvador, Laguna Seca, 8.xii.1992, Pensiero 4372 (SI). Santa Cruz: Cordillera, Aguarati Izozog, 7-13-98, Roca 709 (SI). Tarija: Chaco, Villamontes, 25 km al SE, 29.xi.1989, Saravia Toledo 2206 (SI). BRAZIL. Minas Gerais: Serra da Piedade, 27.iii.1957, Pereira 2672 (SI). Paraíba: Junco de Seridó, 19.vii.1977, Fernández 3337 (SI). Pernambuco: Placas, 12.ix.1954, Falcão 1035 (SI). VENEZUELA. Vargas: Vargas, La Guayra, 20.vii.1997, Debeaux 87 (SI).
2. Lippia origanoides Kunth (Fig. 9)
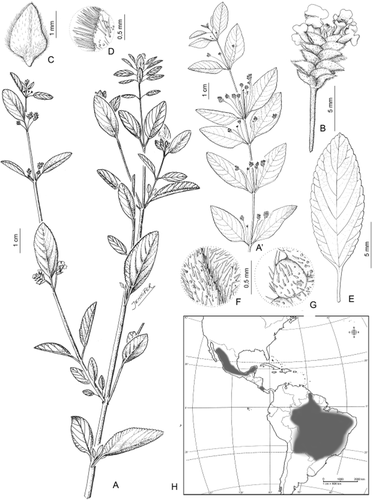
Lippia origanoides: A, plant general aspect; A’, plant general aspect; B, detail of florescence; C, floral bract; D, detail of bract pubescence; E, leaf, adaxial surface; F, leaf pubescence, abaxial surface; G, leaf pubescence, adaxial surface; H, geographical distribution. A’, from Philcox 4736 (U); rest from Schinini 1947 (SI).
Nov. Gen. Sp. (quarto ed.). 2: 267 (1817 [1818]). Type: Venezuela. Sucre. Cumana, Punta Araya, F. Humboldt & A. Bonpland s.n. (holotype: P!; isotype: P!, SI 003562!).
Lippia graveolens Kunth, Nov. Gen. Sp. (quarto ed.) 2: 266 (1817 [1818]). Goniostachyum graveolens (Kunth) Small, Fl. Southeast. U. S.: 1012 (1903), syn. nov. Type: Costa Rica. Guanacaste, alrededores de Liberia, 1910, A. M. Brenes s.n. (neotype, here designated: SI 151910!).
Lippia berteri Spreng., Syst. Veg. 2: 752 (1825). Type: Venezuela. Ad. Fl. Magdalena, C. Bertero s.n. (lectotype, here designated: M!, B †, photograph Field Museum neg. 17496!).
Lippia sidoides Cham., Linnaea 7: 224 (1832), syn. nov. Type: Brazil. F. Sellow s.n. (lectotype, here designated: G!; isotypes: BM!, HAL 98265, K 470796, K 470797, K 470798, NY!, P!, SI 3593!, SI 3594!, SI 3595!, VT!).
Lippia elegans Cham., Linnaea 7: 225 (1832), syn. nov. Type: Brazil. C.F.P. Martius Herb. Fl. Bras. 1037 (neotype, here designated: M!; isotype G!).
Lippia microphylla Cham., Linnaea 7: 226 (1832), non L. microphylla Phil., syn. nov. Type: Brazil. F. Sellow s.n. (lectotype, here designated: HAL!; isotypes G!, K 13628!, K 13629!, P!, SI 3553!, VT 29; B†, photograph Field Museum neg. 17527!).
Lippia salviifolia Cham. ‘salviaefolia’, Linnaea 7: 227 (1832), syn. nov. Type: Brazil. C.F.P. Martius Herb. Fl. Bras. 1032 (neotype, here designated: M!, B†, photograph Field Museum neg. 17541!).
Lantana origanoides Martens & Galeotti, Bull. Acad. Roy. Sci. Bruxelles 11 (2): 327 (1844). Type: Mexico. Oaxaca, Cordillera, xi–iv.1840, H. Galeotti 784 (holotype: BR 550440!)
Lippia velutina Schauer, Prodr. 11: 576 (1847), syn. nov. Type: Brazil. Mato Grosso, Morro do Ernesto, A. Silva Manso s.n. [Herb. Martius 1024] (lectotype, here designated: M!; isotypes: BM 906661, BR 981678!, G!, HAL 106894!, NY 137841, P!, W! SI 3604!, SI 3606!).
Lippia rubiginosa Schauer, Prodr. 11: 574 (1847), syn. nov. Lippia rubiginosa f. dives Schauer, Prodr. 11: 574 (1847). Type: Brazil. F. Sellow s.n. (lectotype, here designated: P 568341!; isotype: SI 3571!).
Lippia rubiginosa f. pauper Schauer, Prodr. 11: 575 (1847), syn. nov. Type: Brazil. Minas Gerais, 1834, Ackerman s.n. (lectotype, here designated: BR 989239!).
Lippia affinis Schauer, Prodr. 11: 576 (1847), syn. nov. Type: Brazil. Schüch s.n. (lectotype, here designated: W!, isotype: SI 3484!).
Lippia berlandieri Schauer, Prodr. 11: 576 (1847), syn. nov. Type: Mexico. De Santander a Vitoria, 1832, J.L.Berlandier 2252 (lectotype, here designated: G!; isotypes: F 74394!, F 74395!, NY 137712!, P!, US 118810!).
Lippia glandulosa Schauer, Prodr. 11: 577 (1847), syn. nov. Type: Brazil. Paramata, J.B. Pohl 132 (lectotype, here designated: K 470786!).
Lippia rigida Schauer, Prodr. 11: 577 (1847), syn. nov. Type: Brazil. Bahia, C.F.P. Martius s.n. (holotype: M!; isotype: SI 3570!).
Lippia schomburgkiana Schauer, Prodr. 11: 577 (1847), syn. nov. Type: Guyana. R. Schomburgk 75 (neotype, here designated: K 470795!; isotypes: BR 550474!, G!, K 470789!, K 470787!, W!).
Lippia palmeri S. Watson, Proceed. Amer. Acad. Arts 24: 67 (1889), syn. nov. Type: Mexico. Sonora, Guymas, E. Palmer 277 (lectotype, here designated: US 118854!; isotype: NY 137723, NY 137724).
Lippia palmeri var. spicata Rose, Contributions from the United States National Herbarium 1(3): 75 (1890), syn. nov. Lippia palmeri f. spicata (Rose) Moldenke, Phytologia 44: 328 (1979). Type: Mexico. Baja California, La Paz, 20.i./5.ii.1890, E. Palmer 62 (holotype: US 118855!).
Lippia obscura Briq., Bull. Herb. Boissier sér. 2, 4: 1155 (1904), syn. nov. Type: Paraguay. ‘in silva, in regione cursus superioris flum Apa’, xi.1901/1902, E. Hassler 8016 (holotype: G!; isotypes: B †, photograph Field Museum neg. 17529!, BM, K 470853!, LIL 1369!, MICH 1108390, MPU 12503!, NY 137789!, NY 137790, P!, SI 3561!, SI 3560!, UC 944345).
Lippia polycephala Briq., Annales Conserv. Jard. Bot. Genève 7–8: 307 (1904), syn. nov. Type: Paraguay. Caacupé, 8.xii.1882, B. Balansa 4624 (holotype: G!; isotypes: BM 98718, K470850, L 3908, L 3909, L 3910, L 3911, L3912, NY 137803, P!, SI 3612!).
Lippia polycephala var. aemilii Briq., Bull. Herb. Boissier, 2 serie, 4: 1155 (1904), syn. nov. Type: Paraguay. In valle fluminis Y-aca in arenosis ad ripam fluminis, xii.1900, E. Hassler 6720 (holotype: G!; isotypes: F 74411F, K 470849, MICH 1108392, MPU 12505, NY 137804, NY 137805).
Lippia candicans Hayek, Fed. Rep. 2: 86 (1906), syn. nov. Type: Brazil. Goyaz, G. Gardner 3942 (holotype: W!; isotypes: K 470864!, K 633682!, NY 83798!, SI 3538!).
Lippia pendula Rusby, Bull. New York Bot. Gard. 8: 116 (1912), syn. nov. Type: Bolivia. La Paz, Apolo, 4800 ft., 27.ii.1902, R. S. Williams 307 (holotype: NY 137796!; isotypes: BM 992656, K 470954, SI 3572, US 118857).
Lippia mattogrossensis Moldenke, Phytologia 1: 468 (1940), syn. nov. Type: Brazil. Mato Grosso, Coxipó da Ponte, Cuiabá, iii.1911, F. Hoehne s.n. Com. Rondon 4541 (holotype: SP!; isotype: NY 83807, NY 137784!).
Lippia elegans var. obtusifolia Moldenke, Phytologia 19: 319 (1970), syn. nov. Type: Brazil. Goiás, Chapada dos Veadeiros, 21.xii.1968, G. M. Barroso, M. J.Lima & A. Lima 568 (holotype: NY 83783!).
Lippia graveolens f. macrophylla Moldenke, Phytologia 49: 431 (1981), syn. nov. Type: Mexico. Michoacán, Zitacuaro – Coyota, 25.viii.1938, G. B. Hinton, 13162 (holotype: NY 137716).
Lippia graveolens f. microphylla Moldenke, Phytologia 50: 13 (1981), syn. nov. Type: Mexico. Puebla, Zapotitlan Valley along the road from Chazumba to Acarapec, 20.vii.1961, C.F.Smith, F.A.Peterson & N.Tejeda 3977 (holotype: MEXU).
Lippia graveolens f. loeseneriana Moldenke, Phytologia 52:130 (1982), syn. nov. Type: Mexico. Chiapas, Gracias A Dios, 19.viii.1896, C. Seler & E. Seler 3043 (holotype: US 118831).
Lippia elegans var. macrophylla Moldenke, Phytologia 55: 42 (1984), syn. nov. Type: Brazil. Distrito Federal, 16.iii.1983, B. A. S. Pereira & R. C. Mendonça 408 (holotype: NY 83784!).
Shrubs 0.8–3.0 m high, stems generally densely strigose, rarely hispid or slightly strigose, internodes (1–)2–9 cm long. Leaves usually opposite, sometimes ternate; petioles 0.1–2.4 cm long, rarely sessile leaves, pubescence strigose rarely hispid; blades 0.5–6.1 × 0.3–3.5 cm, elliptic or ovate, base cuneate rarely round, apex acute rarely obtuse, margin crenate, venation pinnate rarely perfect acrodromous, adaxial surface strigose and abaxial surface sericeous. Inflorescences frondose, (two) three to six florescences per axil, peduncles 0.2–2.6 cm long, strigose, rarely hispid, florescence (0.3–)0.4–1.2(–1.5) cm long, bracts 0.2–0.5 cm long, apex curved or straight, apical bracts free, abaxial surface slightly strigose only on the base and over the central vein, rarely all surface pubescent, adaxial surface mostly all strigose. Calyx 0.1–0.2 cm long, external surface strigose. Corolla 0.2–0.6 cm long, external surface slightly strigose. Nutlets 0.1–0.2 cm long.
Notes
Lippia origanoides is very aromatic; it is employed in medicine and also as a culinary spice. Dry leaves are sold in markets.
Typification
Lippia graveolens is a name frequently used for Mesoamerican specimens, all of which have proved to be L. origanoides. Moldenke (1965: 181) stated that the type material ‘Mexico. Campeche F. Humboldt & A. Bonpland s.n.’ is probably deposited at P, but no original material has been found in P. None of the authors who have used this name have ever seen the type, and no material that could have been original has been found; consequently, a neotype is designated. The neotype is a specimen collected by A. M. Brenes at Costa Rica, seen by Moldenke in 1947 and determined as L. graveolens.
Lippia berteri Spreng. was based on a specimen housed at B now destroyed, and so a well-preserved isotype found in M is chosen here as lectotype.
Lippia sidoides and L. microphylla were described by Chamisso on the basis of two F. Sellow specimens housed at B, now both destroyed. Sellow syntypes of L. sidoides were found at several herbaria; the specimen from G was chosen here as lectotype, given it has the letter ‘n’ which Chamisso used to designate his new taxa. In the case of L. microphylla, several collections were found, but the specimen from HAL was chosen as lectotype, given that this collection was a donation from B and has the same handwriting as the photograph from the destroyed holotype.
No type material for L. elegans Cham. could be found, and so a neotype was designated from the Martius collection housed at M.
The type specimen of L. salviifolia was based on a Sellow specimen housed at B, now destroyed, photograph Field Museum neg. 17541. No Sellow material related to this name could be found, and so a neotype was designated on the basis of a Martius specimen that fits the original description.
Schauer mentioned two syntypes for L. velutina; the specimen from Silva Manso s.n. (Herb. Martius 1024) was chosen as lectotype, given that it is more representative of the species. The name ‘Lippia mollis’ on this specimen sheet is a nom. nudum.
In the protologue of L. rubiginosa and L. rubiginosa f. pauper, Schauer mentioned specimens collected by Sellow, Lund and Ackerman, housed at B and G-DC, but material from B is now destroyed. Sellow specimens related to L. rubiginosa from P and SI could be found, as well as Lund specimens from K, P and G. The Sellow material from P was chosen here as lectotype as it is a better preserved material than the Lund collection. In BR, there is an Ackerman material with the label L. rubiginosa f. pauper, and so this was chosen as lectotype for this taxon.
Schauer mentions two specimens in the protologue of L. affinis, Schüch and Riedel, but only the Schüch material could be found, housed at W, and it is designated here as the lectotype.
Several specimens were mentioned in the protologue of L. berlandieri; the specimen Berlandier 2252 was chosen, given that it is the most complete material and is distributed in several herbaria. The same holds for L. glandulosa, where the specimen Pohl 132 was chosen as lectotype.
The type from L. schomburgkiana was deposited at B, now destroyed. No isotypes were found, and so the specimen Schomburgk 75, housed at K, was chosen as neotype, as this specimen is well represented, being distributed in several herbaria.
Watson (1889) mentioned three specimens in the protologue of L. palmeri; that from US was chosen as the lectotype as Watson worked there.
Synonymy
Type materials of L. candicans Hayek, L. glandulosa Schauer, L. microphylla Cham., L. obscura Briq., L. pendula Rusby, L. polycephala Briq., L. rubiginosa Schauer, L. sidoides Cham. and L. velutina Schauer were included in the data matrix, and they appeared to be grouped within the same cluster in the analysis; they are considered here as synonyms of L. origanoides.
The analysis of the original description and the type material of L. salviifolia allows us to conclude that this taxon is a synonym of L. origanoides because it matches exactly the diagnosable characters of this species.
In the protologue of L. rigida Schauer, the author mentioned that it is similar to L. microphylla and L. glandulosa; all of these taxa are considered here as synonyms of L. origanoides. The same holds with L. schomburgkiana and L. microphylla, both mentioned to be similar taxa and considered here to be synonyms of L. origanoides.
Lippia rubiginosa f. pauper was founded because of its smaller leaf size, but the analysis of type specimens shows that there is no difference from L. origanoides, which can have leaves very variable in size, ranking 0.5–6.0 cm long.
Schauer, in the protologue of L. affinis Schauer, mentioned that it is similar to L. origanoides, but differs because of its round leaf and bract bases. We found that this character is variable in L. origanoides, which has generally cuneate, sometimes round, leaf bases. The taxon accepted here, L. stachyoides var. martiana (Schauer) Salimena & Múlgura, has round leaf bases, but this species has a bracteose inflorescence different from the frondose-bracteose inflorescence of L. origanoides.
Moldenke (1942) was the first author to consider L. berlandieri a synonym of L. graveolens; later Calpouzos (1954) also mentioned L. berlandieri as conspecific with L. graveolens. The analysis of the type material corroborates the previous ideas; L. berlandieri is considered here as a synonym of L. origanoides.
The analysis of the type material and protologue of L. palmeri Rose shows that it is a synonym of L. origanoides. Lippia palmeri var. spicata is defined, but it is longer and has more compact florescences; however, this character is variable in L. origanoides and the length described for this variety falls within the range of variation of the species L. origanoides; therefore, this variety is considered here as a synonym.
Briquet differentiated Lippia polycephala var. aemilii Briq. because of the leaf morphology, but the analysis of multiple specimens shows that this taxon is not different from L. origanoides. Troncoso (1952) already mentioned this taxon as a synonym of L. salviifolia (currently a synonym of L. origanoides).
The analysis of the original description and the type material of L. mattogrossensis Moldenke allows us to conclude that this taxon is a synonym of L. origanoides.
Lippia elegans var. obtusifolia Moldenke and L. elegans var. macrophylla Moldenke were described by Moldenke as different from the typical variety because of the leaf form and size, respectively. The analysis of type material shows that both varieties are synonyms of the typical variety, which, in turn, is a synonym of L. origanoides.
The three infraspecific taxa L. graveolens f. macrophylla Moldenke, L. graveolens f. microphylla Moldenke and L. graveolens f. loesneriana Moldenke were differentiated because of their larger leaves, smaller leaves and leaf pubescence, respectively. The analysis of the type material shows that all of these taxa are synonyms of L. origanoides.
Distribution and habitat
Lippia origanoides is most frequent in South America, growing in Bolivia, Brazil, Guyana, Paraguay and northern Argentina. It is also found in Mesoamerica in Costa Rica, Mexico and Venezuela. Correll & Johnston (1970) mention it in Texas (sub. nom. L. graveolens). It grows in cerrado and caatinga regions, in rocky soils, at 160–1800 m elevation (Fig. 9).
Representative specimens examined
ARGENTINA. Misiones: San Ignacio, Parque Provincial Teyucuaré, entrada al parque, 3.xii.2004, Zuloaga 8403 (SI). Salta: General José de San Martín, 5 km de la ruta nac. 34, camino a Dique Itiyuro, 6.v.2003, Morrone 4627 (SI). BOLIVIA. Santa Cruz: Velazco, San Ignacio, 9.v.1977, Krapovickas 32425 (SI). BRAZIL. Amazonas: Rio Branco, 1.viii.1913, Kuhlman 797 (SI). Bahia: Barreiras, Estrada para Ibotirama, BR 242, 13.vi.1992, Amorim 588 (SI). Ceará: estrada de Fortaleza-Crato, 2.viii.1948, Duarte 1246 (SI). Espírito Santo: Vila Velha, Praia da Costa, 22.ii.1963, Santos 1613 (SI). Goiás: Niquelãndia, 4 km de Muquem, 8.v.1998, Fonseca 1819 (SI). Minas Gerais: Ouro Preto, Serra de Ouro Preto, 15.i.1942, Mendes 1360 (SI). Paraná: Jaguariaiva, Chapada de Santo Antonio, 20.iii.1980, Dombrowski 11233 (SI). Piauí: Pedro II, 24.vi.1972, Sucre 9308 (SI). Rio de Janeiro: Petrápolis, Vale das Vedeiros, 7.i.1973, Martinelli 160 (SI). Roraima: Alto Alegre, Serra de Tepequém, N side of plateau, 23.xii.1987, Hopkins 1025 (SI). São Paulo: Moji-Guaçu, São Paulo, Reserva Biológica, 26.i.1981, Montovani 1560 (SI). COSTA RICA. Guanacaste: alrededores de Liberia, 1.ii.1947, Brenes s.n. (SI). MEXICO. Durango: Lerdo, Presa Fco. Zarco, en ladera W de la presa, 2.x.2005, González 7120 (SI). PARAGUAY. Concepción: Itacurubí, 1.iii.1917, Rojas 2517 (SI). Cordillera: Barrero Grande, 1.ii.1978, Schinini 1947 (SI). Paraguarí: Salto Piraretá, 1.iii.1972, Schinini 4349 (SI). VENEZUELA. Sucre: Cumanacoa, 7.iii.1905, Drake s.n. (SI).
3. Lippia sericea Cham. (Fig. 10)
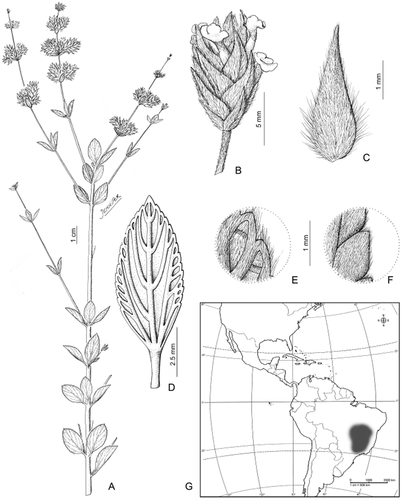
Lippia sericea: A, plant general aspect; B, detail of florescence; C, floral bract; D, leaf, abaxial surface; E, leaf pubescence, abaxial surface; F, leaf pubescence, adaxial surface; G, geographical distribution. From Duarte 9916 (SI).
Linnaea 7: 228 (1832). Type: Brazil. Goiás: Fazenda da Jaguara, F. Sellow 1441 (lectotype, here designated: K 470924!; isotypes: B†, photograph Field Museum neg. 17545!, BR 981675!, SI 3591!, VT 27!).
Shrubs 0.4–0.9 m high, apical stems canescent sericeous, internodes 1.0–5.6 cm long. Leaves usually ternate, sometimes opposite; petioles 0.1–0.4 cm long, sericeous or canescent sericeous; blades 0.6–2.2 × 0.4–1.0 cm, elliptic, base round, apex acute to obtuse, margin crenate, venation perfect acrodromous sometimes pinnate, adaxial and abaxial surfaces canescent sericeous. Inflorescences frondose, two or three florescences per axil, peduncles 0.1–0.4 cm long, sericeous, florescence 0.5–1.4 cm long, bracts 0.3–0.6 cm long, apex straight, apical bracts free, both surfaces canescent sericeous. Calyx 0.15–0.20 cm long, external surface sericeous. Corolla 0.5–0.7 cm long, external surface slightly strigose. Nutlets 0.2 cm long.
Notes
In L. sericea, young and mature plant parts are always canescent sericeous; in other species, only young structures can be canescent sericeous.
Typification
Lippia sericea was described by Chamisso on the basis of a Sellow specimen, housed at B, now destroyed. Consequently, the specimen from K was chosen here as lectotype as it is a good specimen with a label ‘ex Museo Berolinense’.
Distribution and habitat
Lippia sericea is endemic to Brazil, from Minas Gerais, Goiás and Distrito Federal. It grows in wild fields and cerrados (Fig. 10).
Representative specimens examined
BRAZIL. Distrito Federal: Fazenda Água Limpa, 30.v.2000, Munhoz 1642 (IBGE). Goiás: Niquelãndia, 2 km da estrada para Macedo Velho, 21.vi.1995, Fonseca 379 (SI). Minas Gerais: sin localidad, 11.i.1905, Clausen 613 (SI).
4. Lippia stachyoides Cham. (Fig. 11)
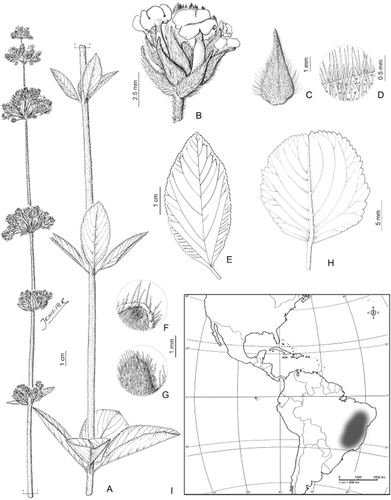
Lippia stachyoides var. stachyoides: A, plant general aspect; B, detail of florescence; C, floral bract; D, detail of bract pubescence; E, leaf, adaxial surface; F, leaf pubescence, abaxial surface; G, leaf pubescence, adaxial surface; H, Lippia stachyoides var. martiana, leaf, abaxial surface; I, geographical distribution. A–G, from Sakane 691 (SI); H, from Arbo & al. 4875 (SI).
Linnaea 7: 227–228 (1832). Type: Brazil. F. Sellow 5297 ‘in Brazilia meridionalis’ (lectotype, here designated: K 470920!).
Shrubs 0.7–2.0 m high, stems hispid or strigose, internodes 1.2–13.0 cm long. Leaves ternate or opposite; petioles 0.1–1.0 cm long, hispid or strigose; blades 0.8–5.6 × 0.7–3 cm, ovate or elliptic, base round or cuneate, apex acute to obtuse, margin crenate, venation pinnate sometimes perfect acrodromous, adaxial and abaxial surfaces strigose or sericeous. Inflorescences frondose-bracteose, three to nine florescences per axil, peduncles 0.2–0.8 cm long, hispid, florescence 0.4–1.0 cm long, bracts 0.2–0.6 cm long, apex straight, rarely curved, apical bracts free, both surfaces strigose. Calyx 0.1–0.2 cm long, external surface hispid. Corolla 0.25–0.70 cm long, external surface strigose. Nutlets 0.15–0.20 cm long.
Notes
This species is characterized by its frondose-bracteose inflorescence. Two varieties are found based on leaf morphological differences.
Typification
Lippia stachyoides was based on a Sellow specimen, housed at B, now destroyed; a Sellow material from K, from a donation from Berlin, was chosen as lectotype.
Distribution and habitat
Lippia stachyoides is endemic to eastern Brazil. Both varieties of this species are sympatrically distributed.
4a. Lippia stachyoides var. stachyoides (Fig. 11A–G)
This variety is distinguished by the larger and more elliptic leaf blades, and larger floral bracts and corolla.
Distribution and habitat
Lippia stachyoides var. stachyoides is found in Brazil, in Minas Gerais, São Paulo and Goiás. It grows in cerrado on rocky soil, at > 600 m elevation, flowering at most times of the year (Fig. 11).
Representative specimens examined
BRAZIL. Goiás: Niquelãndia, 1 km abaixo da mina de níquel, 29.v.1996, Fonseca 959 (SI). Minas Gerais: Serra do Cipó, 17.iv.1950, Duarte 2501 (SI). São Paulo: Moji-Guaçu, Padua Sales, Reserva Biológica, 24.xi.1977, Sakane 691 (SI).
4b. Lippia stachyoides (Cham.) var. martiana (Schauer) Salimena & Múlgura. comb. nov. (Fig. 11H)
Lippia martiana Schauer, Prodr. 11: 578 (1847). Type: Brazil. Minas Gerais, in campis, 1841, C.F.P.Martius 1028 (lectotype, here designated: G!; isotypes: BM 906663!, BM 906662, BR 649481!, K 470927!, MO 503905, NY 137782, SI 3555! SI 3556!, W!, photograph Field Museum neg. 7859!).
Lippia nepetacea Schauer, Prodr. 11: 577 (1847), syn. nov. Type: Brazil. Minas Gerais, Vauthier, herb. bras. 410 (lectotype, here designated: P 568342!; isotypes: G!, P 568343!, SI 3563).
Lippia pohliana Schauer, Prodr. 11: 577 (1847), syn. nov. Type: Brazil. Goiás, ‘Menaponte’, J.B.Pohl 2791 (lectotype, here designated: W!; isotypes: BR 981675! F 87449!, NY!, SI 3596!).
Lippia pohliana var. longibracteolata Moldenke, Phytologia 31: 231 (1975), syn. nov. Type: Brazil. Minas Gerais, north on the road to Diamantina, Serra do Espinhaço, 4.ii.1972, W. R. Anderson, M. Steiber & J. Kirkbride 35371 (holotype: NY 137802!).
Lippia martiana f. campestris Moldenke, Phytologia 41: 346 (1979), syn. nov. Type: Brazil. Brasilia, south sides of campus Universidade de Brasília, 16.xi.1977, Taxonomy Class 518 (holotype: US 118844!).
This variety is distinguished by the smaller and more round leaf blades, and smaller floral bracts and corolla.
Typification
Three syntypes (Martius 1028, Clausen s.n. and Regnell 333) were mentioned in the protologue of L. martiana Schauer; the Martius 1028 material was chosen as lectotype because it is a good specimen and is distributed in several herbaria; the Regnell 333 material could not be found.
Two syntypes were mentioned in the protologue of Lippia nepetacea: Pohl ‘Engenho de São João das Antas e Izidro, Conceição’ (W) and Vauthier 410 ‘herb. bras’ (P); the latter was chosen as lectotype, given that it is a good specimen also distributed in G.
Lippia pohliana was based on two specimens, Pohl 2791 and Riedel s.n.; Pohl 2791 was chosen here as lectotype because it matches exactly the protologue and is distributed in several herbaria.
Synonymy
The type materials of Lippia nepetacea and L. pohliana were included in the data matrix and they appeared to be included within this same group in the CA. Type materials of L. pohliana var. longibracteolata Moldenke and L. martiana f. campestris Moldenke were not included in the analysis, but the study of images of the type material shows that they are synonyms of L. stachyoides var. martiana.
Distribution and habitat
Lippia stachyoides var. martiana grows in Brazil, Distrito Federal, Goiás, Bahía and Minas Gerais. It is found in cerrados, in rocky and sandy soils, at 800–1100 m elevation. (Fig. 11).
Representative specimens examined
BRAZIL. Bahia: Possões, próximo à Vitória da Conquista, estrada para Boa Nova, 5 km, Gottsberger 17-26173 (TEX). Distrito Federal: Reserva Ecológica IBGE, 15.ii.2001, da Silva 4817 (SI). Goiás: Niquelãndia, 18 km de Niquelãndia, Morro do entroncamento para Macedo Velho, 16.iv.1996, Fonseca 921 (SI). Minas Gerais: Santana do Riacho, camino a Lapinha, 11.ii.1991, Arbo 4875 (SI).
Doubtful Taxa
Lippia microphylla var. foliis acutiusculis Hiern, Vidensk. Meddel. Dansk Naturhist. Foren. Kjobenh. 1877–1878: 97 (1877). Type: Brazil. Lagoa Santa, Warming s.n.
Lippia microphylla var. fasciculata Hiern, Vidensk. Meddel. Dansk Naturhist. Foren. Kjobenh. 1877–1878: 97 (1877). Type: Brazil. In Campis ad S. Luiza, Octobri fl., Lund s.n.
Type material of these two taxa is not in C, nor has it been found in other herbaria. They have not been mentioned in any publication, and so these taxa are considered to be doubtful.
Excluded Taxa
Goniostachyum citrosum Small, Bull. Torrey Bot. Club 36: 162. – Type: United States. Florida, Silver Palm Schoolhouse, xi.1904, Small 242 (holotype: NY) = Lantana microcephala A. Rich.
Acknowledgements
We are grateful to the herbaria curators for images of type specimens and for help given to the authors in the search for type material. We are also grateful to the Myndel Botanical Foundation for the fellowship given to F. Salimena in order to visit European herbaria. Special thanks are given to Dr R. Pozner for help with pubescence photographs, and to M. Belgrano for English language editing. An earlier version of the manuscript benefited greatly from suggestions from two anonymous reviewers. This contribution is part of the following projects: PIP 5262/05-05, Consejo Nacional de Investigaciones Científicas y Tecnológicas (Argentina); 490527/2008-6, Programa Sul-Americano de Apoio às Atividades de Cooperação em Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil).
Appendix
List of specimens used in the analysis
Lippia grata Schauer, Biloni 6251 (SI), Biurrun 596 (SI), 4714 (SI), 5444 (SI), Brade 13904 (SI), Debeaux 87 (SI), s.n. (SI), Falçao 1035 (SI), Fernández 3337 (SI), Ferrucci 2663 (SI), Herzog 1108 (SI, Lippia laxibracteata isotype), 1851 (SI, Lippia dumetorum isotype), Hunziker 18382 (SI), Jardim 3475 (CEPEC), Krapovickas 19115 (SI), 30848 (SI), 31316 (SI), 46215 (SI), Martius s.n. (SI-003517, Lippia gracilis isotype), s.n. (SI-003518, Lippia grata isotype), Mendez Magalhaes 14951 (BHCB, CESJ), Parodi 14864 (SI, Lippia hickenii holotype), Pensiero 4372 (SI), Pereira 2672 (SI), Queiroz 6556 (HUEFS), Roca 709 (SI), Saravia Toledo 727 (SI), 2206 (SI), 11766 (SI), Schinini 19563 (SI), Suárez 142 (SI), Vaillant 1422 (HUEFS); Lippia origanoides Kunth, Amorim 588 (SI), Anderson s.n. (SI), Azevedo 1313 (SI), Balansa 4624 (SI, Lippia polycephala isotype), Bandeira 235 (HUEFS), Borges 10 (HUEFS), Braga 2019 (SI), 2060 (SI), Brandao 22813 (BHCB, CESJ), Brenes s.n. (SI), Brochado 203 (SI), Carvalho 3835 (SI), 3987 (SI), Conceiçao 2452 (SPF), Costa 311 (SI), Dechioni s.n. (SI), Dombrowski 11233 (SI), Drake s.n. (SI), Duarte 7849 (SI), 9164 (SI), 9738 (SI), 1043 (SI), 1246 (SI), 8788 (SI), 9011 (SI), Ducke s.n. (SI), Ferreira 377 (CESJ), Filgueiras 2234 (SI), Fonseca 1819 (SI), Funari s.n. (CESJ-55806), Gardner 3942 (SI, Lippia candicans isotype), Gerold 380 (SI), González 7120 (SI), Guedes 5616 (HUEFS), Hahn 1574 (SI), Harley 16269 (SPF), 26235 (SI), Hassler 8016 (SI, Lippia obscura isotype), Hatschbach 12191 (SI), 18859 (SI), 21587 (SI), 38210 (SI), 46377 (CESJ, MBM), 50908 (SI), 71935 (CESJ, MBM), Hoehne s.n. (SI, SPF-12248), s.n. (SI, SPF-12384), s.n. (SI, SPF-12385), 5549 (SPF), Hopkins 1025 (SI), Humboldt & Bonpland s.n. (SI-003562, Lippia origanoides isotype), Irwin 19579 (NY, TEX), 20825 (NY, TEX), 21991 (NY, TEX), Kawasaki s.n. (SI), Krapovickas 32425 (SI), 33029 (SI), Krieger s.n. (CESJ-7050, SI), Kuhlman 797 (SI), Lima s.n. (R-60789, SI), s.n. (R-70003, SI), Lombardi 3772 (BHCB, CESJ), Macedo 3639 (SI), Martinelli 160 (SI), Martius 1024 (SI, Lippia velutina isotype), Matos s.n. (BHCB-100352), Melo 1588 (HUEFS), Mendes 1360 (SI), Mendez Magalhaes 1770 (BHCB, CESJ), Mendonça 1420 (SI), Montovani 1560 (SI), Mori 13431 (HUEFS), Morrone 4627 (SI), Nee 40521 (SI), 40867 (SI), Noblick 3133 (HUEFS), Pastore 880 (CEN), Pereira 1996 (SI), 2794 (SI), Philcox 4736 (SI), Pirani s.n. (SPF), Pohl 132 (K, Lippia glandulosa lectotype), 2956 (SI), Porto 2224 (SI), Queiroz 4617 (HUEFS), Rojas 2517 (SI), Saint-Hilaire 997 (SI), 2675a (SI), Salimena 1274 (CESJ), 2436 (CESJ), 2447 (CESJ), 39360 (CESJ), s.n. (CESJ-31255), s.n. (CESJ-32891), s.n. (CESJ-39362), Santos 1613 (SI), Santos Lima 324 (SI), Schinini 1947 (SI), 4349 (SI), Sellow s.n. (SI-003571, Lippia rubiginosa isotype), s.n. (SI-003593, Lippia sidoides isotype), s.n. (SI-003553, Lippia microphylla isotype), Souza 7805 (CESJ, ESA), 8501 (ESA), Stehman 2410 (BHCB, CESJ), Sucre 9308 (SI), 9468 (SI), Tamayo 3122 (SI), Texeira s.n. (BHCB-90624), Viana 1193 (BHCB, CESJ), Williams 307 (SI, Lippia pendula isotype), Zardini 16449 (SI), Zuloaga 8403 (SI). Lippia sericea Cham, Brade 13905 (SI), Clausen 613 (SI), Dias 223 (CEN), Duarte 8080 (SI), 9916 (SI), Fonseca 379 (SI), Mendonça 2592 (SI), Munhoz 1642 (IBGE), Pereira 2985 (SI), Sellow 1441 (SI-003591, Lippia sericea isotype). Lippia stachyoides var. stachyoides Cham., Barbosa s.n. (HUEFS-45465), Duarte 2501 (SI), 2586 (SI), 7580 (SI), 9643 (SI), Fonseca 959 (SI), Sakane 691 (SI), Sellow 5297 (K-000470920, Lippia stachyoides lectotype), Sine collector 9117 (SI). Lippia stachyoides var. martiana (Schauer) Salimena & Múlgura, Arbo 4875 (SI), Calcavanti 2304 (SPF), Carmo 4187 (BHCB, CESJ), 4637 (BHCB, CESJ), Clausen 1028 (SI-003555, Lippia martiana isotype), da Silva 4817 (SI), Duarte 6467 (SI), 10089 (SI), 10403 (SI), 9114 (SI), Fonseca 921 (SI), 1021 (SI), 4634 (CESJ, IBGE), 4744 (CESJ, IBGE), Gottsberger 17–26173 (TEX), Pereira 8867 (SI), Pohl 2791 (SI-003569, Lippia pohliana isotype), Roth s.n. (CESJ-18311), Salimena 333 (CESJ), 1404 (CESJ), 2438 (CESJ), s.n. (CESJ-32895), s.n. (CFSC-11416, SI), s.n. (SPF-47191, SI), 2442 (CESJ), Tameirao Nieto 578 (BHCB, CESJ), Valente 493 (CESJ, VIC), Vauthier 410 (SI).




