Pollination biology of four sympatric species of Habenaria (Orchidaceae: Orchidinae) from southern Brazil
Abstract
The pollination process and breeding system of the sympatric Habenaria johannensis, H. macronectar, H. megapotamensis and H. montevidensis was documented for native populations from Rio Grande do Sul, Brazil. All species investigated offer a nectar reward (mean values of total sugars ranging from 18 to 26%) concealed in a spur. Habenaria montevidensis is pollinated by butterflies (Hesperiidae, especially of the genus Urbanus) that carry pollinaria on their eyes; the other three species are pollinated by Sphingidae. Habenaria johannensis is pollinated by the moths Manduca rustica and M. sexta that carry the pollinaria at the base of the proboscis. Habenaria macronectar is pollinated by the moths Eumorpha labrusca and M. cf. lucetius, and these bear pollinaria between the palpi. Habenaria megapotamensis is pollinated by moths of M. cf. lucetius that bear the pollinaria on the proboscis. All species studied are self-compatible, but pollinator dependent. They also displayed high reproductive success (ranging from 69.48 to 97.40%) and male efficiency factors slightly higher than 1, suggesting that at least one flower was pollinated for each flower acting as pollen donor. At the study sites, the investigated Habenaria spp. are isolated (in terms of pollination) by a set of factors that includes differing floral morphologies, different pollinators and/or different pollinarium placement on the pollinator. © 2012 The Linnean Society of London, Botanical Journal of the Linnean Society, 2012, ••, ••–••.
Introduction
Comprising some 835 species distributed throughout the temperate and tropical regions of the Old and New Worlds, Habenaria Willd. is the largest terrestrial orchid genus (Batista, Bianchetti & Miranda, 2006), and central and southern Africa, eastern Asia and Brazil are its main centres of diversity (Kurzweil & Weber, 1992). Approximately 165–170 Habenaria spp. have been reported for Brazil (Hoehne, 1940; Pabst & Dungs, 1975; Batista et al., 2006), where the Cerrado Biome (in essence, a tropical Savanna) seems to be particularly species-rich (Batista et al., 2006). Habenaria is a member of subtribe Orchidinae (Chase et al., 2003) and its main distinctive features are the often bifid petals that are not fused to the other floral organs, the usually deeply divided median petal (labellum) that lacks a callus, the distinctly stalked stigmas and the entire stigma lobes, which are usually free and not adnate to the petals or lip (Cribb, 2001). In spite of its diversity, the pollination biology and breeding system of few Habenaria spp. have been studied. Most published reports indicate that Habenaria spp. are mainly pollinated by moths (Nilsson et al., 1985; Galetto, Bernardello & Rivera, 1997; Singer & Cocucci, 1997; Singer, 2001; Singer et al., 2007; Peter et al., 2009) and, to a lesser extent, by crane flies (Singer, 2001) or diurnal Lepidoptera (Moreira, Correa & Mugrabi-Oliveira, 1996). This is supported by floral features. For example, flowers are usually greenish or pale in colour, the nectar is concealed in a spur and crepuscular/nocturnal emission of fragrance (a feature normally associated with pollination by moths) is evident (Singer & Cocucci, 1997; Singer, 2001; Singer et al., 2007; Peter et al., 2009). Pollinaria of Habenaria spp. have been reported to adhere to various smooth body parts of pollinators, such as the surface of the eye, proboscis and distal parts of the forelegs (Singer & Cocucci, 1997; Singer, 2001; Singer et al., 2007; Peter et al., 2009). Little is known of the breeding system of this orchid genus. Habenaria parviflora Lindl. is self-compatible, but pollinator dependent (Singer, 2001), and H. pleiophylla Hoehne & Schltr. appears to be self-compatible, based on the vigorous growth of the pollen tubes following manual self-pollination (Singer et al., 2007).
In southern Brazil, several Habenaria spp. grow sympatrically and, during the local summer (December to March), have overlapping flowering periods. Of these species, four are particularly abundant and conspicuous: H. johannensis Barb. Rodr., H. macronectar (Vell.) Hoehne, H. megapotamensis Hoehne and H. montevidensis Spreng. These species are the subject of this study. Not only do the flowering periods of these species overlap, but these plants may also be found growing in close proximity or separated by short distances. For instance, H. macronectar and H. megapotamensis can often be found growing together. Thus, the aim of this study is to extend our knowledge of the pollination biology and reproductive biology of Neotropical Habenaria spp. More specifically, we set out to document the pollination process, fruiting success and breeding system of H. johannensis, H. macronectar, H. megapotamensis and H. montevidensis.
Material and Methods
Study site
Studies were performed in two neighbouring municipalities (Cambará do Sul, approximately 29°10′S, 50°19′W, and São Francisco de Paula, approximately 29°25′S, 50°23′W) in the Atlantic Rain Forest domain, in the State of Rio Grande do Sul, Southern Brazil. For conservation reasons, we cite here only the coordinates of both municipalities and omit the exact location details of the populations. These data, however, are available on request. This particular region is locally known as Campos de Cima da Serra and consists of a mosaic of native grasslands and forest patches dominated by Araucaria angustifolia (Bertol.) Kuntze (Araucariaceae), at an altitude of c. 900–930 m. Average annual rainfall is c. 2468 mm and annual average temperature is approximately 14.5 °C (Moreno, 1961). The climate is characterized by a cool summer, a cold winter and the lack of a well-defined dry period (Nimer, 1989; Almeida, 2009). All species studied inhabit swamps dominated by Eryngium pandanifolium Cham. & Schltdl. (Apiaceae).
Studied species
Habenaria johannensis and H. macronectar are currently placed in section Macroceratitae Kraenzl. (Kränzlin, 1892; Batista et al., 2006), an easily identifiable assemblage of species with proportionally large, greenish–white, long-spurred (spur length ≥ 6 cm) flowers.
Habenaria megapotamensis and H. montevidensis were never assigned formal sectional classification. According to an unpublished phylogenetic analysis based on DNA sequence data (J. A. N. Batista, pers. comm.), these two species belong in the same clade, which also includes H. exaltata Barb. Rodr., H. henscheniana Barb. Rodr. and H. ekmaniana Kraenzl.
Of the species studied, H. johannensis has the widest geographical distribution, ranging from Bolivia and Paraguay to north-eastern, central western, south-eastern and southern Brazil. It is typical of wet and marshy areas, but occasionally can occupy human-disturbed habitats, growing on damp roadsides or in ditches. Habenaria macronectar is restricted to south-eastern and southern Brazil and Uruguay. This species occurs in wet or marshy areas. Habenaria megapotamensis is known only from southern Brazil, whereas H. montevidensis also occurs in southern Brazil and Uruguay. These last two species are typical of marshy areas or wetlands, and most records are derived from the north-eastern highlands of Rio Grande do Sul. Throughout this paper, we follow the orchid classification of Chase et al. (2003).
Floral features and nectar properties
Ten fresh, intact flowers from each species were used to record and measure flower features (column and pollinarium structure, spur length, nectar-column height, as well as nectar volume and concentration; see Table 1). All flowers came from five bagged specimens of each species, and nectar parameters were always measured for each of these taxa during the period when their pollinators had been recorded in activity. Nectar volume was measured by means of a microsyringe, and nectar concentration (total sugar) was measured using a manual pocket (0–32) refractometer. Because of the small quantity of nectar produced by H. montevidensis flowers, nectar volume in this species was measured by means of a P2 micropipette, and nectar concentration measurements were based on the total accumulated nectar of all the flowers analysed. Data on spur length, nectar volume, height of nectar column and nectar concentration (total sugars) for all studied species were tested for normality by means of a Shapiro–Wilk test and, later, statistically compared by means of a Kruskall–Wallis test (because the data did not display a normal distribution) followed by a Dunn post-hoc test, using the BioEstat software (Ayres et al., 2007) (Table 1).
| H. section Macroceratitae | Section not defined | H | P | d.f. | |||
|---|---|---|---|---|---|---|---|
| H. johannensis | H. macronectar | H. megapotamensis | H. montevidensis | ||||
| Maximum number of flowers observed per inflorescence | 19 | 28 | 87 | 27 | – | – | – |
| Floral lifespan (days) | 12–13 | 16–18 | 14–18 | 17–21 | – | – | – |
| Pollinarium length (mm) | 15 | 9 | 3 | 4 | – | – | – |
| Spur length (cm) | 12.5–14 (13.09 ± 0.51) a | 6.3–7.6 (6.66 ± 0.41) b,c | 6.8–7.8 (7.51 ± 0.37) a,b | 1.6–1.7 (1.62 ± 0.04) c | 36.19 | 0.0001 | 3 |
| Nectar volume (μL) | 20–40 (35 ± 7.07) a | 10–30 (18.8 ± 5.67) a,b | 10–20 (11 ± 3.16) b,c | 0.9–1.7 (1.27 ± 0.28) c | 34.88 | 0.0001 | 3 |
| Nectar column height (cm) | 1.7–3.9 (2.73 ± 0.84) a,b | 0.9–4.3 (2.2 ± 0.98) a,c | 2.4–4 (3.32 ± 0.58) b,c | 0.4–0.6 (0.52 ± 0.08)d | 26.36 | 0.0001 | 3 |
| Total nectar sugar concentration (%) | 20.6–26.6 (24.46 ± 2.01) a | 12–23.6 (18.12 ± 4.04) b | 15–23.6 (19.04 ± 3.13) b | 26.2a | 15.46 | 0.0004 | 2 |
- *Owing to the small volumes of nectar available, nectar concentration in this species was measured by pooling the nectar of ten flowers. In all cases, values outside parentheses represent the range (minimum and maximum) observed; and values in parentheses represent the mean value ± standard deviation.
- †Species not sharing the same letter (a, b or c) within traits differ significantly (Kruskall–Wallis, P < 0.05).
Flower morphology was studied using fresh and alcohol-preserved flowers [70% (v/v) ethanol]. Plant vouchers were deposited at the ICN Herbarium of the Universidade Federal do Rio Grande do Sul (UFRGS) under the following accession numbers: H. johannensis (M. Pedron 5), H. macronectar (M. Pedron 1); H. megapotamensis (M. Pedron 10); H. montevidensis (M. Pedron 9). Throughout this paper, we follow the orchid morphology concepts of Dressler (1993).
Pollination
The pollination biology of H. megapotamensis was studied in the field during the flowering seasons of 2010 and 2011 (Table 2). The other three species were studied in 2011 (Table 2). Generally, the observation period for both years began in late January or early February and was completed by mid March, totalling 181 observation hours (Table 2). Both, crepuscular–nocturnal and diurnal observations were made on each species. Here, we define ‘diurnal observations’ as those made between 06:00 and 18:00 h and ‘crepuscular–nocturnal’ observations as those made from 18:00 h onwards. The specific observation period for each species and the specific number of diurnal and crepuscular–nocturnal hours spent observing each species are detailed in Table 2. When our early observations clearly indicated the prevalence of some kind of pollinator activity (e.g. diurnal vs. crepuscular–nocturnal), our fieldwork schedule was adapted accordingly. Therefore, most observation hours for H. johannensis, H. macronectar and H. megapotamensis were performed during the nocturnal period (Table 2). Conversely, most observation hours for H. montevidensis were made during the day. However, as nocturnal, non-pollinating visitors were also recorded for this species, intact flowers (36 flowers from 21 inflorescences) were marked at the end of the observations for the day and checked at the beginning of the next day, before observations had begun, in order to verify whether they had been pollinated and/or had their pollinaria removed during the night. Pollinator behaviour was documented for all species studied using field notes, photography and, when possible, video. The video record made it possible to gain a better understanding of the pollination process for most of the species studied, especially those visited by crepuscular–nocturnal pollinators. Individuals of both, pollinating and non-pollinating insects were collected and sacrificed for taxonomic identification. These insect vouchers were deposited at the entomological didactic collection of the Insects Ecology Laboratory, Zoology Department, UFRGS.
| Species/locality | Period | Diurnal pollination | Nocturnal pollination | Total | ||
|---|---|---|---|---|---|---|
| Observation period | Observation hours | Observation period | Observation hours | |||
| H. johannensis | ||||||
| Brazil, Rio Grande do Sul, São Francisco de Paula | 24 Janurary and 18–20 February 2011 | 10:00 to 13:00 h | 10 h | 18:00 to 22:30 h | 34 h | 44 h |
| H. macronectar | ||||||
| Brazil, Rio Grande do Sul, Cambará do Sul | 19 February–12 March 2011 | 15:00 to 18:00 h | 9 h | 18:00 to 23:00 h | 55 h | 64 h |
| H. megapotamensis | ||||||
| Brazil, Rio Grande do Sul, Cambará do Sul (two populations) | 10 February 2010; 19–21 February 2011 | 10:20 to 11:20 h; 15:00 to 18:00 h | 5 h | 18:00 to 23:00 h | 30 h | 35 h |
| H. montevidensis | ||||||
| Brazil, Rio Grande do Sul, São Francisco de Paula | 8–19 February 2011 | 06:00 to 18:00 h | 24 h | 18:00 to 22:00 h | 14 h | 38 h |
| Total for all species: | 181 h | |||||
Breeding system, fruiting success and pollination efficiency
Breeding system experiments were performed in situ, by bagging inflorescences in order to exclude natural pollinators. Bags were supported with the help of wooden stakes. Four treatments were applied to these inflorescences: intact flowers (control); emasculation; manual self-pollination; and cross pollination (Table 3). Treatments that set fruit were compared using a χ2-test (α = 0.05). The number of plants per species and flowers used per treatment are shown in Table 3. Intact flowers of these plants were also used to record flower lifespan for each species.
| Section/species | N b | Control | Emasculation | Self-pollination | Cross-pollination | χ2 (self × cross pollination) values, P < 0.2) |
|---|---|---|---|---|---|---|
| H. section Macroceratitae | ||||||
| H. johannensis | 15 | 0 (0/37) | 0 (0/33) | 100 (35/35) | 100 (34/34) | 0 (NS) |
| H. macronectar | 17 | 0 (0/31) | 0 (0/29) | 85.29 (29/34) | 87.5 (28/32) | 0.0093 (NS) |
| Section not defined | ||||||
| H. megapotamensis | 12 | 0 (0/49) | 0 (0/45) | 100 (45/45) | 100 (45/45) | 0 (NS) |
| H. montevidensis | 12 | 0 (0/32) | 0 (0/33) | 100 (30/30) | 100 (30/30) | 0 (NS) |
- a Numbers in parentheses represent the number of fruit obtained over the number of flowers used in each treatment.
- b N represents the number of individuals used in the experiments.
- NS, non-significant.
In order to assess the efficiency of pollination, the fruiting success (number of fruit divided by the number of flowers produced) was calculated for each species, at the end of the observation periods. During 2010, 30 inflorescences of H. johannensis (totalling 269 flowers), 49 inflorescences of H. macronectar (totalling 526 flowers) and 32 inflorescences of H. megapotamensis (totalling 1568 flowers) were available. It was not possible to study the fruiting success for H. montevidensis in 2010, as fruiting inflorescences were destroyed, probably eaten by unidentified animals. During 2011, 45 inflorescences of H. johannensis (totalling 387 flowers), 16 inflorescences of H. macronectar (totalling 154 flowers), 17 inflorescences of H. megapotamensis (totalling 561 flowers) and 35 inflorescences of H. montevidensis (totalling 429 flowers) were available. Fruiting success (mean fruit set per inflorescence) in both years for all species except H. montevidensis (see above) was statistically compared using an independent, two-sample t-test (unequal sample sizes, equal variance) by means of PAST software (Hammer, Harper & Ryan, 2001) (Table 4).
| Species/locality | Pollinators | Proboscis length (cm) | Site of pollinarium attachment | Maximum number of pollinaria observed per insect | Fruiting success (%)a | F | P | d.f. | Male efficiency factor (2011)a | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2010§ | 2011§ | |||||||||
| H. johannensis | Manduca rustica (Sphingidae) | 13.5 | Underside of the base of the proboscis | 15 | 97.4 (97.38 ± 8.13) a | 92.51 (89 ± 16.41) b | 41.464 | 0.0113 | 71 | 1.04 (N: 208, 86.06/82.21) |
| M. sexta (Sphingidae) | 10.3 | Underside of the base of the proboscis | 13 | |||||||
| H. macronectar | Eumorpha labruscae (Sphingidae) | 5.9 | Between the palpi | 12 | 86.12 (84.39 ± 16.35) a | 69.48 (68.62 ± 20.32) b | 15.436 | 0.0027 | 64 | 1.13 (N: 105, 72.38/63.81) |
| M. cf. lucetius (Sphingidae) | 6.4–7.4 | Between the palpi | 3 | |||||||
| H. megapotamensis | M. cf. lucetius (Sphingidae) | 6.4–7.4 | Underside and sides of the base of the proboscis | 15 | 84.69 (85.07 ± 12.2) a | 92.87 (90.84 ± 11.98) a | 10.377 | 0.1175 | 48 | 1.01 (N: 231, 84.42/83.55) |
| H. montevidensis | Cumbre sp. (Hesperiidae) | 1.3 | On the eyes | 2 | a | 78.32 (77.94 ± 28.12) | a | a | a | 1.12 (N: 367, 80.11/71.39) |
| Vehilius clavicula (Hesperiidae) | 1.3 | On the eyes | 3 | |||||||
| Urbanus teleus (Hesperiidae) | 1.1 | On the eyes | 6 | |||||||
| Urbanus zagorius (Hesperiidae) | 1.5 | On the eyes | 14 | |||||||
- *In 2010, the fruiting inflorescences of H. montevidensis had been destroyed, presumably by herbivores.
- †Numbers in parentheses represent mean number of capsules per inflorescence.
- ‡Numbers in parenthesis represent: N, total number of flowers used to calculate Nilsson's male efficiency factor, % of pollinated flowers/% of flowers acting as pollen donors.
- §Species not sharing the same letter (a or b) within years differ significantly according to independent two-sample t-test (P < 0.0005).
In 2011, Nilsson's male efficiency factor (percentage of pollinated flowers divided by the percentage of flowers acting as pollen donors; Nilsson et al., 1992) was also used to calculate pollination effectiveness for all species studied. The total number of flowers used in these calculations and the percentages of flowers that were pollinated and that acted as pollen donors are shown in Table 4.
Results
Floral features
Flowers are resupinate and clustered in long, terminal, racemose inflorescences bearing a maximum of 19 to 87 flowers, depending on the species (Table 1; and see also Supporting Information, Fig. S1). The flowers of H. johannensis, H. macronectar and H. montevidensis are greenish–white and those of H. megapotamensis are light green (Fig. 1A; see also Supporting Information, Fig. S1). The lifespan of intact flowers ranged from 12 to 21 days (Table 1). In all studied species, the dorsal sepal and lateral petals form a hood-like structure that partially hides the column. In H. montevidensis, the lateral petals are entire, and in the other three species they are bifid. The labellum is trilobed, and its posterior part is prolonged to form a spur that is partially filled with nectar (Fig. 1A). Mean spur length ranged from 1.62 to 13.09 cm, and statistically significant differences were found between the studied species (H = 36.19, P < 0.0001, d.f. = 3; see Table 1), except when comparing H. johannensis with H. megapotamenis, H. macronectar with H. megapotamensis and H. macronectar with H. montevidensis (Table 1). The rostellum is trilobed, and the anther canals are adnate to the lateral lobes (rostellar arms) so that a pad-like viscidium is placed at the end of each rostellar arm (Fig. 1B–E). In H. johannensis, the median rostellar lobe is keel-like and anteriorly projecting (Fig. 1B). During floral ontogenesis, the anther undergoes a division that ultimately results in the formation of two separate pollinaria concealed in their respective anther sacs. Each pollinarium is placed alongside a lateral rostellar lobe (Fig. 1B–E). In all studied species, the pollinarium consists of massulate pollinia, an arm-like, hyaline caudicle and a pad-like, terminal viscidium. Pollinarium length ranged from 3 to 15 mm (Table 1). In H. johannensis and H. macronectar, the rostellar arms are long and arched; with both viscidia almost adjacent and upward pointing (Fig. 1B–C). In H. megapotamensis, the rostellar arms are short and curved inwards, so that the spur entrance is closely flanked by the viscidium of each pollinarium (Fig. 1D). In H. montevidensis, the rostellar arms are straight and slightly divergent (Fig. 1E) and the distance between the apices of the rostellar arms is c. 3 mm. In all studied species, the two stigmatic surfaces are placed below the pollen sacs, surrounding the spur entrance (Fig. 1C–E). In H. johannensis and H. macronectar, the stigmatic surfaces are long-stalked and involute (Fig. 1B–C), whereas those of H. megapotamensis and H. montevidensis are short and slightly convex (Fig. 1D–E). In H. megapotamensis, a tooth-like process partially occludes the spur entrance (Fig. 1D). This process is formed by the protruding, erect projection of the apex of the inner margin of the stigmatic lobes, and this is located in front of the spur entrance, thereby dividing the entrance of the spur into two (Fig. 1D). The flowers of H. johannensis, H. macronectar and H. megapotamensis emit a sweet fragrance, the secretion of which is perceptible after 18:00–19:00 h. The flowers of H. montevidensis produce a faint, sweet fragrance throughout the whole day. This fragrance is best perceived by enclosing the flowers in a vial.

Key floral features of Habenaria species. A, comparative floral morphology. (From left to right: H. johannensis, H. macronectar, H. megapotamensis and H. montevidensis). Note the very different spur lengths. B, H. johannensis: dorso-lateral view of rostellar arms. Notice the upward facing viscidia. The arrow points to the projected median rostellar lobe. C, H. macronectar: dorso-lateral view of rostellar arms. Note the upward facing viscidia. D, close-up of H. megapotamensis flowers. The spur entrance is partially occluded by two, tooth-like stigmatic projections, and the viscidia are directed towards the spur entrance. E, close-up of H. montevidensis flowers. Rostellar arms are straight and slightly divergent. Scale bars, 1 cm (A and D–E); 5 mm (B–C).
Nectar volume in intact flowers of the same species is subject to remarkable variation and mean values ranged from 1.27 to 35 μL, depending on the species (Table 1). Mean nectar volumes showed significant differences between the studied species (H = 34.88, P < 0.0001, d.f. = 3; see Table 1), except when comparing H. johannensis with H. macronectar and H. macronectar with H. megapotamensis. In intact flowers of all species, the nectar forms a conspicuous column. Except for H. montevidensis, the height of this nectar column shows remarkable variation in intact flowers of the same species (Table 1). Mean values of nectar column height ranged from 0.52 to 3.32 cm, depending on the species (Table 1). Nectar column heights showed statistically significant differences (H = 26.36, P < 0.0001, d.f. = 3; see Table 1), except when comparing H. macronectar with H. megapotamensis. Mean values of nectar concentration ranged from 18.12 to 26.20% (Table 1). Significant differences were found in nectar concentration (H = 15.46, P < 0.0004, d.f. = 2; see Table 1), except when comparing H. macronectar with H. megapotamensis.
Pollination mechanism
Pollinarium withdrawal takes place when insects insert the proboscis into the spur and press the surface of the viscidia with a scaleless, smooth body part (e.g. proboscis, ventral region between the palpi or the eyes, depending on the species) (Fig. 3), thus dislodging the pollinaria on leaving the flower. Pollination takes place when a pollinarium-laden insect visits a flower; the pollinia contact the stigmatic surface and massulae are left behind (Fig. 3B, E, G). In H. johannensis, H. macronectar and H. montevidensis, the insects are able to insert the proboscis along a straight course. In H. johannensis, pollinator movements are restricted by the projected, keel-like median rostellar lobe (Fig. 1B), and the pollinators are mechanically guided against the upward facing viscidia (Fig. 3A). In H. megapotamensis, two tooth-like stigmatic processes partially block the spur entrance (Fig. 1D). Therefore, the insect has to insert its proboscis laterally, thus pressing it against the viscidia that are orientated toward the spur entrance (Fig. 3C).
Pollinators, pollinator features and behaviour
All species investigated are pollinated by Lepidoptera (Figs 2-4), a fact which is consistent with overall floral morphology. Most insects recorded bearing five or more pollinaria had proboscides that were shorter (see Table 4) than the mean spur length of the orchids that they pollinated (see Table 1). However, a larger pollinator sample is necessary to address this phenomenon adequately.

A–B, H. johannensis and its pollinators. A, Manduca sexta (Sphingidae) pollinating H. johannensis flower. Note the pollinaria attached below the proboscis. B, Manduca rustica (Sphingidae) with H. johannensis pollinaria attached below the proboscis. C–D, H. macronectar and its pollinators. C, Manduca cf. lucetius (Sphingidae) pollinating H. macronectar flowers. D, Eumorpha labruscae (Sphingidae) with H. macronectar pollinaria attached between the palps. Scale bars, 2 cm (A, C); 1 cm (B and D).
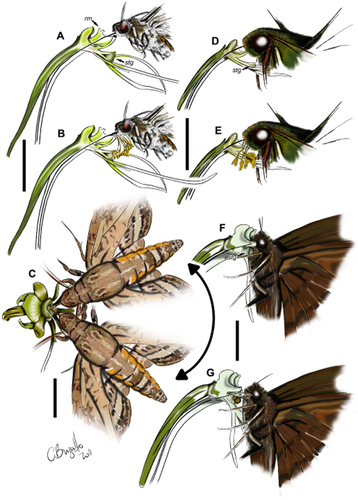
Pollination mechanism of Habenaria species. A–B, H. johannensis. A, a hawkmoth inserts its proboscis into the spur. The keel-like, median rostellar lobe restricts its movement and the insect is physically guided against the rostellum. B, a pollinarium-laden insect visits another flower and brushes the stigmatic surface with the pollinia. C, H. megapotamensis. The spur entrance is partially obstructed by tooth-like projections of the stigma. Therefore, the insect has to insert its proboscis laterally, thus pressing it against the viscidia that are orientated towards the spur entrance. D–E, H. macronectar. D, a hawkmoth inserts its proboscis into the spur and the viscidia come into contact with the region between the palpi. E, a pollinarium-laden insect visits another flower brushing the pollinia against the stigmatic surfaces. F–G, H. montevidensis. F, a butterfly inserts its proboscis into the spur, placing its head between the rostellar arms, and the pollinaria become attached to its eyes. G, pollination takes place when a pollinaria-laden insect visits another flower and brushes the pollinia against the stigmatic surfaces. rm, median rostellar lobe; stg, stigmatic surface. Scale bars, 2 cm (A–B, D–E); 1 cm (C); 5 mm (F–G).

A–B, pollination of H. megapotamensis. A, Manduca cf. lucetius (Sphingidae) inserting its proboscis into the spur. Note that this individual already carries several pollinaria attached to its proboscis. B, a pollinaria-laden moth visits a flower, brushing the pollinia against the stigmatic surfaces. C–D, pollination of H. montevidensis. C, Urbanus teleus (Hesperiidae) inserting its proboscis into the floral spur. Note that this specimen already carries pollinaria that are attached to its eyes. D, a pollinaria-laden insect inserts its proboscis into a floral spur, brushing the pollinia against the stigmatic surfaces. Scale bars, 1 cm.
During our observations, H. johannensis, H. macronectar and H. megapotamensis were pollinated solely by crepuscular–nocturnal hawkmoths (Sphingidae) that were recorded at the flowers from 19:00 to 21:35 h (Figs 2, 4A–B, Table 4). Diurnal visitors or pollinators were never recorded at these three species. Our fieldwork observations and our photographic and film record showed that all Sphingidae displayed similar behavioural patterns. On approaching, the hawkmoth hovers in front the flower and inserts its proboscis into the spur (Fig. 3A, D). In H. johannensis and H. macronectar, the moths use their forelegs to grasp the perianth (Fig. 2A, C), but this behaviour was not observed for H. megapotamensis. During our observations, H. johannensis was pollinated by Manduca rustica (proboscis length: 13.5 cm, n = 1) and M. sexta (proboscis length: 10.3 cm, n = 1) (Fig. 2A). These moths carried pollinaria of H. johannensis (15 and 13, respectively, Table 4) attached to the underside of the base of the proboscis (Fig. 2A, B). Because of weather conditions, the behaviour of the first moth species could not be followed in detail. However, the photographic record suggests that it behaves much like M. sexta. This latter pollinator was seen moving between two neighbouring inflorescences, spending around 25 s at the flowers. Four additional hawkmoth visits were recorded during the observation period, but environmental conditions and the long distance that separated us from the visited inflorescences precluded the unequivocal taxonomic identification of these insects. Also during our observations, Eumorpha labruscae (proboscis length: 5.9 cm, n = 1) (Fig. 2D) and M. cf. lucetius (proboscis length: 6.4–7.4 cm, n = 3) (Fig. 2C) were recorded as the pollinators of H. macronectar. Both moth species carried pollinaria (up to 12 and 3, respectively, see Table 4) attached between the palpi. The first moth species was sighted only once, whereas the second was sighted 13 times. On five of these occasions, the moths carried pollinaria of H. macronectar, and on two occasions were also bearing pollinaria of H. megapotamensis (that was flowering nearby) attached to the base and sides of the proboscis. It was possible to film some individuals of M. cf. lucetius visiting the flowers of H. macronectar (see also Supporting Information, Video S1), and our video record indicates that moths spent between 2 and 17 s probing individual flowers and between 12 and 140 s at each inflorescence. Remarkably, videos revealed that the same moth inserted its proboscis very differently into flowers on the same inflorescence (inserting or not the full length of the proboscis, see also Supporting Information, Video S1).The sphingid Agrius cingulatus (proboscis length: 9–12.8 cm, according to Singer & Cocucci, 1997) was photographed at the flowers. This insect never removed pollinaria, possibly because its proboscis is considerably longer than the spur (see also Supporting Information, Fig. S2). The only pollinators recorded for H. megapotamensis were moths of M. cf. lucetius (Fig. 4A, B, see also Supplorting Information, Video S2). Seven visitation events were recorded and insects were observed carrying pollinaria (up to 15, see Table 4) fixed to the sides and underside of the proboscis base. As stated above, this moth was also observed pollinating flowers of nearby H. macronectar. They visit several flowers per inflorescence, usually starting at the base and proceeding towards the top of the inflorescence. During our observations, the pollinators spent 2–9 s at each flower and 30–125 s at each inflorescence. Also, during our observations, H. montevidensis was pollinated solely by butterflies of the family Hesperiidae (Fig. 4C, D, Table 4). Generally, pollinator activity was observed from 06:00 to 18:00 h. All pollinator species behaved in a similar manner. Insects landed at the base of the inflorescence and proceeded towards the apex, systematically visiting the flowers. Column structure allows pollinators to place their heads between the rostellar arms (Fig. 3F) and all recorded pollinators bore the pollinaria on their eyes (Figs 3G, 4C, D). Cumbre sp. and Vehilius clavicula (proboscis length of both: 13 mm, n = 1) were sighted only once, bearing two and three pollinaria, respectively. Butterflies of Urbanus teleus (proboscis length: 11 mm, n = 1) and U. zagorus (proboscis length: 15 mm, n = 1) were more frequent, bore more pollinaria (up to 6 and 14, respectively, see Table 4) and are considered to be the main pollinators of H. montevidensis in the study area (Fig. 4C, D, see also Supporting Information, Video S3). In total, Urbanus spp. were seen c. 167 times visiting inflorescences. These insects visited one to four inflorescences per visit to the population, spending between 1 and 11 s at each flower and between 14 and 80 s at each inflorescence. These skippers were occasionally recorded actively attempting to remove the pollinaria attached to their eyes with their forelegs. Butterflies of Hesperocharis erota (Pieriidae) and Phocides pialia (Hesperiidae) were photographed and filmed visiting inflorescences, but without dislodging pollinaria (see also Supporting Information, Fig. S2). Although measurements could not be made in these two species, the photographic and film record clearly indicates that the eyes of both insect species do not come into contact with the orchid viscidia. Four visits of the nocturnal hawkmoth Xylophanes fosteri (Sphingidae, proboscis length: 22 mm, n = 1) (see also Supporting Information, Fig. S2 and Video S3) were recorded. This moth consistently visited flowers without removing pollinaria, as would be expected from an insect with a proboscis significantly longer than the nectariferous spur. Furthermore, intact, marked flowers used to ascertain the possibility of nocturnal pollination (see Material and Methods), were still intact the following morning.
Breeding systems
All species studied (Table 3) are self-compatible, but pollinator dependent. No fruit were formed by emasculated or intact flowers, strongly indicating that all species studied require the agency of a pollinator in order to set fruit. All species showed high fruiting success, either by self-pollination or cross-pollination, and there was no significant difference between these two treatments (Table 3).
Fruiting success and pollination efficiency
All species investigated displayed high fruiting successes in both sampling years, ranging from 69.48 to 97.40% (Table 4) (fruit set in H. montevidensis could only be determined for 2011, see Material and Methods). Mean fruit set per inflorescence was also high in both years, ranging from 68.62 to 97.38%. When comparing fruit set per inflorescence for 2010 and 2011, significant differences were found in H. johannensis (F = 41.464, P = 0.0113, d.f. = 71, Table 4) and H. macronectar (F = 15.436, P = 0.0027, d.f. = 64, Table 4). Habenaria megapotamensis was the only species that did not show a significant difference (F = 10.377, P = 0.1175, d.f. = 48, Table 4).
All studied species displayed male efficiency factors slightly higher than 1 (Table 4); i.e. at least one flower was pollinated for each flower acting as pollen donor. Percentages of pollinated flowers ranged from 72.38 to 86.06% and the percentages of flowers acting as pollen donors were also high, ranging from 63.81 to 83.55% (Table 4). However, several flowers used to calculate male efficiency factors contributed only one pollinarium. In H. johannensis, 23.97% of the flowers acting as pollen donors contributed a single pollinarium. In H. macronectar, H. megapotamensis and H. montevidensis, this value reached 38.8, 21.76 and 38.17% of the flowers acting as pollen donors, respectively.
Discussion
On the whole, floral features shown here agree with those already reported for several Neotropical Habenaria spp. (Hoehne, 1942; Dressler, 1993; Singer & Cocucci, 1997; Singer, 2001; Batista et al., 2006; Singer et al., 2007). However, some deserve special mention, as they are relevant to the pollination process. Column features such as the close, convergent rostellar arms and upward facing viscidia in H. johannensis and H. macronectar, the projecting median rostellar lobe in H. johannensis and the stigmatic appendices that partly block the spur entrance in H. megapotamensis restrict the movements of pollinators and physically guide them against the rostellum, thus precipitating pollinarium withdrawal and pollination. Conversely, most Neotropical Habenaria spp. have non-projecting, median rostellar lobes and straight, approximately parallel rostellar arms ending in more or less front-facing viscidia (Hoehne, 1942; Dressler, 1993; Singer & Cocucci, 1997; Singer et al., 2007). The rostellar morphology of H. montevidensis follows this latter pattern. Stigmatic appendices similar to those reported here for H. megapotamensis are also found in other Neotropical Habenaria spp., such as H. secunda Lindl., H. exaltata Barb. Rodr. and the recently described H. psammophila J.A.N.Bat., Bianch. & Carvalho (Batista et al., 2010). Based on the presence of this morphological feature, we suggest that the pollination mechanism of these orchids may be similar to that reported here for H. megapotamensis. The viscidia to H. megapotamensis flank the spur entrance. A similar condition is found in H. parviflora, a species in which the pollinaria are also carried on the proboscis of its pollinators (Singer, 2001). However, in H. parviflora the viscidium is glove-like and clasps the thin proboscides of its moth and crane fly pollinators (Singer, 2001).
Pollination by Sphingidae is confirmed here for three species (H. johannensis, H. macronectar and H. megapotamensis), which share important floral features, such as greenish–white or light green, long-spurred flowers, dilute nectar and crepuscular–nocturnal emission of scent. These features are consistent with those already recorded for other hawkmoth-pollinated orchids of subfamily Orchidoideae (Galetto et al., 1997; Johnson & Liltved, 1997; Hapeman & Inoue, 2000; Westwood & Borkowsky, 2004; Peter et al., 2009) and Epidendroideae (Nilsson et al., 1985; Nilsson & Rabakonandrianina, 1988; Nilsson, 1998; Luyt & Johnson, 2001; Martins & Johnson, 2007). Habenaria johannensis and H. macronectar belong to section Macroceratitae, an orchid group which is characterized by a distinct set of floral features (proportionally large, greenish–white, nocturnally fragrant, long-spurred flowers, etc.) often associated with pollination by hawkmoths (Singer & Cocucci, 1997; Batista et al., 2006). In support of this, hawkmoth pollination has already been documented for H. gourlieana Gillies (also a member of section Macroceratitae) (Singer & Cocucci, 1997). In Central Argentina, this species was found to be pollinated by Manduca sexta (one of two species we recorded pollinating H. johannensis) and visited (but not pollinated) by Agrius cingulatus (a non-pollinating visitor of H. macronectar, according to our observations). On the basis of similar flower features (Batista et al., 2006), we expect all other Habenaria spp. in section Macroceratitae to be pollinated by Sphingidae. Remarkably, pollination solely by Sphingidae was also confirmed in this study for H. megapotamensis, which does not belong to section Macroceratitae. In fact, floral traits often associated with pollination by Sphingidae also occur in some Habenaria spp. of the Laxifloras, Leptoceras and Seticauda groups (sensu Hoehne, 1940; Hoehne, 1942; Singer & Cocucci, 1997), supporting the view that pollination by hawkmoths is not restricted to section Macroceratitae. The present contribution is the second report on butterfly pollination in the genus Habenaria. The first was by Moreira et al. (1996), who documented the pollination of H. pleiophylla by Heliconius erato phyllis (Nymphalidae) in plantations of Eucalyptus L'Hér. in Rio Grande do Sul, southern Brazil. Singer et al. (2007) subsequently demonstrated pollination of the same orchid by short-tongued Sphingidae, Noctuidae and Arctiidae in south-eastern Brazil. Habenaria spp. of section Pratenses Kraenzl. (Cogniaux, 1893; Hoehne, 1942; Pabst & Dungs, 1975) consistently display a set of floral features (diurnally scented, mostly yellow flowers) indicative of diurnal pollination, and we believe that they may also be pollinated by butterflies (see also Singer & Cocucci, 1997).
Moth and butterfly pollinators have most of their bodies covered with scales, and therefore few body parts are sufficiently smooth to carry orchid pollinaria. Moth-pollinated orchids deposit their pollinaria on the proboscis, eyes or, more rarely, the forelegs of the visiting insect (Nilsson et al., 1985; Nilsson, Johnsson & Ralison, 1987; Nilsson & Rabakonandrianina, 1988; Johnson & Liltved, 1997; Luyt & Johnson, 2001; Westwood & Borkowsky, 2004; Martins & Johnson, 2007; Peter et al., 2009). To our knowledge, this is the first report of attachment of Habenaria pollinaria to the area between the palpi of moth pollinators. Earlier reports on Habenaria spp. have documented pollinarium attachment to the eyes (Moreira et al., 1996; Singer & Cocucci, 1997; Singer et al., 2007), the proboscis (Singer, 2001) or the forelegs (Peter et al., 2009). Pollinarium attachment to the eyes, as observed here for H. montevidensis, has already been documented for H. gourlieana, H. hexaptera Lindl. (as H. hieronymi Kraenzl.) and H. pleiophylla (Singer & Cocucci, 1997; Singer et al., 2007). All these species have similar column morphology and have more or less parallel rostellar arms and widely spaced viscidia (Singer & Cocucci, 1997; Singer et al., 2007). As this column structure is widespread among Neotropical Habenaria spp., we expect that pollinarium attachment to the eyes will dominate in these taxa. Pollinarium attachment to the proboscis (as reported here for H. johannensis and H. megapotamensis) was seen in H. parviflora, but involved a differently structured viscidium (see above) (Singer, 2001).
A remarkable finding is that M. cf. lucetius pollinated both H. macronectar and H. megapotamensis, with some individuals simultaneously carrying pollinaria of both species. No hybrids between these species are known and this is consistent with the fact that pollinaria of both orchid species become attached to different parts of the insect, and that the respective flowers differ in their column morphology. These Habenaria spp. did not display statistically significant differences in spur length, nectar volume, nectar column length or nectar concentration (see Results and Table 1). Therefore, floral morphological features and pollinarium placement on the pollinator seem particularly important. The short pollinaria of H. megapotamensis are unlikely to make contact with the stalked, long stigmatic surfaces of H. macronectar. Conversely, the long, pendulous pollinaria of H. macronectar are unlikely to make contact with the short stigmatic surfaces of H. megapotamensis, which are placed just below the spur entrance. Thus, a combination of floral morphology and differing pollinarium placement on the pollinator (also a consequence of column morphology) are probably sufficient to keep the species separate, even when they share the same pollinator. A similar situation was found by Nilsson et al., 1985, 1987) for a community of hawkmoth-pollinated species of Angraecum Bory (Epidendroideae: Angraecinae) from Madagascar. The hawkmoth Panogena lingens was a particularly important pollinator in this community and was shared by some orchids as pollinator. The pollinaria of the different species became attached at different points to its proboscis, thus precluding hybridization events (Nilsson et al., 1985, 1987).
As already stated, most insects bearing several pollinaria had proboscides that were shorter than the mean spur length of the orchids that they pollinate (Table 4). This is the case, for example, in M. sexta (pollinator of H. johannensis), E. labrusca (pollinator of H. macronectar), U. teleus and U. zagorius (pollinators of H. montevidensis) (see Table 4). A similar situation has already been reported for H. gourlieana (Singer & Cocucci, 1997), but, in this case, the pollinating moths (M. sexta) carried only a few pollinaria on their eyes (two). Although a larger pollinator sample is desirable, the above scenario is not unexpected, as insects with proboscides shorter than the floral spurs are more likely to contact the viscidia while probing flowers and therefore dislodging pollinaria. The nectar column varied in length according to species (see Table 1), but it is not essential for the insects to reach the very bottom of the spur to take the nectar. As already stated, some individuals of M. cf. lucetius were filmed visiting flowers of H. macronectar, without pollinating them. This can be partly explained in terms of variation in proboscis and spur length (see Tables 1 and 4, respectively). Insects with longer proboscides are less likely to disturb the rostellum and dislodge pollinaria. However, videos revealed that the same moth may or may not insert the full proboscis into different flowers on the same inflorescence (see also Supporting Information, Video S1). In our opinion, this behaviour may be attributable to nectar columns of different lengths (and thus different volumes of nectar secreted). Insects visiting flowers with longer nectar columns are less likely to disturb the rostellum and dislodge pollinaria, especially if they have proboscides longer than the spur. Conversely, insects visiting flowers with shorter nectar columns are more likely to disturb the rostellum and withdraw pollinaria while trying to reach the nectar, especially if they have proboscides shorter than the floral spur. If these observations are correct, even morphologically compatible moths (e.g. with ‘appropriate’ proboscis length) may visit some flowers without effecting pollination. Pollination of these flowers is more likely to commence when their nectar spurs are partially empty. Morphologically compatible pollinators may then need to insert their proboscides fully into the floral tube, thereby disturbing the rostellum and effecting pollination. It is important to stress that nectar volume and, consequently, the height of the nectar column are already subject to significant variation in intact flowers of the same species (see Table 1).
All species studied are self-compatible, but pollinator dependent. The lack of fruit set in intact or emasculated flowers indicates that no apomixis or autogamy occurs and that the species studied rely on animal pollen vectors to set fruit. Similar results (self-compatibility coupled with pollinator dependence) were found in H. parviflora (Singer, 2001). All studied species displayed high fruit set (see Results), as already reported for other Habenaria spp. (Singer, 2001; Singer et al., 2007). Mean fruit set per inflorescence for 2010 and 2011 showed statistically significant differences for H. johannensis and H. macronectar. As all studied species are pollinator dependent (Table 3), the observed differences may be caused by different visitation rates. The high fruit set observed for all species studied may be partly attributable to the consistent presence of a nectar reward (Neiland & Wilcock, 1998; Tremblay et al., 2005). Indeed, other factors that may be involved include: (1) self-compatibility; (2) pollinators that either are frequent or pollinate several flowers per visit; and (3) the presence of massulate pollinia, where the pollen can be spread onto the stigmatic surface of several flowers.
All species studied displayed male efficiency factors of just greater than 1, indicating that slightly more than one flower was pollinated per flower acting as pollinarium–donor. These results are higher than the values previously recorded for H. gourlieana (0.38), H. parviflora (as H. montevidensis; 0.6) and H. rupicola Barb. Rodr. (0.7) in central Argentina (Singer & Cocucci, 1997), but lower or slightly lower than those recovered for populations of H. pleiophylla (1.8) in eastern Brazil and for H. hexaptera (1.15) (as H. hieronymi) in central Argentina (Singer & Cocucci, 1997; Singer et al., 2007). Although our data suggest that there is some kind of equilibrium (approximately one flower pollinated per flower acting as pollen donor), we believe that there is some wastage of pollinaria. This is based on the fact that pollinarium-laden skippers of the genus Urbanus (the main pollinators of H. montevidensis at the study site) were filmed trying to remove the pollinaria attached to their eyes. Similar behaviour has already been recorded for some noctuid pollinators of H. pleiophylla (Singer et al., 2007). These findings suggest that some pollinaria may be cleaned by the pollinators and, consequently, lost for pollination purposes. It is important to remember that, among the species studied, a proportion of flowers acting as pollen donors contribute only a single pollinarium (see Results).
Concluding remarks
The present study shows that four sympatric southern Brazilian Habenaria spp., the flowering periods of which overlap, are isolated (in terms of pollination) by a set of factors that includes differences in floral morphology (especially spur length and column morphology) and different pollinators and/or different pollinarium placement on the pollinator. Species with similar flower features (e.g. H. macronectar and H. megapotamensis) can share pollinators, but these carry the pollinaria of the different orchid species on different body parts, thereby physically preventing hybridization.
Long-spurred, hawkmoth-pollinated orchids have captivated the attention of scientists ever since Darwin (1862) made his famous comments on the putative pollinators of Angraecum sesquipedale Thouars and proposed a co-evolutionary race between plant and pollinator. Several Neotropical Habenaria spp. rank among the longest spurred orchids in the Americas, with (for instance) all the species of section Macroceratitae having long spurs (≥ 6 cm in length), and that of H. longicauda reaching 25 cm (Renz, 1992; Batista et al., 2006). Therefore, these orchids parallel the mainly African–Madagascan Angraecinae, at least in terms of floral morphology (see Micheneau, Johnson & Fay, 2010). Thus, it is tempting to ask whether the long-spurred Habenaria spp. and their sphingid pollinators co-evolved. Although the present contribution did not set out to address that question, nevertheless some of the results presented here suggest that a co-evolutionary relationship (in its strictest sense, see Ridley, 2004) is unlikely as: (1) pollination by Sphingidae occurs in more than one Habenaria section/species group; and (2) one studied species (H. macronectar) is pollinated by phylogenetically unrelated moths. Well-supported phylogenies are already available for Sphingidae, and these studies indicate that the observed pollinators of H. macronectar are distantly related and belong to different clades (see cladograms in Kawahara et al., 2009). A comprehensive molecular phylogeny of Neotropical Habenaria is currently being prepared (J. A. N. Batista, pers. comm.), and this will be the basis for a more robust and reliable infrageneric classification, prompting a re-evaluation of the characters that are normally used to separate species groups. Furthermore, it could be used as a framework to elucidate the evolution of pollination-related features (e.g. long spurs). Only then will it be possible to ascertain whether the long-spurred Habenaria spp. have a co-evolutionary relationship with their sphingid pollinators.
Acknowledgements
This contribution is part of the first author's MSc Dissertation (in Botany) at the Programa de Pós-Graduação em Botânica-UFRGS. M.P and C.R.B. gratefully acknowledge their CAPES grants (MSc and PhD, respectively). We thank ICMBio for the collecting permit (process 24004-2) and the landowners (especially Mr Mauro, from Fazenda Olaria, Cambará do Sul) for allowing access to the plant populations. Gustavo A. Silva Arias is thanked for his help during fieldwork. Thanks are also due to Dr Kevin L. Davies (Wales, UK) for useful comments and to Pedro M. Abreu Ferreira and Michelle H. Nervo (PPGBOT-UFRGS) for their advice on statistics. We are also grateful for the suggestions of three anonymous reviewers, who greatly improved the manuscript.




