A comparison of the wood anatomy of 11 species from two cerrado habitats (cerrado s.s. and adjacent gallery forest)
Abstract
A comparative study of the secondary xylem (wood) anatomy of 11 species (38 specimens) occurring in cerrado s.s. and the adjacent gallery forest (both cerrado s.l. habitat) was made with the aim of identifying the anatomical characteristics of ecological value and correlating them with the environmental conditions. The anatomical features that vary, in general, between the two habitats are: growth ring distinctness (well or poorly defined); tyloses and deposits (more abundant in cerrado specimens); gelatinous fibres (more evident in cerrado specimens and in different patterns between habitats); variation in paratracheal and banded parenchyma (more abundant in cerrado); and more cells per parenchyma strand in cerrado. In general, gallery forest specimens have wider vessels, fewer vessels per square millimetre and larger intervessel pits, indicating more efficient water conduction, whereas cerrado s.s. specimens are the opposite, with low vulnerability and mesomorphy indices, demonstrating greater safety under conditions of water stress. © 2012 The Linnean Society of London, Botanical Journal of the Linnean Society, 2012, ••, ••–••.
Introduction
Cerrado s.l. is considered to be the most biodiverse savanna in the world (Oliveira & Marquis, 2002), and is one of the 25 global hotspots – priority areas for conservation (Mittermeier et al., 1999). It is mostly restricted to Brazil and merges into a great diversity of biomes, such as Amazon rainforest, Pantanal, Atlantic forest and Caatinga. This enriches its flora, about 11 500 plant species (Lista de Espécies da Flora do Brasil, 2011), 44% of which are endemic (Coutinho, 1990; Castro et al., 1999). The cerrado biome has different habitats varying from open savanna to savanna with scattered trees to cerrado s.s. and cerradão (Coutinho, 2002). Along the rivers there are gallery forests among other formations, which are all considered part of cerrado s.l. (Eiten, 1972). According to Hammerle (2006), few species occur regularly in both cerrado s.s. and gallery forests. In São Paulo state, which includes our study site, the remnants of this vegetation are in scattered patches covering < 1% of the state (Durigan, Franco & Siqueira, 2004b).
Studies on ecological wood anatomy (e.g. Klaassen, 1999; Alves & Angyalossy-Alfonso, 2000, 2002; Lens et al., 2004; Barros et al., 2006; Loepfe et al., 2007; Pratt et al., 2007; see also Carlquist, 2010) are of great importance in understanding the strategies used by species to survive in different environments and the relationship between safety and efficiency in water conduction. Water availability and hydraulic architecture in the wood may strongly influence species distribution (Pockman & Sperry, 2000; Barros et al., 2006), as well as altering photosynthetic capacity, water usage efficiency and storage quantity in dry periods (Hammerle, 2006). However, much is still unknown about the ways in which plants adjust the anatomy of their xylem tissues to meet transpiration needs or to increase mechanical stability, in part because studies have been based on relatively small numbers of species and/or limited geographical regions (Zanne et al., 2010).
Comparative studies of secondary xylem of cerrado and other forest formation species (see Marcati, Angyalossy-Alfonso & Benetati, 2001; Machado et al., 2007) generally show that the main differences are quantitative (vessel diameter and frequency, fibre length and thickness, and ray width and frequency). Qualitative differences are few and, for cerrado species, largely apply to growth rings (Oliveira, 2007).
This article describes the differences in wood anatomy of 11 species that occur in two different habitats of cerrado s.l. (relatively dry cerrado s.s. and wet gallery forest), relating the wood anatomical features to these environments and verifying the strategies used by plants to survive in the different habitats.
Material and Methods
The study was carried out in a private cerrado s.l. reserve of about 180 ha, located in the Palmeira da Serra Ranch in the municipality of Pratânia (22°82′01.8″S and 48°74′02.6″W, 715 m) in the mid-western region of the state of São Paulo, Brazil (Fig. 1; maps were compiled by DIVA-GIS software). The species were collected in the cerrado s.s., which is characterized by short xeromorphic trees and shrubs with thick bark and leathery leaves, and by herbaceous ground cover, and in the adjacent wet gallery forest alongside small streams (the canopy forms a tunnel or gallery) in regions in which the interfluvial zone is covered by cerradão, with standing water even in the dry period.
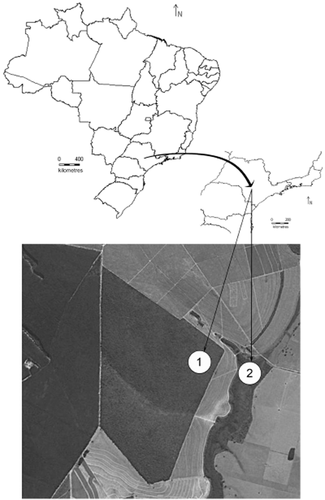
Study area: 1, cerrado s.s.; 2, gallery forest. Maps were compiled by DIVA-GIS software. Satellite image from Google Earth (2010) at an altitude of 3.58 km.
The soils are similar in both habitats: acidic with low organic matter, low phosphorus, potassium, calcium and magnesium and high aluminium (higher in cerrado). Both are considered sandy, but there is a greater quantity of clay in cerrado s.s. (see Milanez, 2007).
The climate diagram shows that both habitats have a dry period of about 4 months with precipitation below 60 mm (Fig. 2); average annual rainfall is approximately 1306 mm and the average temperature is 19.9 °C. Climate parameters were obtained by the same software as described in Hijmans et al. (2005). There are no differences in the climate between the two habitats, which are adjacent.
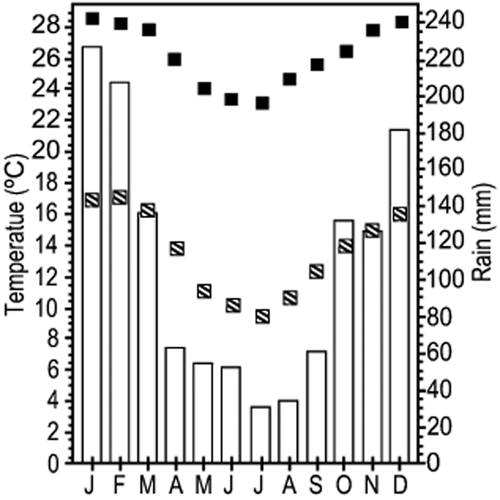
Climatic diagram for cerrado and gallery forest (climate parameters were obtained using the same software as described in Hijmans et al., 2005):  , temperature monthly maximum;
, temperature monthly maximum;  , temperature monthly minimum;
, temperature monthly minimum;  , precipitation.
, precipitation.
Samples of 11 species (adult individuals) from 38 specimens in total, 23 from cerrado s.s. (Ce) and 15 from the gallery forest (GFo), were collected (Table 1), with three to four specimens of each species. Large branches were sampled above the base of the main branch to avoid any irregular formation of that area, and because destructive sampling of trunks is not permitted in environmental reserve areas. Discs of c. 3–6 cm in thickness were obtained from each specimen.
| Family | Species | Area | BOTw | Habit | H (m) | CAP (cm) |
|---|---|---|---|---|---|---|
| Anacardiaceae | Tapirira guianensis Aubl. | GFo | 1952 | T | 4.5 | 17 |
| 1956 | 10.0 | 80 | ||||
| Ce | 1321 | 2.5 | 10 | |||
| 1965 | 1.7 | 10 | ||||
| Apocynaceae | Tabernaemontana catharinensis A.DC. | GFo | 1958 | T | 9.0 | 35 |
| Ce | 1629 | 6.0 | 32 | |||
| 1634 | 4.0 | 30 | ||||
| 1957 | 5.0 | 27 | ||||
| Araliaceae | Schefflera vinosa (Cham. & Schltdl.) Frodin & Fiaschi | GFo | 1963 | ST | 5.5 | 27 |
| Ce | 1324 | 3.5 | 47 | |||
| 1508 | 4.5 | 52 | ||||
| Fabaceae Caesalpinioideae | Dimorphandra mollis Benth. | GFo | 1990 | T | 4.5 | 25 |
| Ce | 1351 | 4.0 | 35 | |||
| 2043 | 3.5 | 38 | ||||
| Fabaceae Mimosoideae | Stryphnodendron polyphyllum Mart. | GFo | 1999 | T | 7.0 | 40 |
| 2000 | 6.0 | 38 | ||||
| Ce | 1352 | 4.0 | 40 | |||
| 1537 | 4.5 | 47 | ||||
| Fabaceae Papilionoideae | Acosmium subelegans (Mohlenbr.) Yakovlev | GFo | 1998 | ST | 4.2 | 30 |
| Ce | 1342 | 3.5 | 35 | |||
| 2044 | 3.3 | 40 | ||||
| Melastomataceae | Tibouchina stenocarpa (DC.) Cogn. | GFo | 2021 | S | 3.0 | 27 |
| Ce | 2018 | 2.1 | 10 | |||
| 1633 | 2.0 | 10 | ||||
| Peraceae (or Euphorbiaceae) | Pera glabrata (Schott) Poepp. ex Baill. | GFo | 1987 | ET | 4.5 | 23 |
| 1989 | 7.5 | 38 | ||||
| Ce | 1339 | 3.5 | 22 | |||
| 1579 | 4.5 | 17 | ||||
| Rutaceae | Zanthoxylum rhoifolium Lam. | GFo | 2036 | T | 4.0 | 17 |
| Ce | 1357 | 2.7 | 14 | |||
| 1560 | 3.5 | 16 | ||||
| Siparunaceae | Siparuna cujabana (Mart. ex Tul.) A.DC. | GFo | 2024 | T | 2.1 | 8 |
| 2159 | 2.2 | 10 | ||||
| Ce | 2022 | 5.0 | 15 | |||
| 2023 | 3.0 | 10 | ||||
| Vochysiaceae | Vochysia tucanorum Mart. | GFo | 2041 | T | 14.0 | 88 |
| Ce | 1368 | 6.4 | 42 | |||
| 2178 | 4.5 | 72 |
- BOTw, ‘Maria Aparecida Mourão Brasil’ Wood Collection; H, tree height; CAP (CBH), tree circumference at breast height; T, tree; ST, small tree; ET, emergent tree; S, shrub.
Habit information follows the classification by 2004) with adaptations: shrub (woody plant branched from the base, without a defined trunk); small tree (similar to a shrub in size, but with a single trunk); tree (tree, the height of which is always lower than the average canopy); emergent tree (tree, the height of which is generally higher than the average canopy). Vouchers were deposited at the ‘Irina Delanova de Gemtchujinicov’ Herbarium (BOTU) of the Institute of Biosciences (IB). The wood samples were deposited at the ‘Maria Aparecida Mourão Brasil’ Wood Collection (BOTw) of the Universidade Estadual Paulista (UNESP) Faculty of Agronomic Sciences (FCA) at Botucatu, São Paulo State.
Transverse (TS), tangential longitudinal (TLS) and radial longitudinal (RLS) sections, 15–20 μm in thickness, were double stained with aqueous 1% safranin and aqueous 1% astra blue (Bukatsch, 1972) (1 : 9). The innermost layer of gelatinous fibres is mainly composed of cellulose, and astra blue staining makes it easier to distinguish the different patterns of distribution. Histological slides were embedded permanently in synthetic resin (Entellan®). The cells were macerated using the method of Franklin (1945), modified by 1997), and stained with aqueous 1% safranin (Sass, 1958). Semi-permanent slides were mounted in glycerin diluted in water (1 : 1).
- Present: the ring boundaries are visible.
- Well defined: the ring boundaries are well defined and easy to observe.
- Poorly defined: the ring boundaries are not well defined and difficult to observe.
- Absent: no rings present.
For quantitative data, we made 30 measurements or counts for fibre length, vessel element length, vessel lumen diameter, fibre wall thickness, and vessel frequency. For intervessel pit diameter, vessel-ray pit diameter and rays per millimeter, we made ten measurements or counts.
For vessel grouping, 100 counts were made for each specimen, represented by one for solitaries, two for pairs, three for multiples of three, and so on successively, and the average of these counts was calculated following the method of Carlquist (1984). Vulnerability (V = vessel element diameter/vessel frequency) and mesomorphy (M = V × vessel element length) indices were calculated following Carlquist (1977).
Statistical analyses were performed with Statistica software version 8.0. The Shapiro–Wilk test was used to assess the normality of the samples (Zar, 1996). Variance analyses with a nested design were used to test variation between specimens of the same species and individuals of the two habitats (Jonsson & Malmqvist, 2000). Honestly significant difference (HSD) Tukey tests were carried out for multiple comparisons. Principal components analysis (PCA) was used to order species, qualitative and quantitative wood anatomy features and climate features, showing the factors with more variance (Ludwig & Reynolds, 1988).
Results
Wood anatomy descriptions
A summarized description with the main anatomical features is given with the relative frequencies in Table 2. A full description of the species is given in Supporting Information, Appendix S1, because there is no information in the literature about the wood anatomy of these species in the cerrado, except for Pera glabrata (Schott) Poepp. ex Baill. and Vochysia tucanorum Mart. by Alves & Angyalossy-Alfonso (2000, 2002). The descriptions cover TS, TLS and RLS, but only TSs are shown in the plates, except for Figure 5.
| Percentage | Vessels | Defined by | Growth rings | CS | Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| More than 4 vessels | 2 or 3 vessels | Solitary vessels | Radial pattern | Diffuse porous | Gelatinous fibres/bands | Radially flattened parenchyma cells | Bands/lines of axial parenchyma | Thick-walled latewood fibres | Thick-walled and radially flattened latewood fibres | Absent | Poorly defined | Well defined | ||
| 2 | 21 | 77 | – | * | – | – | – | – | * | – | * | – | Ce | Tp. guianensis (Fig. 3A) |
| 2 | 22 | 76 | – | * | – | – | – | – | * | – | – | * | GFo | Tp. guianensis (Fig. 3B) |
| 9 | 38 | 53 | * | * | – | – | – | * | – | – | – | * | Ce | Tb. catharinensis (Fig. 3C) |
| 3 | 33 | 64 | * | * | – | – | – | * | – | – | * | – | GFo | Tb. catharinensis (Fig. 3D) |
| 5 | 44 | 51 | – | * | – | – | – | – | * | – | – | * | Ce | Sf. vinosa (Fig. 3E, 5I) |
| 2.5 | 33.5 | 64 | – | * | – | – | – | – | * | – | – | * | GFo | Sf. vinosa (Fig. 3F, 5J) |
| 26 | 28 | 62 | * | * | * | – | – | – | * | – | * | * | Ce | P. glabrata (Fig. 3G) |
| 23 | 42 | 35 | * | * | * | – | – | – | * | – | * | * | GFo | P. glabrata (Fig. 3H) |
| 8 | 34 | 58 | – | * | – | * | – | – | – | * | * | – | Ce | D. mollis (Fig. 4A) |
| 5 | 33 | 62 | – | * | – | * | – | – | – | – | * | – | GFo | D. mollis (Fig. 4B) |
| 2 | 19 | 79 | – | * | – | – | – | – | * | – | * | * | Ce | S. polyphyllum (Fig. 4C) |
| 3 | 20 | 77 | – | * | – | – | – | – | * | – | * | * | GFo | S. polyphyllum (Fig. 4D) |
| 5 | 39 | 56 | – | * | – | – | * | – | – | – | – | * | Ce | A. subelegans (Fig. 4E) |
| 3 | 57 | 40 | – | * | – | – | * | – | – | – | – | * | GFo | A. subelegans (Fig. 4F) |
| 4 | 24 | 72 | – | * | – | – | – | – | * | – | * | – | Ce | T. stenocarpa (Fig. 4G) |
| 4 | 26 | 70 | – | * | – | – | – | – | * | – | * | – | GFo | T. stenocarpa (Figs.4H, 5G) |
| 1.5 | 36.5 | 62 | – | * | – | – | – | – | * | – | – | * | Ce | Z. rhoifolium (Fig. 5A) |
| 3 | 38 | 59 | – | * | – | – | – | – | * | – | * | – | GFo | Z. rhoifolium (Fig. 5B) |
| 10 | 39 | 51 | * | * | – | – | * | – | * | – | * | * | Ce | Sp. cujabana (Fig. 5C) |
| 15 | 43 | 42 | * | * | – | – | – | – | – | * | – | – | GFo | Sp. cujabana (Fig. 5D, 5H) |
| 1 | 23 | 76 | – | * | – | – | * | – | – | – | * | – | Ce | V. tucanorum (Fig. 5E) |
| 0.5 | 38.5 | 61 | – | * | – | – | * | – | – | – | * | – | GFo | V. tucanorum (Fig. 5F) |
| 27 | 100 | 9 | 9 | 27 | 9 | 64 | 9 | 64 | 64 | Ce | Percentage | |||
| 27 | 100 | 9 | 9 | 18 | 9 | 55 | 9 | 64 | 45 | GFo | Percentage | |||
| Fibres | Tyloses | VRP of two distinct sizes in the same ray cell | VRPAS | VRPSI | Intervessel pits | PP simple | CS | Species | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Septate | With minutely bordered pits (visible, but <3 μm) | With simple to minutely bordered pits | Vestured pits | Polygonal | Alternate, circular | Scalariform | PP multiple | |||||||
| * | – | * | a* | * | * | – | – | * | * | – | *a | * | Ce | Tp. guianensis (Fig. 3A) |
| * | – | * | * | * | * | – | – | * | * | – | *a | * | GFo | Tp. guianensis (Fig. 3B) |
| * | * | – | – | – | – | * | – | – | * | – | – | * | Ce | Tb. catharinensis (Fig. 3C) |
| * | * | – | – | – | – | * | – | – | * | – | – | * | GFo | Tb. catharinensis (Fig. 3D) |
| * | – | * | a* | * | * | – | – | – | * | * | * | * | Ce | Sf. vinosa (Fig. 3E, 5I) |
| * | – | * | – | * | * | – | – | – | * | * | * | * | GFo | Sf. vinosa (Fig. 3F, 5J) |
| – | * | – | – | * | * | – | – | – | * | – | – | * | Ce | P. glabrata (Fig. 3G) |
| – | * | – | a* | * | * | – | – | – | * | – | – | * | GFo | P. glabrata (Fig. 3H) |
| – | – | * | – | – | – | * | * | * | – | – | – | * | Ce | D. mollis (Fig. 4A) |
| – | – | * | – | – | – | * | * | * | – | – | – | * | GFo | D. mollis (Fig. 4B) |
| – | – | * | – | – | – | * | * | * | * | – | – | * | Ce | S. polyphyllum (Fig. 4C) |
| – | – | * | – | – | – | * | * | * | * | – | – | * | GFo | S. polyphyllum (Fig. 4D) |
| – | – | * | – | – | – | * | * | – | * | – | – | * | Ce | A. subelegans (Fig. 4E) |
| – | – | * | – | – | – | * | * | * | * | – | – | * | GFo | A. subelegans (Fig. 4F) |
| * | – | * | * | * | * | – | * | – | * | – | – | * | Ce | T. stenocarpa (Fig. 4G) |
| * | – | * | – | * | * | – | * | – | * | – | – | * | GFo | T. stenocarpa (Figs. 4H, 5G) |
| – | – | * | – | – | – | * | – | – | * | – | – | * | Ce | Z. rhoifolium (Fig. 5A) |
| – | – | * | – | – | – | * | – | – | * | – | – | * | GFo | Z. rhoifolium (Fig. 5B) |
| – | * | – | a* | * | * | – | – | – | * | – | * | * | Ce | Sp. cujabana (Fig. 5C) |
| – | * | – | a* | * | * | – | – | – | * | – | * | * | GFo | Sp. cujabana (Fig. 5D, 5H) |
| – | – | * | * | – | – | * | * | – | * | – | – | * | Ce | V. tucanorum (Fig. 5E) |
| – | – | * | * | – | – | * | * | * | – | – | – | * | GFo | V. tucanorum (Fig. 5F) |
| 36 | 27 | 73 | 45 | 45 | 45 | 55 | 45 | 27 | 91 | 9 | 27 | 100 | Ce | Percentage |
| 36 | 27 | 73 | 36 | 45 | 45 | 55 | 45 | 45 | 82 | 9 | 27 | 100 | GFo | Percentage |
| Rays | Parenchyma strand | Axial parenchyma | Fibres | CS | Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–3 cells wide | Exclusively uniseriate | Over 7 cells | Up to 6 cells | 2–4 cells | In bands | Paratracheal | Apotracheal | Extremely rare | Gelatinous fibres | Very thick-walled | Thin- to thick-walled | Very thin-walled | ||
| * | – | * | * | * | – | a* | – | – | * | – | * | * | Ce | Tp. guianensis (Fig. 3A) |
| * | – | * | * | * | – | * | – | – | * | – | – | * | GFo | Tp. guianensis (Fig. 3B) |
| * | – | – | – | – | – | – | – | * | * | – | * | – | Ce | Tb. catharinensis (Fig. 3C) |
| * | – | – | – | – | – | – | – | * | – | – | * | – | GFo | Tb. catharinensis (Fig. 3D) |
| * | – | * | * | * | – | * | – | – | – | – | * | * | Ce | Sf. vinosa (Fig. 3E, 5I) |
| * | – | – | – | * | – | * | – | – | – | – | * | * | GFo | Sf. vinosa (Fig. 3F, 5J) |
| – | * | * | * | * | * | – | * | – | * | – | * | – | Ce | P. glabrata (Fig. 3G) |
| – | * | * | * | * | * | – | * | – | * | – | * | – | GFo | P. glabrata (Fig. 3H) |
| * | – | * | * | * | a* | * | – | – | * | – | * | – | Ce | D. mollis (Fig. 4A) |
| * | – | * | * | * | * | * | – | – | * | * | – | – | GFo | D. mollis (Fig. 4B) |
| – | * | – | * | * | – | a* | – | – | * | – | * | – | Ce | S. polyphyllum (Fig. 4C) |
| – | * | – | – | * | – | * | – | – | * | – | * | – | GFo | S. polyphyllum (Fig. 4D) |
| – | * | – | * | * | a* | * | * | – | * | * | – | – | Ce | A. subelegans (Fig. 4E) |
| * | – | – | – | * | * | * | * | – | * | * | – | – | GFo | A. subelegans (Fig. 4F) |
| * | – | – | – | * | – | * | * | – | * | * | * | – | Ce | T. stenocarpa (Fig. 4G) |
| * | – | – | * | * | – | * | * | – | * | – | * | – | GFo | T. stenocarpa (Figs. 4H, 5G) |
| * | – | – | – | * | – | * | – | – | – | – | * | – | Ce | Z. rhoifolium (Fig. 5A) |
| * | – | – | – | * | – | * | – | – | – | – | * | – | GFo | Z. rhoifolium (Fig. 5B) |
| * | – | – | * | * | – | – | * | – | – | * | * | – | Ce | Sp. cujabana (Fig. 5C) |
| * | – | – | – | * | – | – | * | – | – | – | * | – | GFo | Sp. cujabana (Fig. 5D, 5H) |
| * | – | * | * | * | a* | * | – | – | * | – | * | – | Ce | V. tucanorum (Fig. 5E) |
| * | – | * | * | * | – | * | – | – | * | – | – | * | GFo | V. tucanorum (Fig. 5F) |
| 82 | 18 | 45 | 82 | 91 | 36 | 73 | 36 | 9 | 73 | 27 | 91 | 18 | Ce | Percentage |
| 82 | 18 | 36 | 36 | 91 | 27 | 73 | 36 | 9 | 64 | 18 | 64 | 27 | GFo | Percentage |
| Inorganic inclusions | Organic inclusions | Secretory elements and cambial variants | PRC | Rays | CS | Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deposits in vessels | Canal of traumatic origin | Radial canals | Heterocellular | Homocellular | 4–5 cells wide | |||||||||
| * | – | – | a* | * | * | – | – | Ce | Tp. guianensis (Fig. 3A) | |||||
| * | – | – | a* | * | * | – | – | GFo | Tp. guianensis (Fig. 3B) | |||||
| * | – | – | – | * | * | – | – | Ce | Tb. catharinensis (Fig. 3C) | |||||
| * | – | – | – | * | * | – | – | GFo | Tb. catharinensis (Fig. 3D) | |||||
| – | – | – | a* | * | * | – | * | Ce | Sf. vinosa (Fig. 3E, 5I) | |||||
| – | – | – | * | * | * | – | – | GFo | Sf. vinosa (Fig. 3F, 5J) | |||||
| * | * | – | – | – | * | – | – | Ce | P. glabrata (Fig. 3G) | |||||
| * | * | – | – | – | * | – | – | GFo | P. glabrata (Fig. 3H) | |||||
| * | * | – | – | – | * | * | – | Ce | D. mollis (Fig. 4A) | |||||
| * | a* | – | – | – | * | * | – | GFo | D. mollis (Fig. 4B) | |||||
| * | a* | – | – | – | – | * | – | Ce | S. polyphyllum (Fig. 4C) | |||||
| * | a* | – | – | – | – | * | – | GFo | S. polyphyllum (Fig. 4D) | |||||
| * | * | – | – | * | * | – | – | Ce | A. subelegans (Fig. 4E) | |||||
| * | * | – | – | * | * | – | – | GFo | A. subelegans (Fig. 4F) | |||||
| * | * | – | – | – | * | – | – | Ce | T. stenocarpa (Fig. 4G) | |||||
| – | – | – | – | – | * | – | – | GFo | T. stenocarpa (Figs. 4H, 5G) | |||||
| – | – | * | – | – | * | – | – | Ce | Z. rhoifolium (Fig. 5A) | |||||
| – | – | * | – | – | * | – | – | GFo | Z. rhoifolium (Fig. 5B) | |||||
| – | a* | – | – | * | * | – | – | Ce | Sp. cujabana (Fig. 5C) | |||||
| – | a* | – | – | * | * | – | – | GFo | Sp. cujabana (Fig. 5D, 5H) | |||||
| * | a* | * | – | – | * | – | * | Ce | V. tucanorum (Fig. 5E) | |||||
| * | 1* | * | – | – | * | – | * | GFo | V. tucanorum (Fig. 5F) | |||||
| 73 | 64 | 18 | 18 | 45 | 91 | 18 | 18 | Ce | Percentage | |||||
| 64 | 55 | 18 | 18 | 45 | 91 | 18 | 9 | GFo | Percentage | |||||
- CS, collection site; Ce, cerrado s.s.; GFo, gallery forest; PP, perforation plate; VRPSI, vessel-ray pits with distinct borders; similar to intervessel pits in size and shape throughout the ray cell; VRPAS, vessel-ray pits with much reduced borders to apparently simple; pits rounded or angular/horizontal to vertical; VRP, vessel-ray pits; PRC, perforated ray cells; *present; –, absent; 1few; 2abundant; 3of varying types. The growth ring definition and markers, perforation plate type (simple and multiple), intervessel and ray-vessel pits, fibre thickness, axial parenchyma type and strand, and ray width might occur within a species; therefore, the sum of the percentage exceeds 100%.
General wood anatomical differences between habitats
Individual species show anatomical differences between the two habitats, but these are not universally consistent. Growth ring distinctness was assessed as either well or poorly defined. Only Acosmium subelegans (Mohlenbr.) Yakovlev (Fig. 4E, F) had well-defined growth rings with the same boundary marker in all specimens from both cerrado and the gallery forest. In Siparuna cujabana (Mart. ex Tul.) A.DC. (Fig. 5C, D), growth rings were absent in the gallery forest and present in cerrado. Overall, cerrado samples had narrower vessel elements, higher vessel frequency per square millimetre, smaller intervessel pits (Fig. 6C, E, H, Table 3) and tyloses and deposits in vessels were more frequent. Fibres were longer and thicker walled in cerrado (Fig. 6A, F, Table 3). Gelatinous fibres were also more evident in cerrado specimens (P. glabrata and Tibouchina stenocarpa (DC.) Cogn., Figs 3G, H, 4G, H, Appendix S1), with different patterns and abundance between specimens in the two habitats. Paratracheal axial parenchyma patterns were more diverse in cerrado s.s. and banded parenchyma was more apparent in cerrado s.s. species (Dimorphandra mollis Benth., Stryphnodendron polyphyllum Mart., A. subelegans and V. tucanorum; Figs 4A–F, 5E, F, Table 2, Appendix S1). There were more cells per axial parenchyma strand in cerrado [Schefflera vinosa (Cham. & Schltdl.) Frodin & Fiaschi, S. polyphyllum, A. subelegans, T. stenocarpa and Sp. cujabana; Table 2]. Schefflera vinosa from cerrado had wider radial canals than gallery forest samples (46.7 μm and 21.3 μm, respectively; Fig. 5I, J).
| Species | CS | FL (μm) | VL (μm) | IVPD (μm) | VRPD (μm) | VD (μm) | FWT (μm) | R/mm | V/mm2 | VGI | V | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A | ||||
| Tp. guianensis | GFo | 670 ± 181 B b,c,d | 461 ± 77 A c,d,e,f | 10 ± 1 B c,d | 15.6 ± 5 B f,g | 90 ± 18 B d,e,f | 2.7 ± 1 B a,b | 6 ± 1 B b,c,d | 34 ± 10 B e,f | 1.46 A a,b,c,d | 2.7 | 1241 |
| Ce | 792 ± 169 A d,e,f | 494 ± 116 A e,f | 8.4 ± 1 A b,c | 14 ± 4 A e,f,g | 70 ± 21 A b,c,d,e | 3.5 ± 1 A b,c,d,e | 8.5 ± 2 A g,h,i | 31 ± 14 A d,e,f | 1.58 A a,b,c,d | 2.3 | 1130 | |
| Tb. catharinensis | GFo | 831 ± 174 B d,e,f | 482 ± 101 A d,e,f | 4 ± 0 B a | 3.4 ± 1 B a | 51 ± 6 B a | 4.6 ± 1 B d,e,f,g,h,i | 8 ± 3 B e, f,g,h | 122 ± 11 B i | 1.44A a,b,c,d | 0.4 | 202 |
| Ce | 811 ± 172 A d,e,f | 514 ± 111 A f | 4.4 ± 1 A a | 4 ± 1 A a,b | 43 ± 8 A a | 5.3 ± 2 A i,j | 9.4 ± 3 A h,i | 157 ± 32 A j | 1.75 A c,d,e | 0.3 | 141 | |
| Sf. vinosa | GFo | 820 ± 106 B d,e,f | 689 ± 134 A g,h | 24 ± 11 B f | 15 ± 4 B e,f,g | 71 ± 15 B b,c,d,e | 4 ± 2 B c,d,e,f | 4 ± 1 B a | 31 ± 9 B d,e,f | 1.44 A a,b,c,d | 2.3 | 1596 |
| Ce | 833 ± 175 A e,f | 726 ± 152 A h,i | 13 ± 3 A e | 17.5 ± 9 A f,g | 64 ± 12 A a,b,c | 5.2 ± 1 A h,i,j | 4.6 ± 1 A a,b | 32 ± 9 A e,f | 2.08 A e,f | 2 | 1468 | |
| D. mollis | GFo | 783 ± 146 B d,e | 361 ± 76 A b,c | 7 ± 1 B b | 7 ± 2 B b,c | 137 ± 27 B i | 3.5 ± 1 B a,b,c,d,e | 7 ± 2 B c,d,e,f | 13 ± 5 B b,c | 1.62 A a,b,c,d,e | 10.5 | 3799 |
| Ce | 896 ± 150 A e,f | 365 ± 62 A b,c | 6.3 ± 1 A a,b | 7.5 ± 3 A c | 103 ± 24 A f,g,h | 5.5 ± 1 A i,j | 9 ± 2 A g,h,i | 20 ± 6 A a,b,c | 1.9 A d,e | 5.2 | 1910 | |
| S. polyphyllum | GFo | 555 ± 111 B a | 309 ± 77 A a,b | 8.6 ± 1 B b,c | 6.9 ± 1 B b,c | 115 ± 29 B h,i | 2.4 ± 1 B a | 9.5 ± 2 B h,i | 8 ± 5 B a | 1.3 A a | 13.8 | 4248 |
| Ce | 599 ± 120 A a,b,c | 350 ± 68 A a,b,c | 6.8 ± 1 A a,b | 6.4 ± 1 A a,b,c | 101 ± 22 A e,f,g,h | 3 ± 1 A a,b,c | 7.5 ± 1 A d, e,f,g | 36 ± 8 A f | 1.54 A a,b,c,d | 2.8 | 992 | |
| A. subelegans | GFo | 852 ± 153 B e,f | 221 ± 40 A a | 8 ± 1 B b,c | 8.3 ± 1 B c,d | 88 ± 18 B d,e,f | 5 ± 1 B g,h,i,j | 6.2 ± 1 B b,c,d,e | 27 ± 6 B c,d,e,f | 1.73 A a,b,c,d,e | 3.3 | 721 |
| Ce | 976 ± 207 A f | 233 ± 28 A a | 10.3 ± 2 A c,d,e | 8 ± 2 A c,d | 75 ± 16 A b,c,d,e | 6 ± 1 A j | 8.4 ± 2 A f,g,h,i | 25 ± 7 A c,d,e | 1.74 A b,c,d,e | 3.0 | 693 | |
| T. stenocarpa | GFo | 616 ± 92 B a,b,c,d | 456 ± 97 A c,d,e,f | 11.4 ± 3 B c,d,e | 13 ± 6 B d,e,f,g | 91 ± 17 B d,e,f,g | 4.9 ± 1 B f,g,h,i | 11 ± 2 B i,j | 22 ± 5 B b,c,d | 1.48 A a,b,c,d | 4.1 | 1871 |
| Ce | 572 ± 133 A a,b | 410 ± 119 A b,c,d | 8.2 ± 2 A b,c | 13.3 ± 6 A e,f,g | 83 ± 18 A c,d,e | 4.6 ± 1 A e,f,g,h,i | 9.7 ± 2 A i | 35 ± 9 A f | 1.35 A a | 2.3 | 961 | |
| P. glabrata | GFo | 979 ± 144 B f | 721 ± 159 A h,i | 11.7 ± 3 B d,e | 12 ± 4 B d,e,f | 80 ± 17 B c,d,e | 4.2 ± 2 B d,e,f,g | 13 ± 3 B j | 23 ± 7 B b,c,d | 2.42 A f | 3.4 | 2484 |
| Ce | 964 ± 170 A e,f | 600 ± 133 A f,g | 12 ± 2 A d,e | 12 ± 4 A d,e,f | 77 ± 22 A b,c,d,e | 4.6 ± 1 A d,e,f,g,h,i | 10.5 ± 3 A i | 23 ± 9 A b,c,d | 1.87A c,d,e | 3.4 | 2038 | |
| Z. rhoifolium | GFo | 777 ± 121 B d,e | 416 ± 77 A c,d | 4.5 ± 1 B a | 4 ± 1 B a,b | 80 ± 17 B c,d,e | 3.5 ± 1 B a,b,c,d | 7 ± 1 B d,e,f,g | 34 ± 8 B e,f | 1.43 A a,b,c | 2.4 | 986 |
| Ce | 721 ± 166 A c,d,e | 438 ± 94 A c,d,e,f | 4 ± 1 A a | 3.5 ± 1 A a,b | 66 ± 14 A a,b,c,d | 4 ± 1 A c,d,e | 7 ± 1 A c,d,e,f,g | 35 ± 8 A f | 1.38 A a,b | 1.9 | 827 | |
| Sp. cujabana | GFo | 1495 ± 237 B g | 794 ± 204 A h,i | 7 ± 1 B b | 12 ± 3 B d,e | 65 ± 13 B a,b,c,d | 9.4 ± 2 B k | 7.5 ± 2 B e,f,g | 84 ± 15 B g | 2.04 A e | 0.8 | 617 |
| Ce | 1934 ± 392 A h | 838 ± 179 A i | 4.5 ± 1 A a | 13 ± 6 A d,e,f | 57 ± 11 A a,b | 11 ± 2 A l | 10 ± 1 A i | 105 ± 16 A h | 1.86 A c,d,e | 0.5 | 456 | |
| V. tucanorum | GFo | 769 ± 101 B c,d,e | 428 ± 72 A c,d,e | 7 ± 1 B b | 7.7 ± 2 B c | 115 ± 31 B g,h | 3.6 ± 1 B b,c,d,e | 5.6 ± 1 B a,b,c,d | 27 ± 12 B c,d,e,f | 1.39 A a,b | 4.2 | 1796 |
| Ce | 645 ± 127 A a,b,c,d | 398 ± 101 A b,c,d | 6.2 ± 1 A a,b | 6 ± 2 A a,b,c | 102 ± 24 A e,f,g,h | 4.3 ± 2 A d,e,f,g,h | 5 ± 1 A a,b,c | 16 ± 4 A a,b | 1.26 A a | 6.5 | 2580 | |
| F | 0.000333a | 0.641751 | 0.000155a | 0.048263a | 0.006012a | 0.000001a | 0.029633a | 0.000000a | 0.171712 |
- a Statistically significantly different at a 5% significance level of probability.
- CS, collection site; GFo, gallery forest; Ce, cerrado s.s.; FL, fibre length; VL, vessel element length; IVPD, intervessel pit diameter; VRPD, vessel-ray pit diameter; VD, vessel lumen diameter; FWT, fibre wall thickness; R/mm, ray per millimetre; V/mm2, vessels per square millimetre; VGI, vessel grouping index; V, vulnerability index; M, mesomorphy index; A, average; SD, standard deviation. Uppercase letters indicate the comparison of quantitative data between habitats. Lowercase letters indicate the comparison of quantitative data between species and habitats.
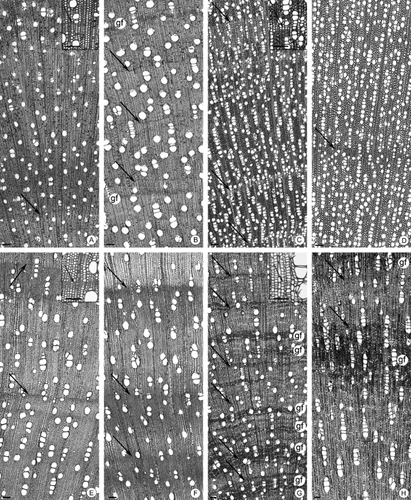
Transverse sections: A, Tapirira guianensis from cerrado (Ce); B, Tp. guianensis from gallery forest (GFo); C, Tabernaemontana catharinensis from Ce; D, Tb. catharinensis from GFo; E, Schefflera vinosa from Ce; F, Sf. vinosa from GFo; G, Pera glabrata from Ce; H, P. glabrata from GFo. Arrows point to the limit of the growth rings; gf, gelatinous fibres. Scale bars, 100 μm.
Transverse sections: A, Dimorphandra mollis from cerrado (Ce); B, D. mollis from gallery forest (GFo); C, Stryphnodendron polyphyllum from Ce; D, S. polyphyllum from GFo; E, Acosmium subelegans from Ce; F, A. subelegans from GFo; G, Tibouchina stenocarpa from Ce; H, T. stenocarpa from GFo. Arrows point to the limit from the growth rings; gf, gelatinous fibres. Scale bars, 100 μm.
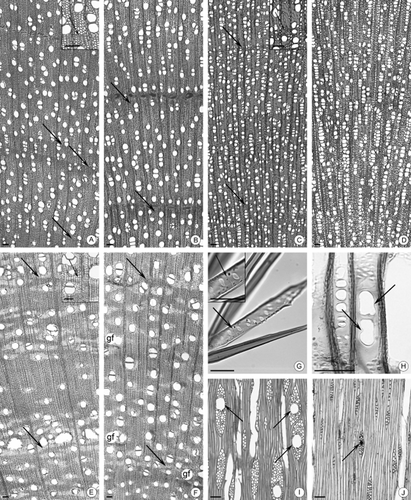
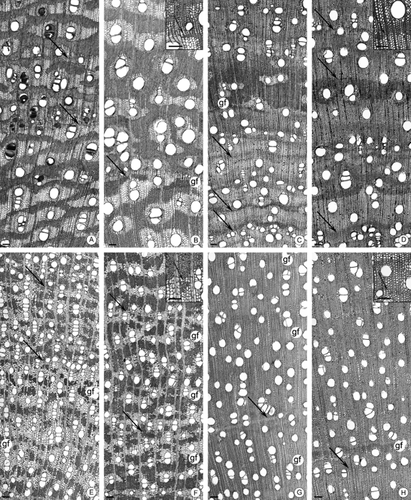
Transverse sections: A, Zanthoxylum rhoifolium from cerrado (Ce); B, Z. rhoifolium from gallery forest (GFo); C, Siparuna cujabana from Ce; D, Sp. cujabana from GFo; E, Vochysia tucanorum from Ce; F, V. tucanorum from GFo. (A–F) Arrows point to the limit from the growth rings; gf, gelatinous fibres. G, Septate parenchyma (arrows) in Tibouchina stenocarpa. H, Dumb-bell-shaped perforation plates (arrows) in Sp. cujabana from GFo. I, Wider radial canals (arrows) in Schefflera vinosa from Ce. J, Narrower radial canals (arrows) in Sf. vinosa from GFo. Scale bars: (A–F, I, J) 100 μm; (G, H) 50 μm.
Vessel arrangement showed a slight variation only in P. glabrata (Fig. 3G, H). Intervessel pitting differed in arrangement in A. subelegans and V. tucanorum. Ray cell composition varied in Sp. cujabana.
Statistical analyses
There were statistically significant differences in quantitative parameters between the two habitats for all specimens, except for vessel element length and vessel grouping index (Table 3, uppercase letters). In general, gallery forest samples had wider vessels at lower frequency per square millimetre, shorter fibres with thinner walls, larger intervessel pits and fewer rays per millimetre than cerrado samples of the same species. Each species, however, showed a limited range within its own taxon (Table 3, lowercase letters) (Fig. 6A–H). This result is best shown in the PCA (Fig. 7A, B), where all cerrado samples of a given species were placed above those from the gallery forest, except for A. subelegans, but they remained close to each other.
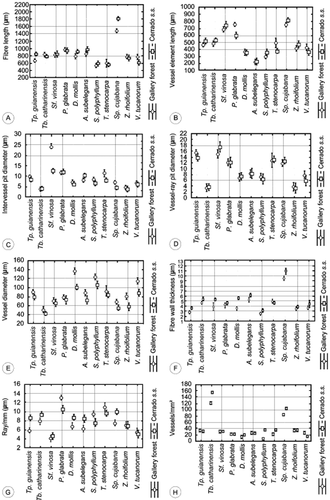
(A–H) Comparison of quantitative data between species in the different habitats: A, fibre length; B, vessel diameter length; C, intervessel pit diameter; D, vessel-ray pit diameter; E, vessel diameter; F, fibre wall thickness; G, ray/mm; H, vessels/mm 2.
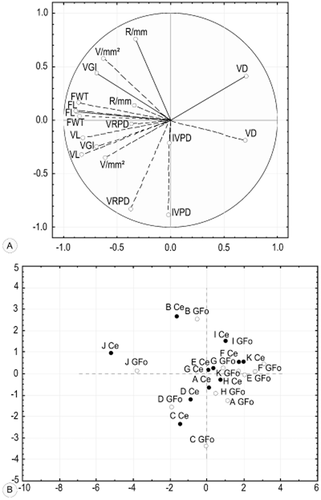
Principal component analysis: Ce (black circles), cerrado s.s.; GFo (white circles), gallery forest. A: FL, fibre length; VL, vessel element length; IVPD, intervessel pit diameter; VRPD, vessel-ray pit diameter; VD, vessel lumen diameter; FWT, fibre wall thickness; R/mm, ray per millimetre; V/mm2, vessels per square millimetre; VGI, vessel grouping index. B: A, Tapirira guianensis; B, Tabernaemontana catharinensis; C, Schefflera vinosa; D, Pera glabrata; E, Dimorphandra mollis; F, Stryphnodendron polyphyllum; G, Acosmium subelegans; H, Tibouchina stenocarpa; I, Zanthoxylum rhoifolium; J, Siparuna cujabana; K, Vochysia tucanorum.
In general, the means of the vulnerability and mesomorphy indices from cerrado were lower (2.9 and 1295, respectively) than those of the gallery forest (5 and 2167, respectively). The vessel grouping index was similar for both habitats (1.7 in cerrado s.s. and 1.6 in gallery forest).
PCA (Table 4) showed that anatomical characteristics vary within factors that together explain 77% of the total variance (Fig. 7A, B). Only quantitative anatomical characters were selected as the qualitative and soil features showed low variance and therefore had a low contribution in PCA. Axis 1 explains 41% of the total variance and is influenced by fibre length, vessel element length and fibre wall thickness. Axis 2 explains 23% of the total variance and is influenced by intervessel and vessel-ray pit diameter, and axis 3 explains 13% of the total variance and is influenced by rays per millimetre. Both Sp. cujabana and Tabernaemontana catharinensis A.DC. remain more distant, as the vessel frequency is higher than that in other species.
| Variable | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Fibre length (μm) | −0.873252a | 0.085033 | 0.078278 |
| Vessel element length (μm) | −0.821138a | −0.322656 | −0.163623 |
| Intervessel pit diameter (μm) | −0.006320 | −0.882585a | −0.206513 |
| Vessel-ray pit diameter (μm) | −0.369329 | −0.832519a | −0.037471 |
| Vessel lumen diameter (μm) | 0.699444 | −0.184896 | 0.410972 |
| Fibre wall thickness (μm) | −0.851011a | 0.161213 | 0.038579 |
| Ray/mm | −0.326246 | 0.143112 | 0.756148a |
| Vessel/mm2 | −0.615054 | 0.585214 | −0.347460 |
| Vessel grouping index | −0.689091 | −0.245837 | 0.439393 |
| Explained variance | 3.746296 | 2.066950 | 1.132893 |
| Proportion of total | 0.416255 | 0.229661 | 0.125877 |
- a Features that contributed most for axes 1, 2 and 3.
Discussion
Most species from cerrado s.s. and gallery forest have growth rings, and this is to be expected, as both areas experience precipitation of < 60 mm for 4 months, which, according to Worbes (1995), is sufficiently long for radial growth to cease and rings to be well defined. Marcati, Oliveira & Machado (2006) also found a high incidence of growth rings which correlated with the presence of a distinct annual dry season in cerrado species. Furthermore, A. subelegans has well-defined growth rings with the same marker in all specimens from both habitats, which suggests that, in some species, growth ring formation could also be partly genetically determined. A forthcoming book with about 90 cerrado species (J. O. Sonsin, P. Gasson, S. R. Machado, C. Caum & C. R. Marcati, unpubl. data) will show many more examples of this phenomenon in cerrado species.
Most of the species do not have a distinct vessel arrangement, i.e. their vessels are diffusely arranged and not in a distinctive tangential, radial or diagonal pattern. This corresponds with c. 80% of dicot woods worldwide (Wheeler, Baas & Rodgers, 2007. Tb. catharinensis, P. glabrata and Sp. cujabana have radial vessel patterns in both habitats, but only P. glabrata shows a more distinct radial pattern in riparian samples. A similar pattern occurs in the same species from the Atlantic forest (Barros et al., 2001), but was not shown by Detienne & Jacquet (1983) in Amazonian samples.
All species have solitary vessels and vessels in short multiples, as do about two-thirds of dicot woods worldwide (Wheeler et al., 2007). The cerrado s.s. and gallery forest species do not have tracheids or vascular tracheids. A few species (Tb. catharinensis, P. glabrata and Sp. cujabana) have fibres with distinctly bordered pits (< 3 μm). Carlquist (1984) speculated that such fibres would provide limited protection against embolism, whereas a radial arrangement of vessels is likely to be safer.
Tapirira guianensis, Sf. vinosa and Sp. cujabana have both simple and multiple perforation plates in both habitats, but, in Tp. guianensis, the multiple perforation plates are less frequent. Scalariform perforation plates are thought to reduce water flow (Carlquist, 1975; Baas & Schweingruber, 1987), and lumen flow resistance has been shown experimentally to be double that of simple perforations by Christman & Sperry (2010). The combination of simple and scalariform plates is seen as an adaptation to environments with seasonal droughts (Dickison & Phend, 1985), although many taxa do not have both.
There are also variations between habitats in axial parenchyma patterns and the number of cells per strand. Axial parenchyma is more abundant in cerrado samples of D. mollis, S. polyphyllum, A. subelegans and V. tucanorum. The greater proportion of axial parenchyma in cerrado is probably for storage and mobilization of metabolites in adverse periods, which are less harsh in riparian samples (see Baas, 1982; Carlquist, 1988; Mauseth, 1988; Alves & Angyalossy-Alfonso, 2002), but this generalization does not apply to all species. Axial parenchyma is scant or absent in both habitats in Tp. guianensis, Sf. vinosa, T. stenocarpa and Tb. catharinensis. These four species have septate fibres which are thought to have a similar storage function to axial parenchyma (Carlquist, 1988; Fahn, 1990; Dickison, 2000). Any storage tissue, either parenchyma or septate fibres, can support rapid flushes of growth, flowering and fruiting when conditions are suitable (Braun, 1984; Carlquist, 1988).
Parenchyma cells in abundance are present in some species, such as S. polyphyllum and V. tucanorum, which have > 70% of solitary vessels in both habitats. Are these parenchyma cells involved in embolism repair in vessels? 2011) hypothesized a process whereby parenchyma cells directly adjacent to an embolized vessel can refill it, even under negative pressure, following changes in carbohydrate metabolism from soluble sugars to insoluble starch. In addition, the authors stated that the decrease in the starch content in the adjacent cell would cause these cells to become strong sinks to phloem, with consequent unloading of sugars and water from the phloem and a bulk flow directed to the refilling conduits. We do not have experimental evidence for this.
Although each species has a suite of qualitative anatomical characters that is consistent between the two habitats, the quantitative features are significantly different. Past studies have linked such differences with climate, soil type and precipitation (Klaassen, 1999; Alves & Angyalossy-Alfonso, 2000; Dickison, 2000). Cerrado samples have more abundant narrower vessels, longer fibres with thicker walls, smaller intervessel pits and more rays than gallery forest samples of the same species. The PCA shows that the same species are close together because, despite their quantitative differences, their anatomy is similar, except with regard to water transport.
The cerrado s.s. and gallery forest species show low vessel indices of 1.7 and 1.6, respectively, suggesting that vessel failure rarely occurs in these species (see Carlquist, 1984). The presence of simple and multiple perforation plates in vessels and perforated ray cells in some species (Tp. guianensis, Sf. vinosa and Sp. cujabana), and abundant axial parenchyma cells in others (D. mollis, S. polyphyllum, A. subelegans and V. tucanorum), may explain the low vessel index for both cerrado s.s. and gallery forest species. They may enhance hydraulic safety for these species, as would a high frequency of narrow vessels.
In general, when present, tyloses are more frequent in cerrado s.s. species (Tp. guianensis, Sf. vinosa and T. stenocarpa) than in gallery forest. Tyloses can be formed in reaction to a wide variety of causes, such as frost, flooding, heartwood formation, wounding and natural senescence (Chattaway, 1949; Davison & Tay, 1985; Parameswaran, Knigge & Liese, 1985; Cochard & Tyree, 1990; Dute, Duncan & Duke, 1999; Sun et al., 2007). All of these phenomena can stimulate ethylene production according to Sun et al. (2007). In grapevine, they found that wound ethylene production was the cause of tylosis formation, and that embolisms in vessels are not directly required for wound-induced tyloses. In cerrado s.s. species, the lack of water in the driest period could stimulate the increase in ethylene production, whereas, in the gallery forest, there is standing water, even in dry periods.
Gelatinous fibre distribution is usually in distinct bands or small groups spread throughout the secondary xylem, but exhibits considerable variation within species between habitats. Gelatinous fibres are usually considered to be a phenomenon of tension wood on the upper side of branches (Esau, 1974 and many other authors), and so their presence is to be expected. The innermost layer of gelatinous fibres is mainly cellulose, which is hydrophilic (Esau, 1965; Mauseth, 1988; Fahn, 1990), suggesting that they might also have a water storage function (Paviani, 1978). Although, to our knowledge, there is no direct experimental evidence for water storage in gelatinous fibres, their abundance in the wood of taxa growing in dry environments suggests that this relationship is worth exploring further. Milanez, Marcati & Machado (2008, 2009) found that aluminium accumulates in the gelatinous fibre walls and septate fibres, both of pectic-cellulosic nature, of Melastomataceae. A hypothesis considered by many authors is that the means by which plants interact with the presence of aluminium seems to prevent its entry into the cell by its deposition on the cell wall, by compartmentalization in vacuoles or plastids, or by the formation of aluminium chelates (Cuenca, Herrera & Merida, 1991; Britez et al., 2002; Milanez et al., 2009). There is therefore some evidence that gelatinous fibres are not only indicative of tension wood, but could also sequester aluminium, which is potentially harmful to plants.
The gallery forest samples are better optimized for conductive efficiency (wider vessels, larger intervessel pits, simple perforation plates) than are cerrado samples, which have a strategy for safety reflected by narrower vessels that are less efficient at water transport, but also less prone to embolism (Baas, Wheeler & Fahn, 1983; Schulte, 1999; Dickison, 2000; Tyree & Zimmermann, 2002; Zanne et al., 2006; Loepfe et al., 2007; Pratt et al., 2007).
The vulnerability and mesomorphy indices of cerrado (2.9 and 1295, respectively) and gallery forest samples (5 and 2167, respectively) correspond with this hypothesis, but are based on observation and not experiment.
Conclusions
We have demonstrated considerable differences in the quantitative characteristics of the secondary xylem among the 11 species which occur in both cerrado s.s. and gallery forests, particularly in the vessel features. The wood of the species from gallery forest is better optimized for conductive efficiency (wider vessels, larger intervessel pits, simple perforation plates and higher vulnerability and mesomorphy indices) than the wood from cerrado species, which have a strategy for safety reflected in a higher frequency of narrower vessels that are less efficient at transporting water, but less prone to embolism. If, as seems likely, these characteristics are at least partly genetically controlled, when restoring cerrado habitats, it is important to consider the ecological provenance of the trees to be reintroduced, especially if they have a broad habitat tolerance.
Acknowledgements
Thanks are due to the Coordination for the Improvement of Higher Level Personnel (CAPES) and the National Council of Technological and Scientific Development (CNPq) for the sponsorship of this research, to Liliane C. Pereira for technical assistance in the laboratory, to Clemente José Campos who provided assistance in the field and to the Royal Botanic Gardens, Kew, for general support and for hosting the first author.




