A ballistic pollen dispersal system influences pollination success and fruit-set pattern in pollinator-excluded environments for the endangered species Synaphea stenoloba (Proteaceae)
Abstract
The critically endangered Synaphea stenoloba (Proteaceae) has numerous scentless flowers clustered in dense inflorescences and deploys a ballistic pollen ejection mechanism to release pollen. We examined the hypothesis that active pollen ejection and flowering patterns within an inflorescence influence the reproductive success (i.e. fruit formation) of individual flowers within or among inflorescences of S. stenoloba in a pollinator-excluded environment. Our results showed that: (1) no pollen grains were observed deposited on the stigma of their own flower after the pollen ejection system was manually activated, indicating self-pollination within an individual flower is improbable in S. stenoloba; (2) fruit set in the indoor open pollination treatment and the inflorescence-closed pollination treatment indicated that S. stenoloba is self-compatible and pollen ejection can potentially result in inter-floral pollination success; (3) fruit set in the inflorescence-closed pollination treatment was significantly lower than that of indoor open pollination, indicating within- and between-flower pollination events in an inflorescence are most likely limited, with pollination between inflorescences providing the highest reproductive opportunity; and (4) analysis of the spatial distribution of cumulative fruit set on inflorescences showed that pollen could reach any flower within an inflorescence and there was no functional limitation on seed set among flowers located at various positions within the inflorescence. These data suggest that the pollen ejection mechanism in S. stenoloba can enhance inter-plant pollination in pollinator-excluded environments and may suggest adaptation to pollinator scarcity attributable to habitat disturbance or competition for pollinators in a diverse flora. © 2012 The Linnean Society of London, Botanical Journal of the Linnean Society, 2012, 170, 59–68.
INTRODUCTION
Flowers and inflorescences are the functional units of angiosperm mating and exhibit a tremendous diversity of size, shape, colour, scent, rewards, symmetry, placement of sexual organs, number of flowers open concurrently, arrangement in inflorescences and gender (Barrett, 1998, 2003). Since Darwin, most floral traits have been interpreted as mechanisms that passively encourage cross-pollination by preventing or discouraging self-pollination, thereby allowing more opportunities for ovules to be outcrossed (Barrett & Harder, 1996). It is now widely recognized that many features of flowers and inflorescences influence patterns of pollen dispersal and pollination success (Harder & Wilson, 1994; Barrett, 2003).
Most plant species require vectors to transfer pollen to achieve pollination success, and some species have evolved active pollen release mechanisms to enhance pollination success to cope with various pollinator-poor environments. For example, a sudden movement of the anther walls of Ricinus communis L. launches pollen (Bianchini & Pacini, 1996) and a sudden movement of filaments under tension launches pollen in the Mediterranean herb Parietaria judaica L. and in certain tropical trees of Moraceae (Williams & Adam, 1999; Pacini & Hesse, 2004). The Chinese perennial herb Caulokaempferia coenobialis (Hance) K.Larsen uses a mechanism in which a film of pollen is transported from the anther by an oily emulsion that slides sideways along the style and into the stigma of the same individual to cope with a scarcity of pollinators in extremely shady and humid habitats (Wang et al., 2004). The self-incompatible bunchberry dogwood (Cornus Canadensis L.) catapults pollen into the air as closed flowers open explosively to enhance cross-pollination (Edwards et al., 2005).
In plants where flowers are grouped in inflorescences, each flower may offer differing pollination opportunities (Hiei & Ohara, 2002). A large number of studies have investigated variations in the reproductive success of individual flowers within an inflorescence. Differences in flower position and flowering phenology within an inflorescence have a significant effect on the reproductive success of individual flowers across many species (e.g. Vaughton, 1993; Diggle, 1995, 1997; Whelan & Denham, 2009). Various non-exclusive hypotheses, such as ‘non-uniform pollination hypothesis’, ‘resource competition hypothesis’ and ‘architectural effects hypothesis’, have developed to explain reproductive success variation within inflorescences of different species (Medrano, Guitian & Guitian, 2000). However, few experimental studies have investigated whether the active pollen release mechanisms influence the reproductive success of individual flowers within or among inflorescences of plants employing this pollination-facilitation mechanism.
Synaphea stenoloba A.S.George is a critically endangered endemic taxon, known from only four small and fragmented populations within a 6-km radius near Pinjarra, Western Australia in the global biodiversity hotspot of south-west Australia. Synaphea stenoloba occurs in sites that are threatened with human activities (the largest populations are on remnant vegetation on lands leased for mining) and other sites are composed of remnant vegetation on road verges or adjacent to farmland and prone to invasive weeds and vulnerable to disturbance (e.g. roadworks, etc.). An active pollen release mechanism is deployed by flowers of S. stenoloba and has been noted for the genus Synaphea R.Br. by George (1995). Synaphea flowers share a similar type of active pollen release involving a moving style mechanism as Conospermum with pollination by native solitary bees (Colletidae, Leioproctus spp.), but appear not to be species specific, unlike some native bees that pollinate Conospermum (Carolin, 1960; Holm, 1978; Houston, 2000), have nectar but no appreciable scent (also noted by George, 1995) and appear to be self-incompatible with low and highly variable seed set under natural conditions (G. Keighery, pers. comm.).
Flowers of cultivated S. stenoloba are clustered in dense inflorescences and we hypothesized that the ‘pollen ejection mechanism’ (PEM) might encourage neighbouring pollination within or among inflorescences in habitats experiencing pollinator scarcity. This study aimed to: (1) examine whether pollen ejection facilitates pollination success within and among inflorescences under pollinator-excluded environments; (2) investigate the spatial patterns of fruit set on inflorescences to determine whether pollen dispersal and the flowering pattern within inflorescences influence the patterns of fruit set within and among inflorescences.
MATERIAL AND METHODS
Study species
Synaphea stenoloba is a compact shrub to 50 cm high. The leaves are 5–40 cm long and tri-pinnate. The inflorescences are yellow and borne above the leaves to a height of 15 cm. The flowers appear to be scentless. The perianth of S. stenloba is tubular, zygomorphic and yellow, opening in the upper third to half of its total length; the adaxial tepal is the longest and broadest, 4.5–5.0 mm long, 2 mm wide and distinctively hooded; the lateral pair of tepals falcate; abaxial tepal smallest, 3.5 mm long, usually with a small, reflexed tip. Stamens are on short, thick filaments; adaxial stamen is sterile; one pair of lateral stamens is unilocular; abaxial stamen bilocular; with the average number of pollen grains per flower 142 ± 11.19. The stigma is plate-like, bilobed with a narrow semi-translucent border dorsally attached from the base to a sterile filament by a narrow column. Anthers are located on the back of the stigma receptive area. The ovary is unilocular, sessile with an apical ring of large translucent glands. There is one ovule per flower. Flowering occurs in the Southern Hemisphere spring from August to October (George, 1995).
The S. stenoloba plants used in this study were clonally micropropagated from plants in the wild (Bunn et al., 2010) and grown in 10-cm diameter pots in Kings Park and Botanic Garden (KPBG), Perth, Western Australia (Fig. 1A, B). From prior studies, cultivated plants were observed to produce from 12–24 inflorescences per plant (mean of 18.50 ± 4.04); inflorescence height varied from 16 to 40 cm (mean of 28.84 ± 11.30 cm) and exceeds the foliage with the spikes elongating markedly during floral development; flowers tended to be clustered in the upper 7/10ths of the inflorescence; with varying numbers of flowers per inflorescence (mean 39.5 ± 16.59).
(A) Wild and (B) cultivated micropropagated plants of Synaphea stenoloba in the nursery at Kings Park and Botanic Garden (scale bar, 5 cm).
Flowering phenology within inflorescences
To observe the flowering pattern within inflorescences, five inflorescences with flowers in the bud stage of each of six plants were selected and tagged. A total of 30 inflorescences and 1175 flowers were observed. Individual flower and inflorescence flowering phenology was determined by following flower development until senescence.
Pollen viability
To determine pollen viability of different pollen ages, pollen was collected 1 day before anthesis and at 1, 3, 5, 7, 9 and 11 days after anthesis (but prior to any pollen ejection event) and six flowers were collected at each pollen age. A drop of 1% w/v 2, 3, 5-triphenyl-2H-tetrazolium chloride (TTC) in 0.2 m phosphate buffer solution was placed on a slide and flowers placed in the solution (the mouth of the flower placed down into the solution) and then the perianth squeezed using a pair of forceps to cause the PEM to deposit pollen into the solution. Pollen grains were counted under a compound microscope. Pollen viability was estimated using the TTC solution method described above with at least 100 grains assessed (for each of six flowers/pollen age) for presence or absence of a stain reaction.
Timing of stigma receptivity
To determine the timing of stigma receptivity, ten inflorescences with flowers in the bud stage were tagged and bagged using bags of fine nylon organza. Six flowers were selected at 1, 2, 3, 5, 7, 9 and 11 days after anthesis (but prior to PEM activation) and were pollinated immediately with fresh pollen from flowers of other inflorescences from clones of the same plant. This procedure was repeated three times. Flowers not used for the experiment were removed from the inflorescence. To collect fresh pollen, a fine brush was placed over the flower mouth and thumb and forefinger were used to slightly compress the perianth, causing the pollen to be ejected onto the brush. Fruit set was recorded 40 days after pollination.
Pollination treatment
To examine whether the PEM enhances pollination success, we compared pollen deposition and pollen germination on the stigma after pollen ejection at different times. The PEM was manipulated on different days after anthesis and up to the stage of automatic PEM activation. The PEMs of each of four bagged flowers were manipulated by pressing the perianth using thumb and forefinger at 1, 3, 5, 7, 9 and 11 days following anthesis. Flowers were collected 24 h later (including four flowers where pollen had been automatically ejected) and fixed in Copenhagen fixative for later observation of pollen grains and pollen tubes.
To determine whether the PEM might enhance between-flower crossing within inflorescences, we enclosed 13 inflorescences in various bud stages in bags made of fine nylon organza. To examine open pollination we did an indoor open pollination where a doublet of potted plants with 23 neighbouring inflorescences were placed close together, with foliage just touching in an insect-proof greenhouse at KPBG. To detect the influence of potential pollinators, we performed outdoor open pollination with the same experimental set-up (doublet of potted plants) in the general (outdoor) nursery area, where it is known that pollination of other Synaphaea spp. occurs regularly (A. Shade, pers. comm.). To preclude the effect of outcrossing between different genotypes, all experimental plants were the same genotype obtained from tissue culture.
Spatial patterns of fruit set
To test the hypothesis that fruit set along the rachis may reflect the pollen deposition patterns, we tested for spatial patterns of fruit set on the rachis, whereby the positions of developing fruits were scored for each inflorescence, following the method of Denham & Whelan (2000). Each flower on an inflorescence was scored as occurring at a point along the rachis relative to other flowers. The position of each flower on an inflorescence was given a number from the lowermost flower to the uppermost (i.e. 1, 2, 3, etc.), with the assumption that fruits could occur at any flower position. As inflorescences varied in their numbers of flowers, a measure of proportional relative position (the serial number of a flower divided by the total number of flowers on the respective inflorescence) along the rachis was used. Thus, the lowest flower on a 24-flower inflorescence would have a relative position of 0.0417 (1/24) and the uppermost flower would have a relative position of 1 (24/24). Thirteen plants were randomly chosen from an open pollination treatment carried out in the general (outdoor) nursery area to determine the fruit set pattern on individual inflorescences. Measurements from fruiting inflorescences were pooled and the cumulative frequency of fruits plotted against proportional relative position (a line with a slope of one representing an even distribution of fruits). Actual fruit distributions were then compared using Kolmogorov–Smirnov goodness-of-fit tests for continuous data.
Statistical analysis
One-way analyses of variance (ANOVA) combined with the Bonferroni test for multiple comparisons at P < 0.05 was applied to the data of pollination treatment. Correlations were assessed using linear regression analysis. All statistical analysis was performed using SPSS version 10.0 (SPSS Inc., 1999).
RESULTS
Flowering pattern within inflorescences
From August to November, flowers opened acropetally. During the flowering period for any inflorescence, there were opened flowers (mean of 9.5 ± 4.0) that remained in good condition (pollen was not ejected) on the inflorescence after the pollen of the lowermost flower was ejected and before the uppermost flower opened. On the largest inflorescences, the lowermost flowers produced fruits while the uppermost flowers were still in bud. Inflorescences with many flowers tended to flower longer, begin flowering earlier and end later than those with fewer flowers. There was a significant positive correlation between the number of flowers per inflorescence and flowering duration of an inflorescence (R2 = 0.506, P = 0.002). There were 0.99 ± 0.31 (range 0–5) flowers opened per inflorescence each day. Typically 11–70 days elapsed before inflorescences completed anthesis. Figure 2 shows an example of the flowering pattern for both the number of flowers opened and the number of flowers from which pollen was ejected in a 27-flower inflorescence.
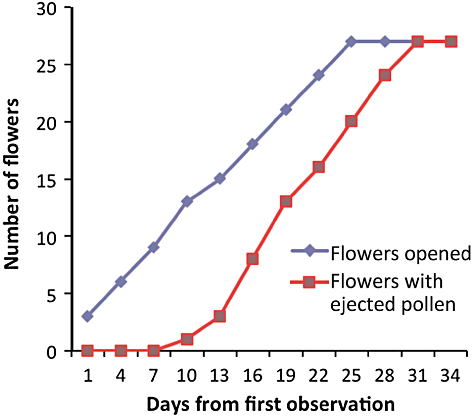
An example of the pattern of flowering phenology in Synaphea stenoloba for cumulative numbers of flowers opened from the day of first observation and the number of flowers with ejected pollen in a 27-flower inflorescence.
Pollen viability
The present study found that pollen viability was not affected by the time of collection. Treatment means for pollen germination given in Figure 3, illustrate that pollen collected 1 day before anthesis compared with pollen collected 1–11 days after anthesis (but before the PEM occurred spontaneously) showed no significant difference (ANOVA, F6, 35 = 2.21, P = 0.07) in viability. These data suggest that pollen in the anther matures from the day of flower anthesis and retains viability up to the day before pollen is ejected automatically.
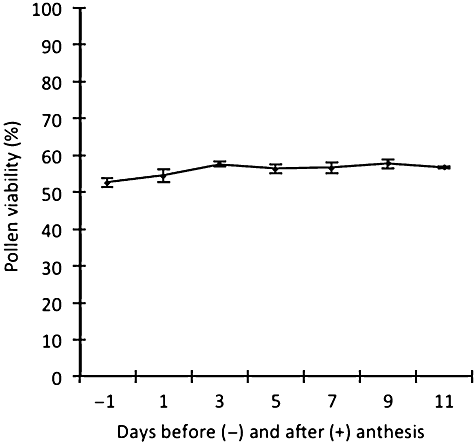
Viability of pollen of Synaphea stenoloba collected 1 day before anthesis and 1–11 days after anthesis (and prior to pollen ejection). Bars indicate the standard errors of means (SE).
In preliminary studies on cultivated plants of S. stenoloba, we observed that, if the stigma is not disturbed and the anther remains un-dehisced, flowering typically lasts 9 days, after which the stigma flicks and ejects pollen without mechanical stimulation. Figure 4A–H illustrates the floral morphology and PEM in S. stenoloba.
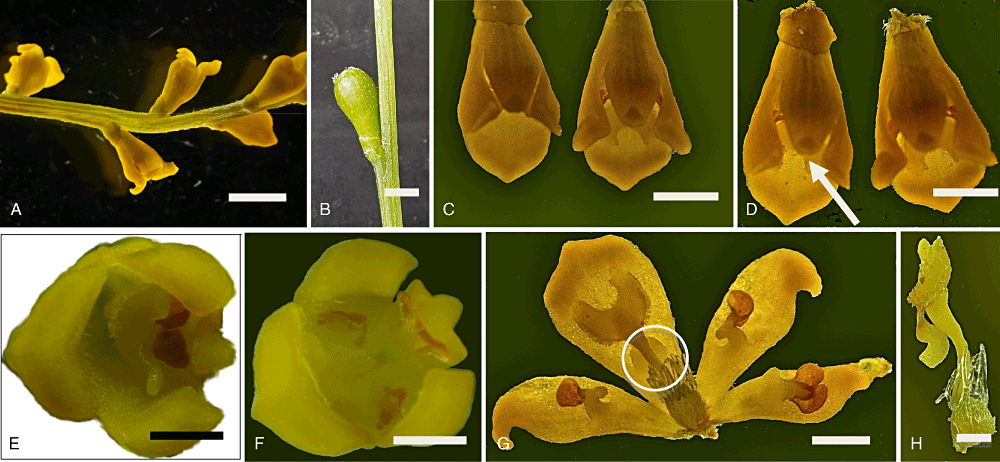
A, part of floret of Synaphea stenoloba with flowers (scale bar, 3 mm). B, immature fruit (scale bar, 2 mm). C, pair of flowers showing flower on the left prior to stigma flick (scale bar, 2 mm). D, same pair of flowers with the flower on the left stimulated to release stigma (scale bar, 2 mm). E, top view of flower prior to stigma flick (scale bar, 1 mm). F, top view of flower with stigma flick activated (scale bar, 1 mm). G, opened flower showing released bilobed stigma adhered to perianth segment and three stamens (scale bar, 1 mm). H, excised stigma (scale bar, 0.5 mm).
Fruit set after anthesis (and prior to pollen ejection)
Results of the controlled pollination experiment (Fig. 5) indicate that fruit set is high from the day of anthesis to 11 days before the PEM is spontaneously activated. There was no significant difference among treatments (ANOVA, F6, 14 = 0.13, P = 0.99). This result suggests that the stigma is receptive from the day of anthesis to the day before pollen is ejected automatically.
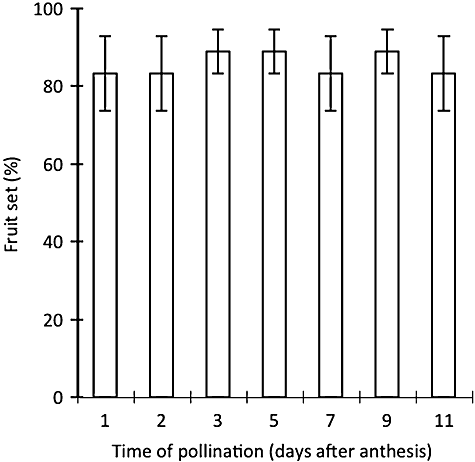
Percentage fruit set from controlled inter-inflorescence pollination at different days after anthesis (and prior to triggering of the stigma) on bagged flowers of Synaphea stenoloba. Bars indicate the standard errors of means (SE).
Pollination treatment
When the PEM was artificially manipulated at differing periods post-anthesis, or following automatic pollen ejection events in older flowers, no pollen grains were observed to deposit and germinate on the receptive area of their sister stigma (i.e. no between-flower selfing). Fruit was set in all the pollination treatments: (1) indoor open; (2) outdoor open; (3) inflorescence closed (Fig. 6); and there were significant differences among treatments (F2, 56 = 10.98, P < 0.0001). Bonferroni post-hoc tests revealed that there was no significant difference between treatments 1 and 2, but there were significant differences between treatments 3 and 1 or 3 and 2 (Fig. 4).
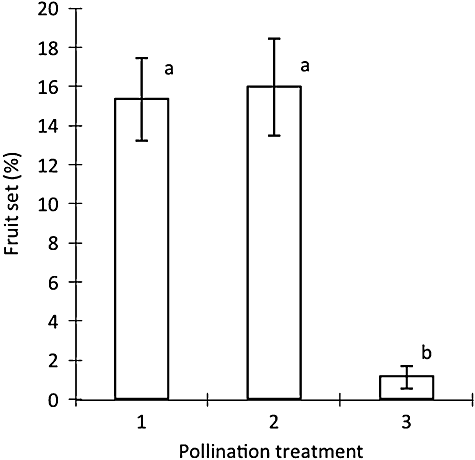
Percentage fruit-set in Synaphea stenoloba plants for different pollination treatments: (1) indoor open pollination; (2) outdoor open pollination; (3) inflorescence-closed. Different letters above columns indicate significant treatment differences, as determined by Bonferroni post-hoc tests. The mean difference is significant at the 0.05 level. Bars indicate the standard errors of means (SE).
Spatial patterns of fruit set
Fruit set per inflorescence was strongly related to the number of flowers/inflorescence with significantly increased fruiting on inflorescences with larger numbers of flowers (R2 = 0.09, F1,44 = 4.43, P = 0.041) compared with fewer fruits set on inflorescences with lower numbers of flowers (R2 = 0.25, F1,44 = 14.66, P < 0.0001). The spatial distribution of fruits on inflorescences showed variable clustering of fruits on the rachis (Fig. 7). The spatial distribution of the cumulative fruits (N = 185) for a total of 44 inflorescences is represented in Figure 8, where line A indicates an even distribution. The distribution of these initial fruits was significant, even along the inflorescence (Kolmogorov–Smirnov test; D = 0.23, P = 0.004) (Fig. 8), although there was a trend towards clumping of initial fruits towards the middle part of the rachis.
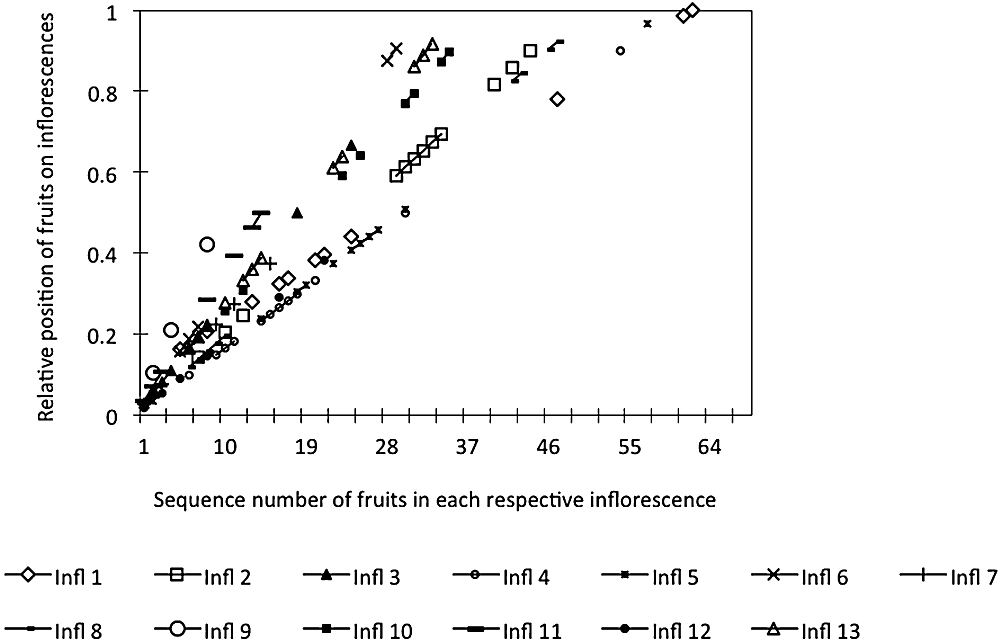
The spatial distribution of each fruit on each respective inflorescence in Synaphea stenoloba. Thirteen inflorescences (Infl 1–Infl 13) that set fruits were randomly chosen from all outdoor and indoor open pollination treatments.
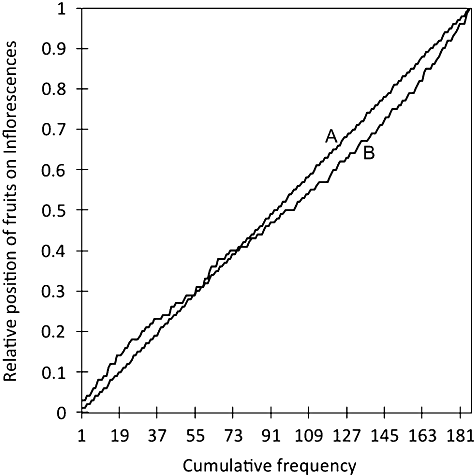
The cumulative frequency of Synaphea stenoloba fruits against their relative position on the rachis (line B). Line A represents the expected pattern if fruit was distributed evenly. The distribution of fruits in relation to position on the rachis was not significantly different from an even one (Kolmogorov–Smirnov test; D = 0.0649, P = 0.819). Fruits were sourced from all outdoor and indoor open-pollination treatments.
DISCUSSION
Pollination success and pollen ejection mechanism in synaphea
Our results showed that the PEM in S. stenoloba enhances between-flower pollination success in pollinator-excluded environments. Fruit set in the pollinator-excluded part of the study indicated that S. stenoloba is self-compatible and the PEM can result in inter-floral pollination success.
Pollen in the anther matures from the day of flower anthesis, and pollen viability and stigma receptivity were constant during flowering, which suggested that, once pollen deposits on a stigma, reproductive success may follow.
Fruit set in the inflorescence-closed pollination treatment was significantly lower than that of indoor open pollination, indicating within- and between-flower pollination within an inflorescence was limited and pollination between inflorescences was elevated. No pollen grains were observed deposited on the stigma of their own flower, either by ejection of pollen attributable to flower manipulation or pollen released spontaneously, therefore pollination within an indi vidual flower (autogamy) seems improbable in S. stenoloba. The failure of within-flower pollination resulted from the avoidance of interference between the presentation of pollen and stigmas. The anthers on the back of the plate-like stigma receptive area in flowers of S. stenoloba showed that the floral mechanism is herkogamous (spatial separation of male and female organs within flowers) (Webb & Lloyd, 1986). Once the stigmatic ‘catapult’ is triggered, the stigma receptive area presses firmly to the perianth and it is unlikely to accept pollen after this event. The inter-floral pollination limitation within an inflorescence may result from the height and the direction of the PEM facilitating pollen deposition among immediate neighbouring inflorescences.
Flowers of S. stenoloba possessed some of the features usually associated with anemophily (e.g. tendency to be clustered in dense inflorescences, small size, anthers that often open with explosive dehiscence, dry pollen, expanded stigma surface and sexes separated temporally, i.e. dichogamy and/or spatially, i.e. herkogamy) (Stebbins, 1970; Ackerman, 2000; Culley, Weller & Sakai, 2002), especially the floral feature of separation of sexes and forceful ejection of pollen suggestive of a wind pollination mechanism in this species. Our results with S. stenoloba showed that pollen ejection via an automatic stigma ‘flip’ or ‘catapult’ action is capable of inducing between-inflorescence pollination under pollinator-excluded conditions. This raises the possibility that pollen grains emitted from the tepal opening could be carried by air currents and transported some distance from the flower. Could S. stenoloba be wind-pollinated in some circumstances? The ability to achieve pollination in this way, even if occasional and opportunistic, could be important for pollination success in pollinator-poor environments and is particularly important in conservation planning for rare species where pollinator scarcity because of habitat fragmentation may compromise reproductive success. However, S. stenoloba possesses other floral traits uncharacteristic of wind pollination (e.g. low pollen : ovule ratio, showy yellow flowers), which are more typical of an animal-pollinated species. Wind pollination (anemophily) of angiosperms may possibly have evolved from insect pollination (entomophily) in response to pollinator limitation and changes in the abiotic environment. Recent evidence suggests that ambophily (a combination of both wind and insect pollination) might be more common than previously assumed and could represent either a stable or transitional state (Culley et al., 2002). Synaphea stenoloba flowers are nectar producing but unscented and the species grows in a region of high biodiversity, therefore S. stenoloba may utilize multiple pollination mechanisms that can offset lack of insect pollination vectors. Although the present study shows that anemophily is possible under controlled pollination conditions for S. stenoloba, it is clear that the high level of floral zygomorphy, low pollen : ovule ratio, bright yellow colour and mechanical triggering of pollen release indicates that insect visitation still plays a primary role in pollination.
The lack of significant differences between indoor and outdoor pollination in KPBG may indicate pollinator limitation (or inappropriate pollinator species under open nursery conditions at KPBG), but cannot preclude the possibility that insect pollination in the home range of S. stenoloba may be higher than recorded at KPBG. Further investigations on the nature and level of involvement of pollinators in the wild will help to reveal more details of the pollination mechanism and reproductive strategies of Synaphea and other members of Proteaceae (e.g. Conospermum spp.) demonstrating pollen ejection.
Fruit-set pattern
Although the total number of fruits was significantly higher in large vs. small inflorescences, fruit set expressed as a percentage of the total number of flowers decreased significantly as inflorescence size increased. This may be the result of most late-opening flowers in large-size inflorescences merely serving as pollen donors because of reduced chances of pollination success for these flowers in a pollinator-limited habitat.
The clustering patterns exhibited in fruits on individual inflorescences may reflect the pattern of flower opening in each inflorescence. During the flowering period for an inflorescence, there was consistency in a mean of 9.5 ± 4.0 of immediate neighbouring opened flowers that remained in good condition on the inflorescence after the pollen of the lowermost flower was ejected and before the uppermost flower opened. Thus, some of these adjacent flowers may potentially be pollinated simultaneously once a pollination event occurs.
Analysis of the spatial distribution of cumulative fruit production in 44 inflorescences showed that fruit set was evenly distributed along the rachis rather than being clustered in any particular section. This suggests that pollen can reach various positions within an inflorescence, with floral position posing no functional limitation in seed-set among flowers.
There appeared to be no ‘architectural effects’ (Wyatt, 1982; Thomson, 1989; Wolfe, 1992; Diggle, 1995, 1997) or ‘resource limitation’ (Stephenson, 1980, 1981; Bawa & Webb, 1984; Nakamura, 1986, 1988; Thomson, 1989; Medrano et al., 2000; Hiei & Ohara, 2002) for fruit set within inflorescences of S. stenoloba. This result agrees with the pattern of fruit set found in Lomatia silaifolia (Sm.) R.Br., which was explained as an expanded conflorescence with relatively few flowers, compared with many groups of Proteaceae where the inflorescences (pair of flowers) are effectively independent with respect to nutrient supply (Denham & Whelan, 2000). Our finding of this even fruit set could have been influenced by ample water and nutrient supply under nursery conditions in KPBG. However, this study emphasizes that pollen can disperse to any position of an inflorescence in plants of S. stenoloba through deployment of a PEM under pollinator-excluded conditions.
CONCLUSION
In plants of S. stenoloba growing under cultivated conditions, a pollen ejection mechanism (PEM) facilitates between-flower pollination success within and among inflorescences under pollinator-excluded environments, but we did not see significant effects concerning spatial patterns of fruit set on inflorescences that could be attributed to pollen dispersal, and flowering pattern within inflorescences did not appear to shape the patterns of fruit set within and among inflorescences significantly. Consequences for conservation planning of this study indicate that this species may cope with pollination limitation by invoking pollen ejection to move pollen between flowers and between plants. From the perspective of recovery operations for this critically endangered species, it is conceivable that seed set could still occur in sites where pollinator abundance may be limited or erratic. However, this study did not investigate the genetic consequences of selfing and further conservation research should include an examination of the genetic composition of offspring from wild, open-pollinated plants.
ACKNOWLEDGEMENTS
We wish to thank Dr Deanna Rokich and Dr TianHua He and technical staff of the Botanic Gardens and Parks Authority (especially Ms Amanda Shade) for their valuable assistance. We also thank Mr Greg Keighery (Principal Research Scientist, Dept Environment and Conservation, Como, Perth, Western Australia) for botanical information on Synaphea.




