Female gametophyte development in Aristolochia labiata Willd. (Aristolochiaceae)
Abstract
Piperales are an extremely diverse angiosperm lineage in terms of female gametophyte structure and, as such, an excellent candidate for comparative investigations of developmental evolution. In Piperales, Aristolochiaceae are sister to clades in which several divergent patterns of female gametophyte development are prevalent. Outgroup comparisons and explicit analyses of character evolution demonstrate that Polygonum-type female gametophyte development and structure in Aristolochiaceae appears to represent the plesiomorphic condition from which the divergent ontogenies of Piperales evolved. Here, we present detailed analyses of female gametophyte development in Aristolochia labiata that combine light and confocal microscopy with three-dimensional computer reconstruction. Our reconstructions demonstrate that, at the two-nucleate stage of development, separation of nuclei to opposite poles of the female gametophyte does not appear to be dependent on vacuolar expansion as generally hypothesized. We also found a decrease in antipodal volume following formation of the secondary nucleus. Our data provide a baseline for future efforts to describe developmental modifications responsible for evolutionary transitions in female gametophyte ontogeny throughout Piperales. © 2008 The Linnean Society of London, Botanical Journal of the Linnean Society, 2008, 158, 19–29.
INTRODUCTION
Piperales may have the most variable female gametophyte developmental patterns of any major angiosperm lineage. There are reports of transitions from monospory to bispory (Johnson, 1900a; Quibell, 1941; Raju, 1961) and tetraspory (Johnson, 1900b, 1914; Swamy, 1944; Lei, Wu & Liang, 2002). There are at least four different patterns of tetrasporic female gametophyte development that produce central cells containing from two to fourteen nuclei, with corresponding endosperm levels ranging from triploid to decapentaploid (Johnson, 1900a, b, 1914; Swamy, 1944; Maheshwari, 1950; Murty, 1960; Davis, 1966; Plyushch, 1982a, b; Haig, 1990; Johri, Ambegaokar & Srivastava, 1992; Lei et al., 2002). Of the ten basic patterns of female gametophyte development among angiosperms circumscribed by Maheshwari (1950), at least six occur in Piperales. Piperales is truly a ‘hotspot’ of female gametophyte developmental evolution and, as such, constitutes the premier group for comparative investigations of divergent patterns of female gametophyte ontogeny.
Embryological investigations in Cannelales (Winterales), Laurales and Magnoliales indicate that female gametophyte development in the common ancestor of the eumagnoliids was monosporic Polygonum-type with eight nuclei and seven cells at maturity (Mauritzon, 1935; Davis, 1966; Tobe & Sampson, 2000; Kimoto & Tobe, 2001, 2003; Heo et al., 2004; Williams & Friedman, 2004; Kimoto, Utami & Tobe, 2006). Therefore, members of Piperales with Polygonum-type female gametophyte development are likely to represent the plesiomorphic condition for the clade. Aristolochiaceae are sister to lineages of Piperales with a remarkable degree of female gametophyte developmental diversity (Nickrent et al., 2002; Hilu et al., 2003; Davis et al., 2004, 2006; Wanke et al., 2007a). Five of the seven currently recognized genera are reported to form Polygonum-type female gametophytes (Hofmeister, 1858; Chodat, 1916; Jacobsson-Stiasny, 1918; Dastur & Bombay, 1921; Johri & Bhatnagar, 1955; Nair & Narayanan, 1961; Davis, 1966; Tobe et al., 1993; González & Rudall, 2003; Wyatt, 1955). However, few studies have focused on early ontogenetic events, at synctial stages of development, that may be critical for cell-level patterning.
In this study, we combine light and confocal microscopy of serially sectioned material with three-dimensional computer-imaging to describe female gametophyte development in Aristolochia labiata Willd. In particular, we focus on vacuole morphology and key nuclear positioning events in the syncytial stages of embryo sac formation. This is the first study to combine such techniques and produce high-resolution computer-generated models of female gametophyte development.
Our goals in this study were to combine recent systematic insights into Piperales (Jaramillo & Manos, 2001; Jaramillo, Manos & Zimmer, 2004; Wanke, González & Neinhuis, 2006a; Wanke et al., 2006b, 2007a, b) with developmental data, in order to reconstruct the ancestral characteristics of the female gametophyte in Piperales. These data will provide a baseline for future comparative evolutionary developmental analyses that aim to describe modifications responsible for evolutionary transitions to apomorphic bisporic and tetrasporic ontogenies of Piperaceae and Saururaceae.
MATERIAL AND METHODS
Aristolochia labiata is a vigorous climbing vine native to Brazil. It has flowers up to 25 cm long that are a mottled mix of white and dark burgundy in colour. Flowers of A. labiata were collected from individuals in greenhouses at the University of Colorado in Boulder throughout the summer of 2006.
The calyx was removed from whole flowers and the gynoecium immersed in a modified PIPES buffer (PIPES 60 mM, Hepes 25 mM, CaCl2 5.4 mM, MgCl2 1.97 mM). Ovules were dissected away from the gynoecium and placed in a fixative solution containing 4% paraformaldehyde, 2.5% glutaraldehye and 4% acrolein in the modified PIPES buffer (above). Ovules were dehydrated in a graded ethanol series, infiltrated, and embedded with glycol methacrylate (JB-4 embedding kit; Polysciences, Warrington, PA, USA). Embedded ovules were sectioned into 5-µm thick ribbons using a Microm HM330 rotary microtome and glass knives. Sections were stained with 0.1% toluidine blue and photographed with a Zeiss Axiophot microscope equipped with a Zeiss Axiocam digital camera using both brightfield and differential interference contrast optics.
Selected serial sections were optically sectioned and photographed using a Leica TCS SP2 AOBS laser scanning confocal microscope. Images were created by exciting the toluidine blue stain at 633 nm with a helium/neon laser, with detectors set to absorb within the 640–700 nm range. Each Z-stack of a single serial section consisted of 8 to 10 0.5-µm thick optical sections. The Z-stacks from each serial section were aligned and modelled using the IMOD software package (Kremer, Mastronarde & McIntosh, 1996).
Post-processing of all images was carried out with the Adobe Creative Suite 2 software package and image manipulations were restricted to processes that applied to the entire image unless otherwise noted in specific figure legends.
RESULTS
Megasporogenesis
In Aristolochia labiata, the megasporocyte is densely cytoplasmic, approximately 30 µm long and has a large centrally located nucleus (Fig. 1). Meiotic division of this cell produces a usually linear tetrad of megaspores. The chalazal-most cell becomes the functional megaspore, while the other three cells degenerate (Fig. 2). Initiation of female gametophyte development begins when a vacuole expands at the chalazal end of the functional megaspore (Fig. 3). Vacuole expansion correlates with an increase in size of the functional megaspore (Figs 4, 5 and supporting Fig. 27).
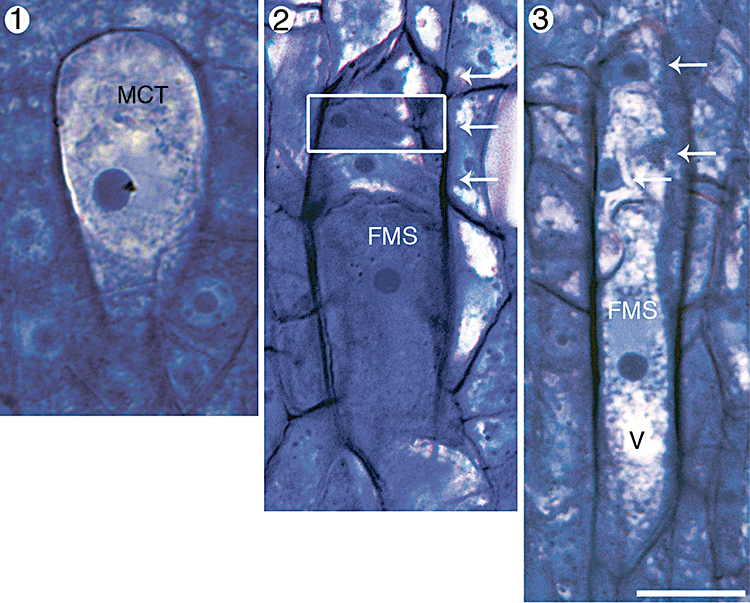
Megasporogenesis in Aristolochia labiata. Each image is oriented so that the micropyle is towards the top of the page and the chalaza is towards the bottom. Portions of other serial sections that have been inserted into these composite images are outlined with a white box. Abbreviations: FMS, functional megaspore; MCT, megasporocyte; V, vacuole. Arrows indicate degenerating megaspore cells. Scale bar, 10 µm. Fig. 1. Megasporocyte. Fig. 2. Megaspore tetrad. The larger chalazal-most megaspore is the functional one. Fig. 3. Megaspore tetrad. Vacuolar expansion has begun in the chalazal end of the functional megaspore.
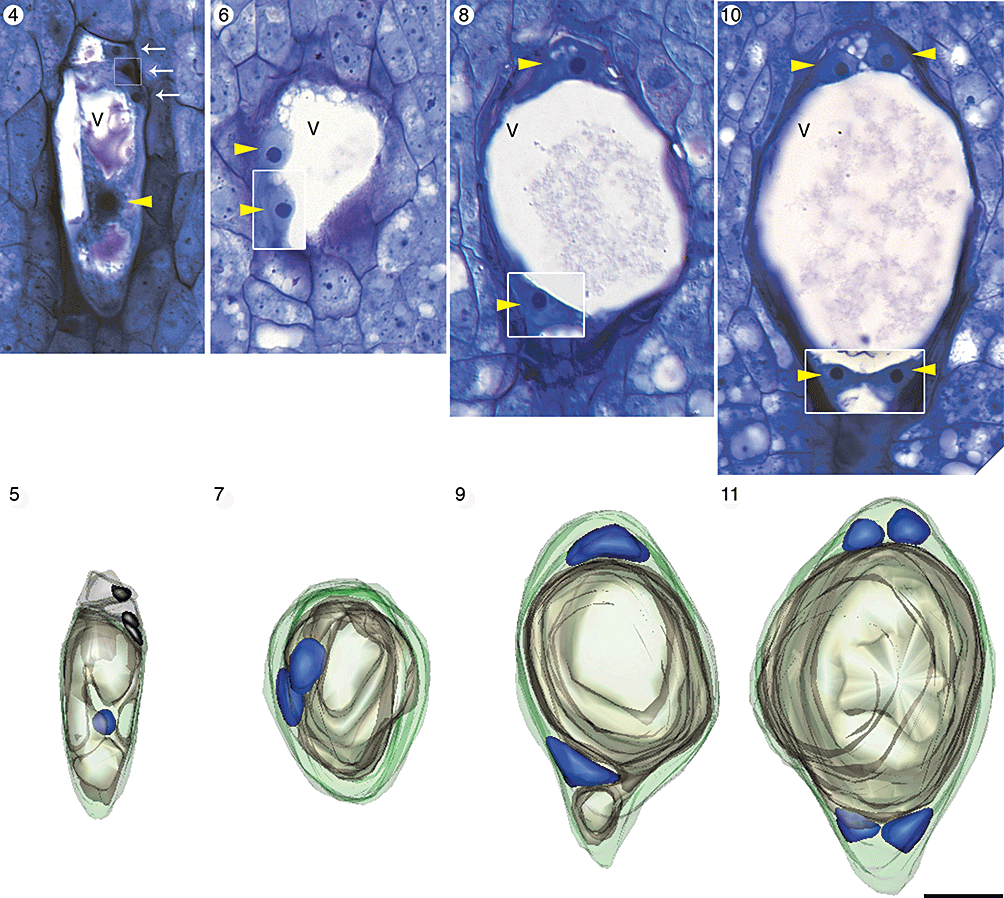
Light micrographs and three-dimensional computer reconstructions of megagametogenesis in Aristolochia labiata up to the four-nucleate stage of development. Each image is oriented so that the micropyle is towards the top of the page and the chalaza is towards the bottom. The same ovule has been used to make the composite longitudinal section and computer reconstruction in each column. Portions of other serial sections that have been inserted into the composite micrographs are outlined with a white box. In each model nuclei are blue, the central vacuole is tan and the wall of the megasporangium is green. Abbreviations: N, nucleus; V, central vacuole. Yellow arrowheads indicate nuclei. White arrows indicate degenerating megaspore cells. Scale bar, 10 µm. Figs 4, 5. Vacuolate functional megaspore. Degenerating megaspores are still visible. Figs 6, 7. Two-nucleate female gametophyte immediately following the first free-nuclear mitotic division. Figs 8, 9. Later two-nucleate stage in which each nucleus has migrated to an opposing pole of the female gametophyte. Figs 10, 11. Four-nucleate stage of development.
Female gametophyte development and cytokinesis
The first free-nuclear mitotic division of the female gametophyte occurs within a parietal band of cytoplasm, midway along the micropylar–chalazal axis, when the functional megaspore is approximately 40 µm long (Figs 6, 7 and supporting Fig. 28). Following this division, each nucleus migrates to an opposing pole of the female gametophyte – one to a location nearest the micropyle and the other closest to the chalaza (Figs 8, 9 and supporting Fig. 29). A single dominant vacuole (Fig. 5) fills the majority of cell volume prior to the first free-nuclear mitotic division (Figs 6, 7) and occupies the space between the two nuclei once they reach the micropylar and chalazal poles (Figs 8, 9).
Further cellular and vacuolar expansion brings the female gametophyte to approximately 50 µm in length and a second round of free-nuclear mitosis ensues. These mitotic divisions are not followed by nuclear migration events. The four resulting nuclei are arranged in two pairs at the micropylar and chalazal poles (Figs 10, 11 and supporting Fig. 30). In every four-nucleate embryo sac observed, the nuclei at each pole of the female gametophyte were arranged in a single plane along the micropylar–chalazal axis (N = 25).
When the gametophyte is approximately 75 µm long, a final round of free-nuclear mitosis produces eight nuclei (Figs 12–14 and supporting Fig. 31). We observed no eight-nucleate syncytial female gametophytes, making it reasonable to assume that cytokinesis immediately follows this set of mitotic divisions. We found six gametophytes in which the last round of mitosis had recently occurred and cytokinesis had cleaved the syncytium into seven cells. In these female gametophytes, the nuclei were partitioned into three antipodal cells, two synergid cells, an egg cell and a binucleate central cell with several trans-vacuolar strands of cytoplasm (Figs 12–14). Thus, the female gametophyte of A. labiata is Polygonum type, with eight nuclei and seven cells.
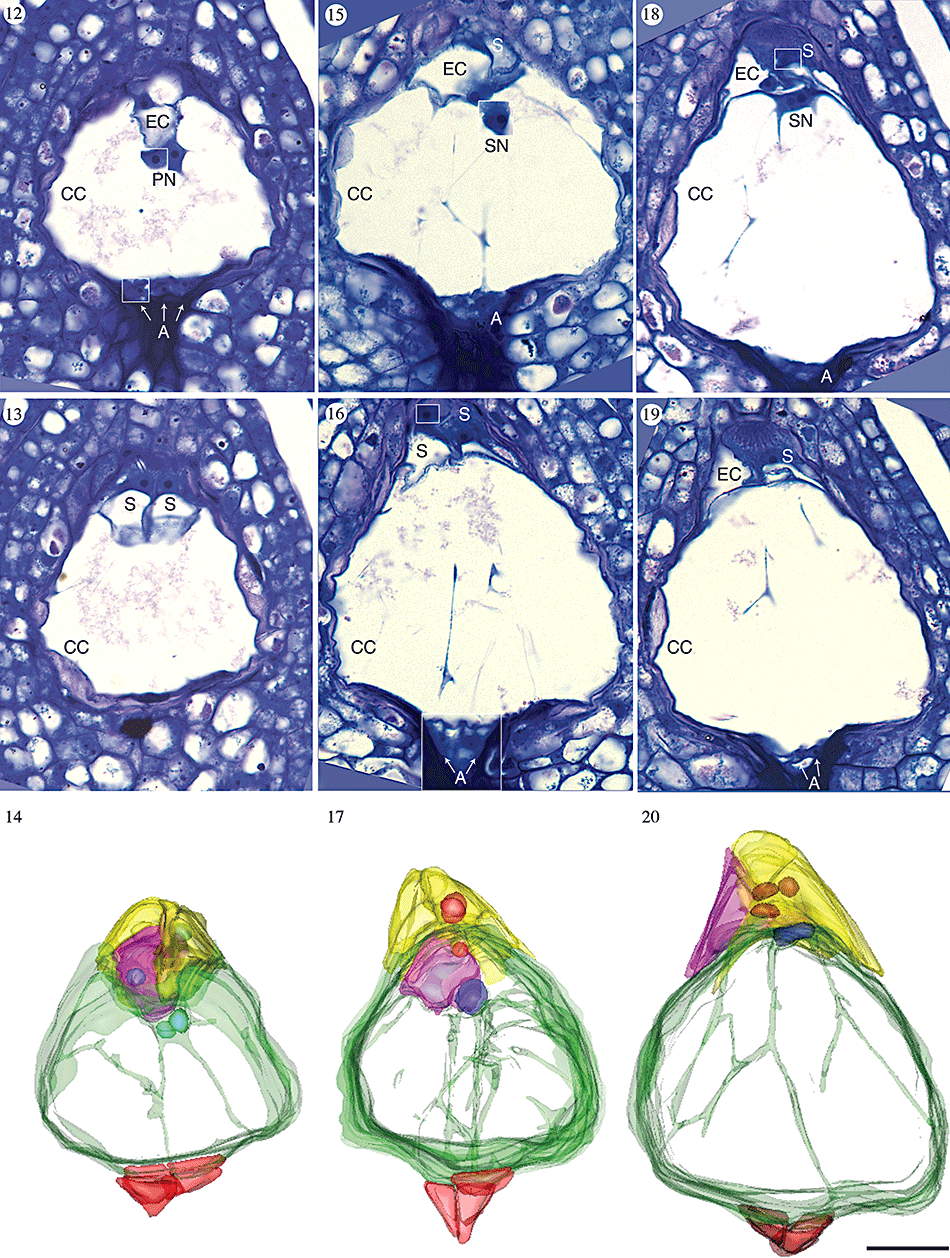
Light micrographs and three-dimensional computer reconstructions of cellularized female gametophytes in Aristolochia labiata. Each image is oriented so that the micropyle is towards the top of the page and the chalaza is towards the bottom. The same ovule has been used to make the composite longitudinal section and computer reconstruction in each column. Portions of other serial sections that have been inserted into the composite micrographs are outlined with a white box. In each model synergids are yellow, the egg cell is pink, the central cell is green, antipodals are red, nuclei in the egg apparatus are pink and the polar nuclei/secondary nucleus is blue. The vacuole of the central cell is clear, allowing trans-vacuolar cytoplasmic strands to be visualized. Abbreviations: A, antipodal; CC, central cell; EC, egg cell; S, synergid. Scale bar, 10 µm. Figs 12–14. Female gametophyte immediately following cytokinesis. The polar nuclei remain unfused and suspended in cytoplasm immediately below the egg apparatus. Figs 15–17. Female gametophyte immediately following fusion of polar nuclei. Figs 18–20. Female gametophyte at latest stage of development observed, with highly vacuolate central cell.
When first formed, the egg apparatus is diamond shaped and each cell has a face in contact with the micropylar boundary of the female gametophyte (Fig. 14). The individual cells of the egg apparatus can be identified by their cytology. The synergids are densely cytoplasmic and their nuclei are located in the micropylar-most region of the cells (Fig. 13). In contrast, the egg cell is largely vacuolate with a centrally located nucleus, stabilized by strands of cytoplasm (Fig. 12). All three cells of the egg apparatus fit within a depression of the otherwise ovoid highly vacuolate central cell (Fig. 14).
The two polar nuclei are positioned near the egg apparatus, within a small volume of cytoplasm along the boundary of the central cell (Fig. 12). In all ovules examined, the antipodals were densely cytoplasmic, triangular in sectional view, and grew in size from their initial formation until the fusion of the polar nuclei (Figs 14, 17, 20; N = 14).
Female gametophyte maturation
Following cellularization, the female gametophyte continues to increase in size, primarily because of expansion of the central cell. This transforms the micropylar-most boundary of the central cell from concave (Fig. 14) to convex (Figs 15–17, 32). Eventually the micropylar end of the central cell becomes completely rounded and the egg apparatus is pushed into the surrounding nucellus (Fig. 20). The egg apparatus assumes a pyramidal shape (Figs 18–20, 33) and the female gametophyte increases in length from approximately 75 µm (immediately following cytokinesis) to 100 µm at the most mature stages observed.
After cellularization of the syncytial gametophyte, each synergid develops a dark, cytoplasmically dense zone within the micropylar-most region of the cell (Fig. 21) that will become the filiform apparatus. This region attains a characteristic fibrillar texture prior to the fusion of the polar nuclei (Fig. 22) and, by the time the polar nuclei fuse, it is not possible to distinguish between the filiform apparatus of each synergid cell (Fig. 23). In the latest stages of development observed, the filiform apparatus occupies approximately one-tenth of the synergid cell volume and the cell wall infoldings are prominent (Fig. 24).
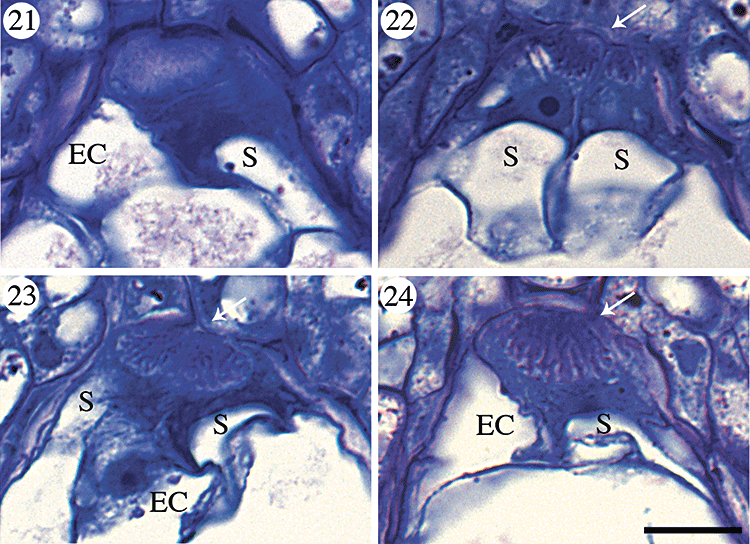
Filiform apparatus development in Aristolochia labiata. Each image is oriented so that the micropyle is towards the top of the page and the chalaza is towards the bottom. Abbreviations: EC, egg cell; S, synergid; arrows indicate the filiform apparatus in synergid cells. Scale bar, 10 µm. Fig. 21. Dark cytoplasmically dense zone within synergid cell immediately following cellularization of the female gametophyte. Fig. 22. Characteristic fibrillar texture of filiform apparatus within synergid cells of the egg apparatus. Fig. 23. Filiform apparatus within synergid cells immediately following fusion of the polar nuclei. Fig. 24. Largest filiform apparatus observed within synergid.
As the central cell expands, the polar nuclei fuse to form the secondary nucleus (Fig. 15). The nucleoli of each polar nucleus remain visible within the initially round secondary nucleus (Fig. 15). The secondary nucleus eventually assumes a cylindrical shape (Figs 18, 20). After fusion of the polar nuclei, the antipodals decrease in volume, their walls appear wrinkled and the cells become slightly difficult to identify in sectional view (Figs 18–20). In all mature female gametophytes observed, the antipodals were always visible although sometimes they were extremely small (N = 14).
DISCUSSION
Megasporogenesis
Multiple megasporocytes and even female gametophytes within a single ovule have been reported in Aristolochia bracteata (Johri & Bhatnagar, 1955), Asarum europaeum (Jacobsson-Stiasny, 1918; González & Rudall, 2003) and Thottea siliquosa (Nair & Narayanan, 1961; González & Rudall, 2003). However, in each case, these phenomena were rare and not part of the standard mode of embryo sac formation. Reports of fluctuating megaspore orientation between linear and T-shape are also common in Aristolochiaceae (Jacobsson-Stiasny, 1918; Johri & Bhatnagar, 1955; Nair & Narayanan, 1961; González & Rudall, 2003). We did not observe multiple megasporocytes or female gametophytes in any of the ovules of A. labiata we examined (N = 87) and consistently found megaspores in a linear arrangement.
Recent molecular phylogenetic analyses place the monotypic Lactoris (Qiu et al., 1999, 2000; Soltis et al., 2000; Hilu et al., 2003; Davis et al., 2004, 2006; Wanke et al., 2007a) and holoparasitic subterranean lineages Hydnora and Prosopanche within Aristolochiaceae (Nickrent et al., 2002). Megasporogenesis in Lactoris is similar to that of genera that have long been considered members of Aristolochiaceae (Thottea, Saruma, Aristolochia and Asarum). Female gametophyte development in Lactoris is monosporic Polygonum-type and originates from the chalazal-most megaspore (Tobe et al., 1993). Megasporogenesis in Hydnora and Prosopanche is very different from that of other genera in Aristolochiaceae. Prosopanche is reportedly bisporic (Chodat, 1916; Cocucci, 1976, 1983). Dastur & Bombay (1921) concluded that megasporogenesis in Hydnora africana was tetrasporic; however, their drawings can easily be interpreted as showing bisporic development. For now, it is best to view megasporogenesis in Hydnora as unknown but likely polysporic.
Syncytial development
All previous descriptions of the syncytial stages of female gametophyte development in monosporic members of Aristolochiaceae, including A. labiata, are fundamentally similar (Jacobsson-Stiasny, 1918; Johri & Bhatnagar, 1955; Wyatt, 1955; Nair & Narayanan, 1961; González & Rudall, 2003). Development begins when the functional megaspore nucleus mitotically divides without cytokinesis. The two resulting sister nuclei become positioned at opposite poles of the embryo sac, where they each go through two rounds of free-nuclear mitosis to produce eight nuclei that will be partitioned into seven cells.
We modelled vacuole morphology throughout development in A. labiata (Figs 5, 7, 9) and found that vacuolar expansion begins at the one-nucleate stage of development, before any free-nuclear mitotic divisions have occurred. Several reviews of female gametophyte development across angiosperms suggest that expansion of the central vacuole between the two nuclei following the first mitosis is the mechanistic force behind proper nuclear positioning to two poles of the female gametophyte (Schnarf, 1936; Haig, 1990; Johri et al., 1992). However, vacuolar expansion occurs independently of nuclear migration in A. labiata, indicating that the central vacuole alone cannot be the causal force behind this critical nuclear migration event. Other studies of female gametophyte development in monosporic members of Aristolochiaceae (Jacobsson-Stiasny, 1918; Wyatt, 1955; Tobe et al., 1993; González & Rudall, 2003), monocots (Huang & Sheridan, 1994, 1996), eudicots (Folsom & Cass, 1989; Floyd, Lerner & Friedman, 1999) and eumagnoliids (Sampson, 1969; Tobe & Sampson, 2000; Heo et al., 2004) contain drawings or photographs similar to our three-dimensional computer-generated images. These data indicate that our findings are not unique to Aristolochiaceae, but widespread throughout angiosperms.
It is important to note, however, that timing of central vacuole expansion during early female gametophyte development is variable among angiosperms. In Arabidopsis thaliana, vacuolization occurs only after the first free-nuclear mitotic division, at the two-nucleate stage of development (Misra, 1962; Poliakova, 1964; Schneitz, Hulskamp & Pruitt, 1995; Schneitz et al., 1997; Webb & Gunning, 1990). Although it is not clear what role, if any, vacuolar expansion plays in female gametophyte development (other than to drive growth), its implied causal function to separate nuclei to opposite poles of the female gametophyte (Schnarf, 1936; Haig, 1990; Johri et al., 1992) may lack empirical support.
Antipodals
Most studies of female gametophyte development in Aristolochiaceae report that antipodals are present at the time of fertilization (Jacobsson-Stiasny, 1918; Johri & Bhatnagar, 1955; Wyatt, 1955; Tobe et al., 1993; González & Rudall, 2003). Nair & Narayanan (1961) indicated that antipodals degenerate in Bragantia wallichii (now Thottea siliquosa) shortly after being formed, but González & Rudall (2003) re-examined female gametophyte development in Thottea and found that antipodals persist through fertilization. Dastur & Bombay (1921) reported antipodals may degenerate before fertilization in Hydnora; however, they did not provide micrographic evidence of this phenomenon. For now, antipodal persistence in Hydnora is best viewed as unknown.
Our data show a decrease in antipodal volume after fusion of the polar nuclei in A. labiata, but we did not observe complete antipodal degeneration in any of our preparations. In Laurales, Magnoliales and Cannelales, antipodals are absent at fertilization (Davis, 1966; Sampson, 1969; Tobe & Sampson, 2000; Kimoto & Tobe, 2001, 2003; Heo et al., 2004; Williams & Friedman, 2004; Kimoto et al., 2006). Antipodal persistence in Aristolochiaceae appears to represent a derived condition from the plesiomorphic state (antipodal degeneration; see Williams & Friedman, 2004) within the eumagnoliids.
Reconstructing the ancestral pattern of female gametophyte development in Piperales
General features of female gametophyte development among monosporic members of Aristolochiaceae include vacuole expansion at the one-nucleate stage of development, antipodal persistence through the time of fertilization and Polygonum-type female gametophyte development. If transitions between female gametophyte ontogenies are assumed to be unordered and reversible, an unweighted strict parsimony analysis combining known mature female gametophyte structure (Johnson, 1900a, b, 1914; Chodat, 1916; Jacobsson-Stiasny, 1918; Dastur & Bombay, 1921; Quibell, 1941; Swamy, 1944; Maheshwari, 1950; Johri & Bhatnagar, 1955; Wyatt, 1955; Murty, 1960; Raju, 1961; Davis, 1966; Cocucci, 1976, 1983; Plyushch, 1982a, b;Johri et al., 1992; Tobe et al., 1993; Lei et al., 2002; González & Rudall, 2003; Arias & Williams, 2008) and the most recent molecular phylogenies (Jaramillo & Manos, 2001; Wanke et al., 2006a, b, 2007a, b) leaves female gametophyte development in the common ancestor of Piperales equivocal – it is equally parsimonious for the common ancestor to be monosporic or bisporic (Fig. 25). However, basic long-standing assumptions about female gametophyte evolution can help to generate reasonable conclusions concerning character state polarities and transitions within Piperales.
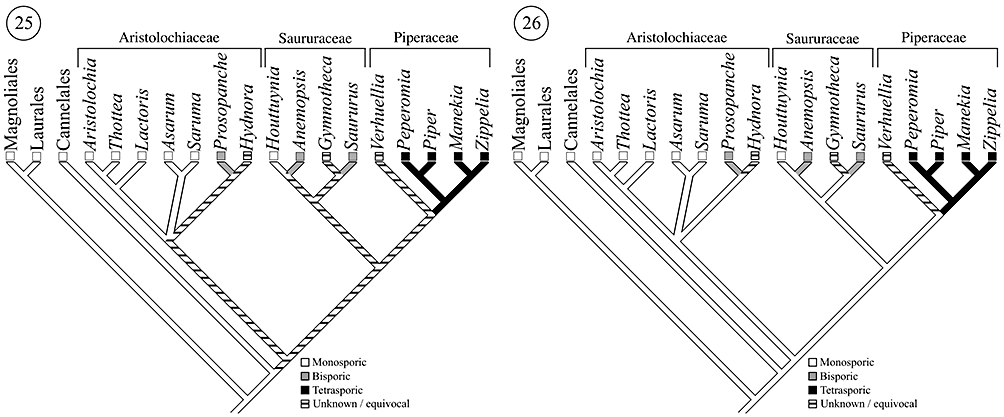
Systematic relationships adapted from (Nickrent et al., 2002; APG II, 2003;Jaramillo et al., 2004; Zanis et al., 2003; Wanke et al., 2006a, b, 2007a, b). Fig. 25. Unweighted strict parsimony analysis of megasporogenesis throughout eumagnoliids, assuming transitions to polyspory are reversible. Fig. 26. Developmental evolution of megasporogenesis throughout eumagnoliids assuming transitions to polyspory are irreversible.
It has long been assumed that bispory and tetraspory evolve from monosporic ancestors (Porsch, 1907; Coulter, 1908; Johnson, 1914; Maneval, 1914; Haig, 1990; Donoghue & Scheiner, 1992) and implicit in these ideas is that the transition from monospory to polyspory is irreversible. Recent theoretical analyses concerning female gametophyte developmental evolution also predict that the potentially higher ploidy, higher levels of heterozygosity and reduced levels of genetic conflict (associated with interparental and/or parent–offspring genetic conflict; see Charnov, 1979; Westoby & Rice, 1982; Queller, 1983, 1994; Wilson & Burley, 1983; Haig, 1986, 1987; Haig & Westoby, 1988; Friedman, 1995) in endosperms derived from polysporic female gametophytes may have potentially adaptive consequences (Friedman, Madrid & Williams, 2008). If the transition to polyspory is irreversible, the common ancestor of Piperales was almost certainly monosporic and Polygonum type; bispory evolved at least three times and tetraspory at least once (depending on the actual condition in Hydnora) (Fig. 26).
Piperales represents one of the largest developmental radiations of female gametophyte structure in all of angiosperms. Based on our analysis, we have demonstrated that monosporic female gametophyte development in Aristolochiaceae can serve as a starting point for the reconstruction of the evolution of derived female gametophyte ontogenies among Piperales. In particular, as will be seen (E. Madrid, unpubl. data), the timing and positional aspects of vacuole expansion and nuclear migration events in female gametophyte development will be important components of future comparative studies within derived members of Piperales.
ACKNOWLEDGMENTS
We thank Eileen O'Toole of the CU-Boulder laboratory for the 3-D electron microscopy of cells for assistance with the IMOD software package. This work was supported by a grant from the National Science Foundation to WEF (IOB-0446191).




