Molecular phylogeography reveals an antitropical distribution and local diversification of Solenogyne (Asteraceae) in the Ryukyu Archipelago of Japan and Australia
Abstract
An antitropical distribution represents an intriguing disjunction, in which a given species or sister lineages occupy regions north and south of the tropics but are absent from the intervening areas. Solenogyne mikadoi endemic to the Ryukyu Archipelago is regarded as an Australian element. Testing the phylogenetic relationship with Australian congeners and discussing the onset timing and causes of the disjunction would potentially enhance the understanding of antitropical distribution. A nuclear ribosomal DNA phylogeny was reconstructed using Bayesian and most parsimonious criteria with allied genera. Solenogyne was monophyletic and clustered with Lagenophora huegelii endemic to Australia, indicating the antitropical distribution and Australian origin of Solenogyne. Multispecies coalescent analysis based on nuclear ribosomal DNA and chloroplast DNA indicated the divergence of S. mikadoi and Australian congeners in the Plio-Pleistocene. Phylogenetic network analyses suggested that the ancestral lineage of S. mikadoi first colonized the southernmost island in the archipelago and then dispersed northward. The migration to the archipelago likely followed the flourishing of Solenogyne in open vegetation communities that radiated in south-eastern Australia during the late Pliocene. This disjunction might arise through long-distance dispersal across the tropics or, alternatively, through extinction in the tropics as a result of unsuitably high temperatures during climate oscillation and/or competitions from diverse tropical flora surviving since the early Tertiary. © 2011 The Linnean Society of London, Biological Journal of the Linnean Society, 2012, 105, 197–217.
INTRODUCTION
An antitropical (or amphitropical) distribution represents an intriguing pattern of disjunct occurrence of organisms at a global scale, in which the same taxon or sister taxa occupy regions north and south of the tropics but are absent from the intervening tropical regions (Hubbs, 1952; Randall, 1981; Donoghue, 2011). Such a distribution has mostly been observed for marine organisms (e.g. algae, fishes, molluscs and seals in North and South America, Asia and Australasia, and Europe and Africa; Ekman, 1953; Pielou, 1979; Santelices, 1980; Briggs, 1995; Hilbish et al., 2000; Burridge, 2002; Mabuchi, Nakabo & Nishida, 2004). Reports of land plants showing antitropical distributions are mainly limited to disjunctions between North and South America (Constance, 1963; Raven, 1963; Wen & Ickert-Bond, 2009; Spalik et al., 2010; Popp, Mirré & Brochmann, 2011). To increase our understanding of this phenomenon in plants, more studies in other regions are needed. These would potentially increase our knowledge on intercontinental disjunctions, for which taxonomists and biogeographers have attempted to elucidate their onset timing and causes (Gray, 1878; Raven, 1963; Thorne, 1972; Raven & Axelrod, 1974; Wu, 1983; Wen, 1999; Parris, 2001; Wen & Ickert-Bond, 2009).
The Ryukyu Archipelago is an assemblage of continental islands between the main Japanese islands and Taiwan (Fig. 1). The archipelago lies in the subtropics and, as a result of the moderate climate throughout the year and an annual precipitation of > 2000 mm with no dry season, the dominant natural vegetation of the islands is broad-leaved and evergreen (Maekawa, 1974; Nakamura et al., 2009). The flora of the Ryukyu Archipelago can be divided approximately into six groups according to their assumed origins: from main Japanese islands, the East Asian continent, the East Asian continent via migration initially to the island of Taiwan, from South-east Asia without migration via Taiwan, from Pacific islands, and from Australia (Hatusima, 1975, 1980). Among the six groups, the most intriguing are the Australian elements because the Ryukyu Archipelago and Australia are more than 7000 km apart, in the northern and southern hemisphere, respectively. Among the Australian components, multiple species show disjunct distributions or have close relatives in eastern Australia; for example Cassytha glabella R.Br. and Cassytha pubescens R.Br. (Lauraceae), Oxalis exilis Cunn. (Oxalidaceae), Lobelia loochooensis Koidz. (Campanulaceae), Solenogyne mikadoi Koidz. (Asteraceae), and Eriachne armitii F.Muell. ex Benth. (Poaceae) (Adams, 1979; Hatusima, 1985; Murata, 1992; Shinjyo & Ikehara, 2006; Yokota & Hiraiwa, 2006; Yokota & Shinzato, 2006a, b). Thus, these are typical examples of antitropical distributions.
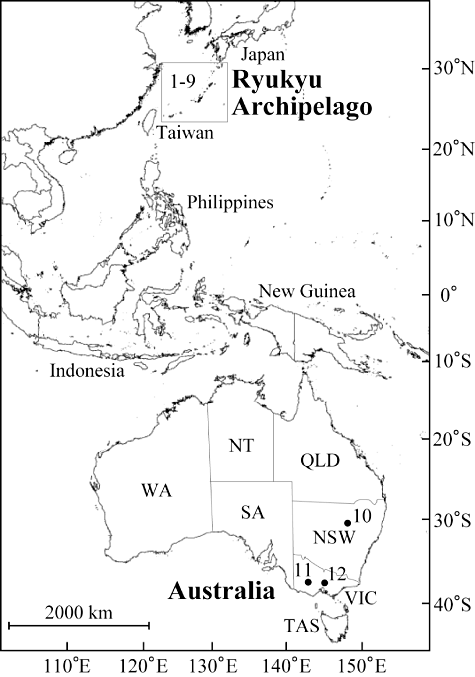
In the present study, we investigated in detail the genus Solenogyne Cass., a small genus in the tribe Astereae that comprises four species: Solenogyne bellioides Cass., Solenogyne dominii L.G.Adams, Solenogyne gunnii (Hook.f.) Cabrera, and S. mikadoi Koidz. (Adams, 1979). These are all small rosette-forming perennial herbs. The genus exhibits an extremely disjunct distribution (Fig. 1); S. mikadoi is endemic to the Ryukyu Archipelago, whereas the other three species are endemic to temperate south-eastern Australia (Brown & Porteners, 1992). Solenogyne mikadoi occurs on four relatively large islands with well-developed river systems, namely, Amamioshima, Tokunoshima, Okinawajima, and Iriomotejima (Fig. 2, Table 1), and grows on rocky beds of mountain streams (Yokota & Hiraiwa, 2006). In Australia, S. bellioides is reported from New South Wales and Queensland, S. dominii from New South Wales and Victoria, and S. gunnii from New South Wales, Victoria, and Tasmania (Fig. 1) (Brown & Porteners, 1992). The three Australian species grow in open sclerophyll vegetation with sparse overstorey trees or in anthropogenic pastures, and their habitats are drier than those of S. mikadoi in the Ryukyu Archipelago. The achenes (fruits) of Solenogyne have no pappi (feather-like appendages) commonly observed in Asteraceae (Davis, 1950), and thus do not appear to be adapted for long-distance dispersal. Solenogyne is considered to be closely allied to Lagenophora Cass. (Davis, 1950; Cabrera, 1966; Adams, 1979) and some studies have synonymized Solenogyne with Lagenophora (Bentham, 1867; Maiden & Betche, 1916; Drury, 1974). Therefore, to represent an antitropical distribution, the monophyly of Solenogyne needs to be demonstrated. Lagenophora comprises 15–36 species and is broadly distributed in India, South-east and East Asia, Australia, New Zealand, the Pacific islands, and Central and South America (Cabrera, 1966; Porteners & Brown, 1992; Mabberley, 1997; Mill, 1999). Lagenifera Cass. is an etymological variant of Lagenophora, and the latter is the conserved name for this genus (Australian National Botanic Gardens, 2009). Although S. mikadoi was once named L. mikadoi (Koidz.) Koidz. ex H.Koyama (Koyama, 1995), the two genera are morphologically distinct; in Solenogyne, ray florets are tubular and the achenes have neither beaks nor glands, while in Lagenophora, ray florets are ligulate and the achenes have glandular beaks (Davis, 1950; Cabrera, 1966; Adams, 1979). However, their phylogenetic relationship has not been studied using molecular data. Molecular phylogenetic studies can reveal intercontinental disjunct distributions and can provide information on the timing of the onset of disjunctions (Vijverberg, Mes & Bachmann, 1999; Xiang et al., 2000; Givnish & Renner, 2004; Barker et al., 2007; Namoff et al., 2010; Rowe et al., 2010). In previous studies, it was found that some taxa, which had been assumed to be typical examples of intercontinental disjunct distributions, were not monophyletic (Soltis et al., 1991; Qiu, Chase & Parks, 1995; Soltis & Kuzoff, 1995; Soltis, Xiang & Hufford, 1995; Kim & Jansen, 1998), hence reinforcing the need for phylogenetic analyses of taxa exhibiting global scale disjunct distributions. Antitropical distributions for vascular plants in the Ryukyu Archipelago and eastern Australia have not previously been tested with molecular phylogenies.
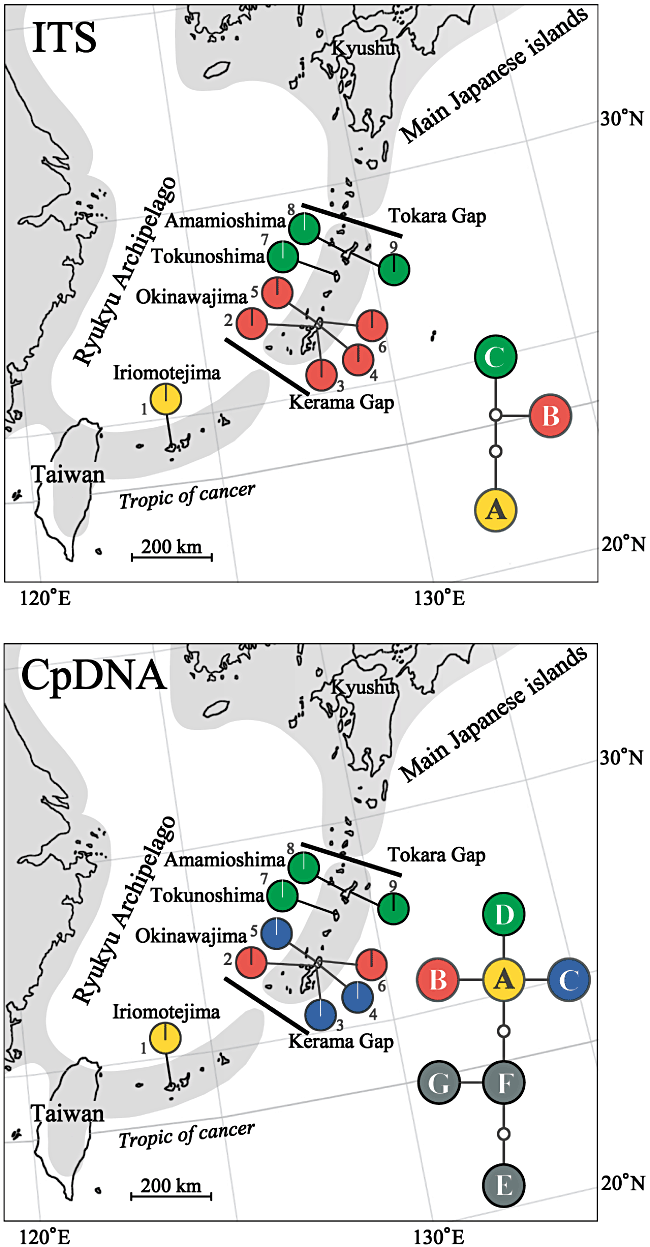
Geographical distributions of the internal transcribed spacer (ITS) types (A–C) and chloroplast DNA (cpDNA) haplotypes (A–D) of Solenogyne mikadoi in the Ryukyu Archipelago and their statistical parsimony networks. CpDNA haplotypes E, F, and G were found in S. bellioides, S. dominii, and S. gunnii, respectively. Small open circles indicate hypothetical (i.e. extinct or not sampled) types, and each line connecting the ITS and cpDNA types indicates one mutational change. The numbers indicate the collection localities; for details, see Table 1. Shaded areas in the map indicate land configuration in the early Pleistocene after splitting of the land bridge by the formation of the Tokara and Kerama gaps (Ota, 1998).
| Locality code* | Locality | Sample number | CpDNA haplotype | Accession | nrDNA type | Accession | Voucher no. | |
|---|---|---|---|---|---|---|---|---|
| atpB-rbcL | trnK3′ intron | ITS | ||||||
| Solenogyne mikadoi Koidz. | ||||||||
| 1 | Urauchigawa River, Iriomotejima Island, Japan. 30 m alt. | 5 | A | AB543921 | AB543925 | A | AB543934 | NK20100009a – NK20100009e |
| 2 | Taihogawa River, Okinawajima Island, Japan. 200 m alt. | 6 | B | AB543921 | AB543926 | B | AB543935 | NK20100004a – NK20100004f |
| 3 | Haramatagawa River, Okinawajima Island, Japan. 180 m alt. | 4 | C | AB543921 | AB543927 | B | AB543935 | NK20100006a – NK20100006d |
| 4 | Hijigawa River, Okinawajima Island, Japan. 150 m alt. | 3 | C | AB543921 | AB543927 | B | AB543935 | NK20100007a – NK20100007c |
| 5 | Ukagawa River, Okinawajima Island, Japan. 140 m alt. | 3 | C | AB543921 | AB543927 | B | AB543935 | NK20100008a – NK20100008c |
| 6 | Arakawa River, Okinawajima Island, Japan. 160 m alt. | 4 | B | AB543921 | AB543926 | B | AB543935 | NK20100005a – NK20100005d |
| 7 | Akirikamigawa River, Tokunoshima Island, Japan. 200 m alt. | 6 | D | AB543920 | AB543925 | C | AB543936 | NK20100003a – NK20100003f |
| 8 | Kawauchigawa River, Amamioshima Island, Japan. 80 m alt. | 2 | D | AB543920 | AB543925 | C | AB543936 | NK20100002a – NK20100002b |
| 9 | Sumiyogawa River, Amamioshima Island, Japan. 100 m alt. | 5 | D | AB543920 | AB543925 | C | AB543936 | NK20100001a – NK20100001e |
| Solenogyne bellioides Cass. | ||||||||
| 10 | 4.7 km south-east of Nevertire on Route 32, New South Wales, Australia. 335 m alt. | 3 | E | AB543924 | AB543928 | D | AB543933 | NK20100010a – NK20100010c |
| AB604756 | ||||||||
| AB604757 | ||||||||
| Solenogyne dominii L.G.Adams | ||||||||
| 11 | 20 km east of Dadswells Bridge on Western Highway, Victoria, Australia. 260 m alt. | 2 | F | AB543923 | AB543929 | E | AB543932 | NK20100011a – NK20100011b |
| AB604758 | ||||||||
| Solenogyne gunnii (Hook.f.) Cabrera | ||||||||
| 12 | Glen Waverlay Garrisson Drive, Melbourne, Victoria, Australia. 80 m alt. | 4 | G | AB543922 | AB543930 | F | AB543931 | NK20100012a – NK20100012d |
| AB604759 | ||||||||
| AB604760 | ||||||||
| AB604761 | ||||||||
In the Ryukyu Archipelago, the distribution of S. mikadoi is scattered on four islands and their mode of migration into the archipelago and range expansion is also of interest. The archipelago warrants attention from a biogeographical perspective because of the geohistory of island connections and separations (Ota, 1998; Otsuka, Ota & Hotta, 2000; Kimura, 2002a; Nakamura et al., 2009, 2010). These islands are of continental origin, and represent exposed portions of the Ryukyu Cordillera. This region underwent extensive changes in land configuration during the Cenozoic as a consequence of the subduction of the Philippine plate at the Ryukyu trench and subsidence of the Ryukyu trough (Ota, 1998), leading to crustal deformation and faulting. In addition, climatic oscillations have resulted in eustatic sea level changes. In the Neogene, the Ryukyu region formed the eastern margin of the East Asian continent. Subsequently, the island arc began to take its present day shape (Ota, 1998). Any discussion on plant migratory processes requires consideration of this geohistory.
In the present study, we conducted molecular phylogenetic analyses based on nuclear ribosomal DNA (nrDNA) and chloroplast DNA (cpDNA) sequences, aiming to: (1) test the Ryukyu–Australia antitropical distribution of Solenogyne; (2) discuss its onset timing and causes; and (3) elucidate the migratory processes of S. mikadoi in the Ryukyu Archipelago.
MATERIAL AND METHODS
Plant materials and DNA sampling
Solenogyne mikadoi is reported from nine river systems (Yokota & Hiraiwa, 2006) and we collected samples from almost all known localities, from one river system on Iriomotejima, from five on Okinawajima, from one on Tokunoshima, and from two on Amamioshima, respectively (Table 1). Because of the scarcity of the plants in each river system, we collected two to six plants per river system; in total 38 plants were collected. For the three Australian species, the collection of plants from many localities was difficult because the known sites (gathered from herbarium specimen data) were scattered very widely. Instead, multiple plants were collected from one locality for each species (three plants for S. bellioides, two for S. dominii, and four for S. gunnii, respectively; Fig. 1, Table 1). The three species were collected from different localities where only one species occurred; this was carried out to avoid collecting possible hybrids between them in mixed populations (Adams, 1979). These 47 plants in total were used for the analyses of nrDNA and cpDNA sequences.
To test the monophyly of Solenogyne, the internal transcribed spacer (ITS) region of nrDNA was used. For Lagenophora, eight species and one variety were analyzed (Table 2). We collected three species: Lagenophora gracilis Steetz, Lagenophora huegelii Benth., and Lagenophora lanata A.Cunn. For the other five species and the variety, the ITS sequences were obtained from databases (DDBJ/EMBL/GenBank). The analysis included all species of Lagenophora reported from Japan (one species; Hatusima, 1975), Australia (four species; Porteners & Brown, 1992; Australian National Botanic Gardens, 2009), and surrounding areas, namely, Taiwan, continental China, India, New Zealand, and Java (Hooker, 1881; Backer & van den Brink, 1965; Soejima & Peng, 1998; Chen & Jin, 2005), except Lagenophora pinnatifida Hook.f., which is endemic to New Zealand (five species, Allan, 1982). In addition, the analysis included 25 species from 24 Astereae genera (Table 2) to ensure that Lagenophora is the sister genus of Solenogyne and appropriate to test the monophyly of Solenogyne. It was reported that the major genera of Astereae are clearly separated into two groups based on the ITS sequence (Noyes & Rieseberg, 1999); we selected the genera from the group that included Lagenophora. We added some other genera not included in the above study, referring to the results of a phylogenetic study of the tribe based on the ITS sequences (Wagstaff & Breitwieser, 2002). The ITS sequences for genera other than Solenogyne and Lagenophora were obtained from databases (DDBJ/EMBL/GenBank). As outgroups, we selected Chiliotrichum diffusum and Olearia argophylla of basal Astereae clades (Noyes & Rieseberg, 1999) and phylogenetic trees were rooted on C. diffusum.
| Species | Subtribe* | Locality** or source | ITS accession |
|---|---|---|---|
| Lagenophora Cass. | |||
| L. cuneata Petrie | LAG | New Zealand, South Island | EU352246 |
| L. gracilis Steetz | LAG | Australia, Queensland. 650 m alt. NK20100013 (RYU) | AB550254 |
| L. huegelii Benth. | LAG | Australia, Victoria. 260 m alt. NK20100014 (RYU) | AB550255 |
| L. lanata A.Cunningham | LAG | Japan, Amamioshima Island 670 m alt. NK20100015 (RYU) | AB550256 |
| L. panamensis S. F. Blake | LAG | Panama, Chiriqui | AF046965 |
| L. pumila (G.Forst.) Cheeseman | LAG | New Zealand, South Island | AF422124 |
| L. pumila (G.Forst.) Cheeseman | LAG | New Zealand, S. A. Norton 598 (NO) | DQ479037 |
| L. pumila (G.Forst.) Cheeseman | LAG | New Zealand | EU352244 |
| L. stipitata (Labill.) Druce var. stipitata | LAG | Australia | AB435145 |
| L. stipitata (Labill.) Druce var. montana (Hook.f.) Cabrera | LAG | New Zealand | EU352243 |
| L. strangulata Colenso*** | LAG | New Zealand, North Island, Erua | EU352245 |
| Other genera | |||
| Aster amellus L. | AST | Russia, Northern Caucasus | AF046961 |
| Baccharis dracunculifolia DC. | BAC | Bolivia, La Paz | AF046958 |
| Bellis perennis L. | BEL | Bolivia, La Paz | AF046950 |
| Brachycome breviscapis C.R.Carter | BRA | Australia, South Australia | AB435100 |
| Calotis dentex R.Br. | BRA | Australia, Queensland | AF046956 |
| Celmisia mackaui Raoul | HIN | New Zealand, South Island | AF422115 |
| Chiliotrichum diffusum (Forst.) O.Kuntze | HIN | Chile, Cape Horn Island | AF046945 |
| Conyza gouanii (L.) Willd. | CON | Tanzania, Iringa | AF046948 |
| Conyza pyrrhopappa A.Rich. | CON | Tanzania, Tanga | AF046953 |
| Crinitaria linosyris (L.) Less. | AST | Russia, Saratov | AF046949 |
| Damnamenia vernicosa (Hook.f.) Given | HIN | New Zealand, Campbell Island | AF422119 |
| Diplostephium rupestre (H.B.K.) Wedd. | HIN | Ecuador, Napo | AF046962 |
| Kalimeris integrifolia Turcz. ex DC. | AST | China, Jiangsu | AF046960 |
| Laennecia sophiifolia (Kunth) Nesom | POD | Mexico, Oaxaca | AF046964 |
| Minuria integerrima (DC.) Benth. | POD | Australia, Queensland | AF046957 |
| Myriactis humilis Merr. | LAG | Taiwan, Pingtun Hsien | AF046959 |
| Nidorella resedifolia DC. | GRA | South Africa, Cape | AF046952 |
| Olearia argophylla (Labill.) F.Muell. ex Benth. | HIN | Australia, New South Wales | AF046944 |
| Oritrophium peruvianum (Lam.) Cuatrec. | HIN | J. Jaramillo et al. 21020 (QCA) | DQ479117 |
| Pachystegia insignis (Hook.f.) Cheeseman | HIN | New Zealand, South Island | AF422132 |
| Pleurophyllum speciosum Hook.f. | HIN | New Zealand, Campbell Island | AF422133 |
| Podocoma notobellidiastrum (Griseb.) Nesom | POD | Paraguay, Caazapa | AF046963 |
| Psiadia punctulata (DC.) Vatke | BAC | South Africa, Transvaal | AF046954 |
| Pteronia incana (Burm.) DC. | HIN | South Africa, Cape | AF046947 |
| Vittadinia australis A.Rich. | POD | New Zealand, South Island | AF422140 |
- * Subtribes sensuNesom & Robinson (2007): AST, Asterinae; BAC, Baccharidinae; BEL, Bellidinae; BRA, Brachycominae; CHR, Chrysopsidinae; CON, Conyzinae; FEL, Feliciinae; GRA, Grangeinae; HIN, Hinterhuberinae; LAG, Lageniferinae; MAC, Machaerantherinae; POD, Podocominae; SOL, Solidagininae; SYM, Symphyotrichinae.
- ** For cited sequences, localities were referred from Noyes & Rieseberg (1999), Wagstaff & Breitwieser (2002), and the DDBJ/EMBL/GenBank databases.
- *** Lagenophora strangulata is synonymized with Lagenophora petiolata Hook.f. in Cheeseman (1925).
Voucher specimens of our collections were deposited in the herbarium of the Faculty of Science, University of the Ryukyus (RYU; Tables 1, 2).
DNA extraction, amplification, and sequencing
Total DNA was isolated from leaf tissue by using the cetyl trimethyl ammonium bromide method of Doyle & Doyle (1987). The following three molecular markers were amplified using polymerase chain reaction (PCR): the ITS region (including ITS1 and ITS2 spacer regions and the 5.8S rRNA gene) of nrDNA, the intergenic spacer of the atpB and rbcL genes (atpB–rbcL), and the 3′ intron of the trnK gene (trnK3′ intron) of cpDNA. The PCR mixtures consisted of the reagents: 10–40 ng of genomic DNA, 1.0 unit of rTaq polymerase (TOYOBO, Osaka, Japan), 10 µL of rTaq DNA polymerase buffer containing 1.5 mM magnesium chloride, 0.2 mM of each dNTP, 0.25 µM of each primer, and 70.5 µL sterile water. With respect to the primers used for PCR amplification and the PCR cycle conditions: for the ITS region, we used universal primers 1 and 4 (White et al., 1990), with an initial template denaturation at 95 °C for 5 min. This was followed by one cycle at 97 °C for 2 min, 50 °C for 1 min, and 72 °C for 1 min; 25 cycles at 95 °C for 1 min, 50 °C for 2 min, and 72 °C for 3 min; and a final extension at 72 °C for 10 min. For the atpB–rbcL spacer, the primers atpB2F and rbcL2R were used (Nakamura et al., 2006), with an initial template denaturation at 95 °C for 5 min. This was followed by 25 cycles at 95 °C for 1 min, 57 °C for 1 min, and 72 °C for 2 min, and a final extension at 72 °C for 10 min (Nakamura et al., 2007). For the trnK3′ intron region, the universal primers trnK-3914F and trnK-2R (Johnson & Soltis, 1994) were used, with an initial template denaturation at 95 °C for 5 min, followed by 30 cycles at 94 °C for 1.5 min, 51 °C for 2 min, 72 °C for 3 min, and a final extension at 72 °C for 10 min. The PCR fragments were purified with shrimp alkaline phosphatase and exonuclease I (Promega). These were used as templates for cycle sequencing reactions, and direct sequencing was performed in accordance with the manufacturer's protocol on an ABI Prism 3730 DNA analyzer (Applied Biosystems). The sequences were deposited in the DDBJ/EMBL/GenBank databases (Tables 1, 2).
Analytical scheme and DNA data sets
To test the monophyly of Solenogyne, the ITS data set of Solenogyne plus the other Astereae genera was constructed (henceforth the Astereae data set) and analyzed based on Bayesian and maximum parsimony (MP) criteria. Because there was no variation in ITS within populations (Table 1), we used a single sample from each population as sufficient to test the monophyly of Solenogyne. To elucidate phylogenetic relationships among Solenogyne species and their divergence times, ITS and cpDNA data sets comprising only Solenogyne were made (including all the samples in Table 1; henceforth the Solenogyne data set). The analyses of the Solenogyne data set were conducted using Bayesian methods implemented in BEAST, version 1.6.1 (Drummond et al., 2006), which estimates the root of a phylogeny without using an outgroup by enforcing the (relaxed) molecular clock constraint (Drummond & Rambaut, 2007). BEAST calculates the proportion of trees that have a particular root based on Markov chain Monte Carlo (MCMC) simulation, and obtains a posterior probability for this root position. The ITS and cpDNA Solenogyne data sets were also used to elucidate phylogeny of S. mikadoi samples and their divergence times.
In the data set construction, the DNA sequences were aligned using CLUSTALX version 1.8 (Thompson et al., 1997) and then adjusted manually. The combinability of the atpB–rbcL and trnK3′ intron regions was determined with the incongruence length difference test (P = 1.00) (Farris et al., 1994) using the partition homogeneity test implemented in PAUP* version 4.0b10 (Swofford, 2002) and subsequent analyses were conducted on the combined data.
Tests of DNA substitution model and molecular clock hypothesis
Estimation of the appropriate DNA substitution models were conducted based on the Akaike information criterion using MODELTEST version 3.7 (Posada & Crandall, 1998); the model GTR+I+G was selected for the Astereae data set of ITS, the model TrN (also called TN93 in some software) for the Solenogyne data set of ITS, and the model HKY for the Solenogyne data set of cpDNA. Note the model TrN was also selected for the Astereae data set by hierarchical likelihood rate tests implemented in MODELTEST.
The molecular clock hypothesis was tested for each data set using the two-cluster test (Takezaki, Rzhetsky & Nei, 1995) and a molecular clock likelihood ratio test (LRT) (Felsenstein, 1988). The two-cluster test, implemented in LINTREE (Takezaki et al., 1995), examines whether there are significant differences in substitution rates in sister lineages in a given tree. Identical sequences in each data set were collapsed (Quek et al., 2004) and Neighbour-joining trees were constructed using the model TrN, because the models GTR+I+G and HKY were not implemented in the LINTREE software and the model TrN was similar to the models. The hypothesis of rate constancy for all sequences in a tree was tested simultaneously by Q-values of the U-statistic, which approximately follows a χ2 distribution with n − 1 degrees of freedom (d.f.), where n is the number of sequences. The molecular clock hypothesis was rejected at the significance level of P = 0.05 for the Astereae data set for ITS (Q = 1103.8, d.f. = 41, P < 0.0001) but not rejected for the Solenogyne data sets (ITS, Q = 7.8, d.f. = 5, P = 0.17; cpDNA, Q = 3.0, d.f. = 6, P = 0.70). The molecular clock LRT was conducted using PAUP*, by comparing the log likelihood (L) of maximum likelihood trees with and without assuming a molecular clock, based on the determined DNA substitution models. The likelihood ratio was calculated as 2(ln Lnoclock − ln Lclock) and assumed to follow a χ2 distribution with n − 2 degrees of freedom, where n is the number of sequences (Muse & Weir, 1992). Identical sequences in each data set were collapsed in the analyses. The results of the LRT agreed with the results of the two-cluster test. The molecular clock hypothesis was rejected for the Astereae data set (−ln Lnoclock = 4587.48, −ln Lclock = 4647.28, LRT = 119.60, d.f. = 40, P < 0.0001) but not rejected for the Solenogyne data set (ITS, −ln Lnoclock = 1064.83, −ln Lclock = 1069.60, LRT = 9.54, d.f. = 4, P = 0.05; cpDNA, −ln Lnoclock = 2389.11, −ln Lclock = 2393.57, LRT = 8.93, d.f. = 5, P = 0.11). Note, however, that the Solenogyne data set for ITS showed the marginal value of P = 0.05.
Phylogenetic analysis based on astereae data set
The first Bayesian phylogenetic analysis was conducted using MrBayes version 3.1.2 (Ronquist & Huelsenbeck, 2003) and the second analysis was conducted using BEAST version 1.6.1 (Drummond et al., 2006). MrBayes does not consider across-lineage variability of substitution rate, whereas BEAST can incorporate this into tree construction (Drummond & Rambaut, 2007). Both the complex (GTR+I+G) and simple (TrN) models were used for comparison.
With MrBayes, two separate runs of Metropolis-coupled Metropolis-coupled Markov chain Monte Carlo (MCMCMC) analyses were performed based on the models of GTR+I+G or TrN, with a random starting tree and four chains (one cold and three heated). The MCMCMC length was two million generations, and the chain was sampled every 100th generation from the cold chain. The mixing and convergence of the MCMC chains of the two runs was assessed by inspection of the trace plots of parameters using TRACER version 1.5.0 (Drummond & Rambaut, 2007); the first 2000 sample trees (10% of the total 20 000 sampled trees) were discarded as burn-in. After the burn-in, the effective sample sizes (ESS) of all parameters were > 200, indicating that the analyses sampled the posterior distributions of each parameter satisfactorily, and the values of Average Standard Deviation of Split Frequency (ASDSF) were below 0.01. The 50% majority rule consensus tree of all the post-burn-in trees was generated using TREEVIEW version 1.6.6 (Page, 1996).
To determine the models for BEAST analyses, we compared clock and relaxed-clock models to avoid over-parameterization although the molecular clock hypothesis was rejected (see above), and compared Yule and birth-death speciation priors for the branching rates. We used the Bayes factor (BF) [i.e. the ratio of the marginal likelihoods (L) of two models] (Kass & Raftery, 1995; Suchard, Weiss & Sinsheimer, 2001). The marginal likelihood for each model was estimated by calculating the harmonic mean of the sampled likelihoods from a MCMC chain with 1000 bootstrap replications. The calculations were performed using BEAST and TRACER version 1.5. An unweighted pair-group method of arithmetic averages (UPGMA) was used to construct the starting trees and the MCMC chain length was 10 million (with 10% burn-in). Evidence against the null model (i.e. the one with lower marginal likelihood) is indicated by 2ln (BF) > 2 (positive) and > 10 (very strong) (Kass & Raftery, 1995). As a relaxed-clock model, we used an uncorrelated lognormal distribution (UCLN) model for rate variation among lineages, which was, in a simulation study, robust in rate estimation even when the data are generated based on a lognormally autocorrelated rate variation model (Drummond et al., 2006). The strict clock and UCLN models were compared under the conditions of the Yule model plus the GTR+I+G or TrN models; the UCLN model was selected [under the GTR+I+G model, ln L = −4676.329 in the strict model, ln L = −4642.553 in the UCLN model, 2ln (BF) = 67.552; under the TrN model, ln L = −4965.295 in the strict model, ln L = −4932.799 in the UCLN model, 2ln (BF) = 64.990]. In addition, the frequency histogram of the SD of the uncorrelated lognormal relaxed clock (ucld.stdev parameter) did not include or abut 0 under the GTR+I+G and TrN models, hence rejecting a strict molecular clock (Drummond et al., 2007). The Yule and the birth-death models were compared under the conditions of the UCLN model plus the GTR+I+G or TrN models. The Yule model is parameterized by a rate of lineage birth (i.e. bifurcation) and the birth-death model is parameterized by lineage birth and death rates. Under the GTR+I+G model, both models indicated insignificantly different marginal likelihood values [ln L = −4642.553 in the Yule model, ln L = −4643.046 in the birth-death model, 2ln (BF) = 0.986] and the Yule model was selected because it is simpler than the birth-death model. Under the TrN model, the birth-death model was selected [ln L = −4932.799 in the Yule model, ln L = −4931.390 in the birth-death model, 2ln (BF) = 2.818]. Based on these results, the BEAST analyses used the UCLN and Yule models with the GTR+I+G model, and the UCLN and birth-death models with the TrN model. Empirical base frequency setting was applied, and the UPGMA starting tree was used. Default priors were used for the remaining parameters. MCMC chains were run for 10 million generations and sampled every 1000 generations. We ran two separate analyses. We checked the convergence of all parameters using TRACER and the first 1000 of the 10 000 sampled generations in each run were discarded as burn-in. The log files from the two runs were combined using LOGCOMBINER version 1.5.4 (Drummond & Rambaut, 2007), and the ESS of all parameters were > 200 after the burn-in. A maximum clade credibility tree was estimated with a burn-in of 10% of the sampled trees and a posterior probability limit of 0.5 by TREEANNOTATOR version 1.5.4 (Drummond & Rambaut, 2007), and generated by FIGTREE version 1.3.1 (Drummond & Rambaut, 2007).
The MP analysis was conducted using PAUP*. Indels were treated as missing data. Characters were treated as unordered, and character transformations were weighted equally. The branch collapse option was set to collapse at a minimum length of zero. A heuristic parsimony search was performed with 200 replicates of random additions of sequences, with the ACCTRAN character optimization, tree bisection–reconnection (TBR) branch swapping, MULTREES, and STEEPEST DESCENT options on. Statistical support for each clade was assessed by bootstrap analysis (Felsenstein, 1985). One thousand replicates of heuristic searches, with the TBR branch swapping and MULTREES options off, were performed to calculate bootstrap values.
Phylogenetic and dating analysis ofSolenogynedata sets
To elucidate phylogenetic relationships among the four Solenogyne species and to conduct molecular dating analyses simultaneously based on ITS and cpDNA, the Solenogyne data sets were analyzed using *BEAST (Heled & Drummond, 2010) implemented in BEAST version 1.6.1. *BEAST conducts multispecies coalescent analysis to estimate a species tree that is most probable given the unlinked multi-locus sequence data (i.e. the ITS data and the combined cpDNA data). Multispecies coalescent analysis considers that gene trees are embedded in a shared species tree by following the stochastic coalescent process. This analysis requires multiple samples per species to examine coalescent events for that species (Heled & Drummond, 2010). Because we could not use multiple samples for most Lagenophora species except Lagenophora pumila, and because the monophyly of Solenogyne was indicated by the analyses of the Astereae data set (see Results), we used only the Solenogyne samples in this analysis. Thus, the root of a phylogeny was estimated without using an outgroup by enforcing the molecular clock constraint (Drummond & Rambaut, 2007).
The models of TrN and HKY were used for ITS and cpDNA data, respectively, with empirical base frequency setting and UPGMA starting trees. The piecewise linear population size model, which is more realistic for natural populations than piecewise constant population size model (Heled & Drummond, 2010), was used. Because the Solenogyne data set for ITS showed the marginal value in the molecular clock LRT for the molecular clock hypothesis (P = 0.05), we compared the strict clock and UCLN relaxed-clock models for ITS under the strict clock model for cpDNA and the Yule model using the BF. The result selected the simpler strict clock model [ln L = −3480.868 in the strict model, ln L = −3480.214 in the UCLN model, 2ln (BF) = 1.306]. Also, the frequency histogram of the ucld.stdev parameter abutted 0, failing to reject a strict molecular clock (Drummond et al., 2007). The Yule and the birth-death models were compared under the conditions of the strict clock models for ITS and cpDNA; the simpler Yule model was selected [ln L = −3480.868 in the Yule model, ln L = −3480.393 in the birth-death model, 2ln (BF) = 0.948]. Based on these preliminary tests, the multilocus estimation of species tree was performed under the strict clock models for ITS and cpDNA and the Yule model.
There are no fossils to calibrate the molecular clock for Solenogyne and its allied taxa, and thus divergence times were calculated using reported substitution rates. There are multiple estimates of ITS substitution rates in Asteraceae with a minimum generation time of 2–3 years. These range from 7.83 × 10−9 (the highest) to 2.51 × 10−9 (the lowest) substitutions per site per year (Richardson et al., 2001; Kay, Whittall & Hodges, 2006). Because it is inappropriate to apply one of these rates from a different lineage, we used a normal distribution prior with the mean (5.17 × 10−9) equal to the mean of the highest and lowest values and with a SD of 1.36 × 10−9, which includes the highest and lowest values in the 95% range (mean ± 1.69 SD) of the distribution. In the analysis of cpDNA data, we used a normal distribution prior with the mean (4.36 × 10−9) and SD of 2.29 × 10−9, which covered a range from 4.87 × 10−10 to 8.24 × 10−9 (the lowest and highest rates for cpDNA noncoding region of plants with a minimum generation time of 2–3 years, Richardson et al., 2001) in the 95% range of the distribution.
MCMC chains were run for 30 million generations and sampled every 1000 generations. We ran two separate analyses. We checked the convergence of all parameters using TRACER and the first 3000 of the 30 000 sampled generations in each run were discarded as burn-in. The log files from the two runs were combined, and the ESS of all parameters were greater than 200 after burn-in. A maximum clade credibility tree was estimated with a burn-in of 10% of the sampled trees and a posterior probability limit of 0.5.
Divergence time estimation forS. mikadoi
Bayesian estimation of divergence times among the lineages of S. mikadoi was performed based on each ITS and cpDNA sequence, using BEAST. The analyses employed the strict clock models and the models TrN for ITS and HKY for cpDNA with empirical base frequency settings. For ITS and cpDNA substitution rates, the normal distribution priors described above were used. The analyses used a coalescent tree prior, which is adequate to study intraspecific diversification (Drummond et al., 2007), under the most simple assumption of a constant population size. Because the coalescent tree prior is suited for within species data, the sequences of the other three Solenogyne species were excluded from the analyses, and the root of a phylogeny was estimated by enforcing the molecular clock constraint. Random starting trees were used for ITS and cpDNA. Default priors were used for the remaining parameters. MCMC chains were run for 10 million generations and sampled every 1000 generations. We ran two separate analyses for each ITS and cpDNA. We checked the convergence of all parameters using TRACER and the first 1000 of the 10 000 sampled generations in each run were discarded as burn-in. The log files from the two runs were combined, and the ESS of all parameters were greater than 200 after the burn-in. A maximum clade credibility tree was estimated with a burn-in of 10% of the sampled trees and a posterior probability limit of 0.5.
Phylogeographical analysis ofS. mikadoi
A statistical parsimony network, suited to the analysis of intraspecific and/or recently diverged genetic lineages, was estimated based on each of the Solenogyne data sets of ITS and cpDNA. The analyses were conducted by a 95% parsimony criterion using TCS version 1.21 (Clement, Posada & Crandall, 2000). Indels were scored as binary states indicating presence/absence, in accordance with the simple indel coding strategy of Simmons & Ochoterena (2000). The other three Solenogyne species were used as outgroup taxa because an Australian origin of Solenogyne was indicated by the analyses of the Astereae data set (see Discussion).
RESULTS
Data matrix characteristics
The length of the ITS sequences was in the range 627–634 bp among Solenogyne and the other species, and the aligned sequence length was 650 bp. In Solenogyne, 30 nucleotide substitutions were found in 29 variable sites. Within S. mikadoi, four nucleotide substitutions were found in four variable sites and three unique ITS types were found (A–C), and each of the three Australian Solenogyne species had an unique ITS type (D–F) (Table 1). The length of the atpB–rbcL spacer and trnK3′ intron region was 994–1001 bp and 752 bp in the four Solenogyne species, and their alignment resulted in matrices of 1001 and 752 characters, respectively, resulting in the combined data of 1753 bp. Across all Solenogyne samples, seven nucleotide substitutions and one length polymorphism were found. In all accessions of S. mikadoi, three nucleotide substitutions were found and four haplotypes (A–D) were recognized. Each of the three Australian species had one unique haplotype (E–G).
nr DNA phylogeny ofSolenogyneand allied genera
The 50% majority rule consensus tree by MrBayes based on the model TrN is depicted with mean branch length of all the post-burn-in trees and Bayesian posterior probabilities (PP) (Fig. 3).
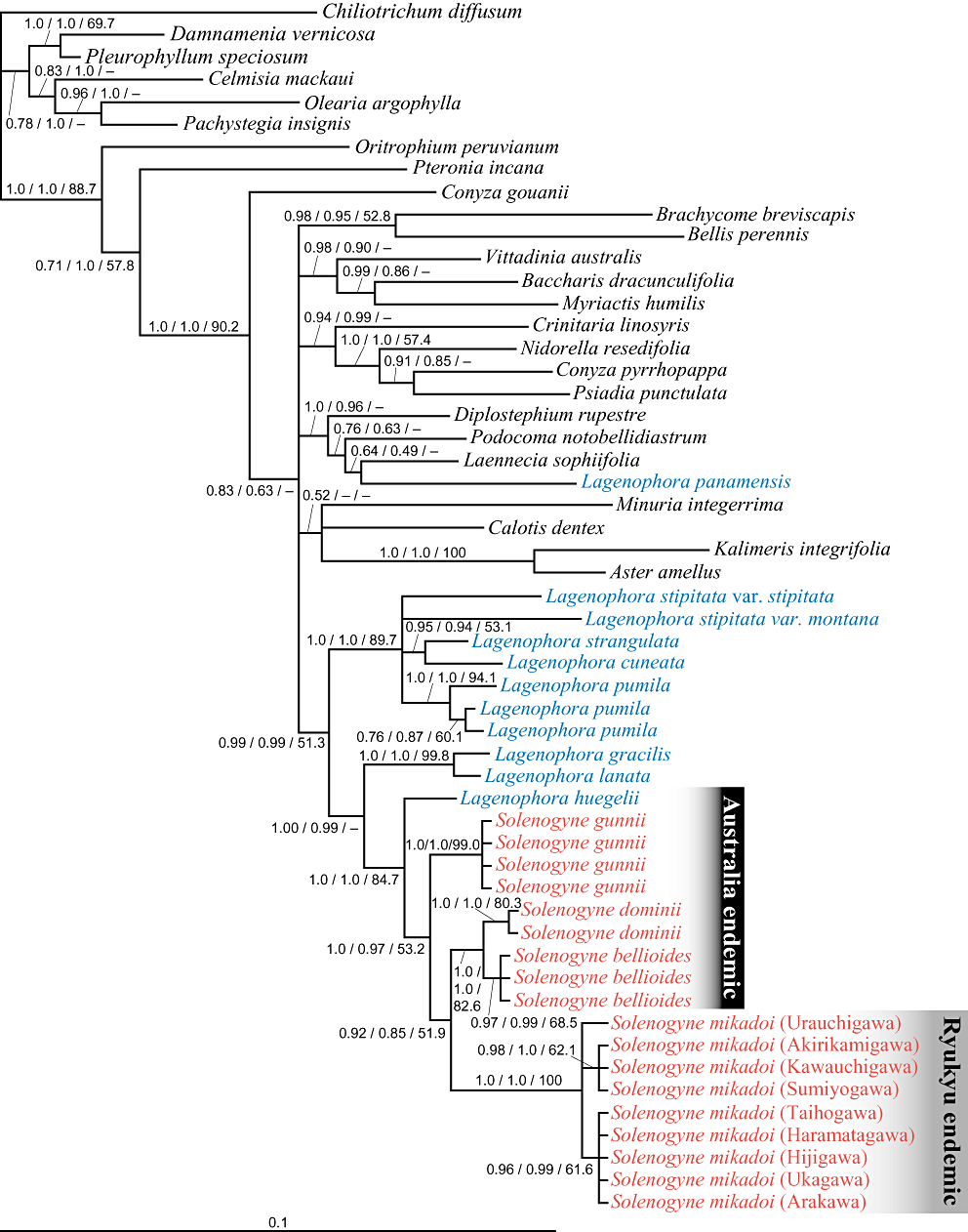
The 50% majority rule consensus tree of Solenogyne, Lagenophora, and 34 taxa from 25 genera of tribe Astereae by MrBayes based on internal transcribed spacer sequences under the model TrN. The analysis based on the model GTR+I+G resulted in the same topology (not shown). The maximum clade credibility trees by BEAST based on the models GTR+I+G and TrN and the maximum parsimony (MP) strict consensus tree were all topologically compatible (not shown). Numbers along the branches indicate Bayesian posterior probabilities (PP) with MrBayes and BEAST under the model TrN and bootstrap percentages (BP) by the MP analysis (PPMrBayes/PPBEAST/BP).
The maximum clade credibility tree by BEAST based on the model TrN were topologically compatible with the 50% majority rule consensus tree (not shown). The MrBayes and BEAST analyses based on the model GTR+I+G indicated lower posterior probability values for some nodes but showed the same topologies (not shown) as the model TrN. In the MP analysis, 107 of 275 variable sites were parsimoniously informative in Solenogyne and the other Astereae taxa. Seventy-three equally parsimonious trees were obtained (709 steps, consistency index = 0.536, retention index = 0.666, rescaled consistency index = 0.357, homoplasy index = 0.464). The topology of the MP strict consensus tree (not shown) was compatible with those of the Bayesian trees by MrBayes and BEAST. The PP for clade support by BEAST based on the model TrN and bootstrap percentages (BP) by the MP analysis are shown on the 50% majority rule consensus tree (Fig. 3). In what follows, we consider clades supported by BP > 70 and/or PP > 0.95. The Solenogyne and Lagenophora samples together formed a clade (PPMrBayes/PPBEAST/BP = 0.99/0.99/51.3), except Lagenophora panamensis S.F.Blake from Central America that fell in a clade containing a sample each of three other genera (1.0/0.96/ < 50). Within the Solenogyne/Lagenophora clade, all Solenogyne samples and L. huegelii formed a clade (1.0/1.0/84.7), and Solenogyne was monophyletic (1.0/0.97/53.2). Each species, S. gunnii (1.0/1.0/99.0), S. dominii (1.0/1.0/80.3), S. bellioides (0.97/0.99/68.5), and S. mikadoi (1.0/1.0/100), was monophyletic, and S. dominii and S. bellioides formed a monophyletic clade (1.0/1.0/82.6). In the S. mikadoi clade, a clade composed of plants from Amamioshima and Tokunoshima islands (0.98/1.0/62.1) and a clade of plants from Okinawajima Islands (0.96/0.99/61.6) clustered in a polytomy with the plant from Iriomotejima Island.
Phylogeny and divergence time ofSolenogyne
Species phylogeny of the four Solenogyne species estimated using multispecies coalescent analysis based on ITS and cpDNA is depicted with clade posterior probabilities above branches (Fig. 4). Clade depth indicates mean nodal age and bars indicate 95% highest posterior density (HPD) intervals for nodal ages. The phylogenetic tree was rooted at the branch separating S. mikadoi from the three Australian congeners (PP = 1.0). The three Australian species were monophyletic (PP = 0.98) and S. bellioides and S. dominii formed a clade (PP = 0.95). The estimated age of the most recent common ancestor (MRCA) of Solenogyne was 2.38 Mya (95% HPD interval = 0.93–4.16 Mya). The age of the MRCA of the three Australian species was 1.15 Mya (95% HPD interval = 0.30–2.21 Mya). The age of the MRCA of S. bellioides plus S. dominii was 0.63 Mya (95% HPD interval = 0.15–1.22 Mya).

Species phylogeny of the four Solenogyne species estimated using multispecies coalescent analysis based on internal transcribed spacer (ITS) and chloroplast DNA (cpDNA). Clade posterior probabilities (PP) are indicated above branches. Clade depth indicates mean nodal age (million years) and bars indicate 95% highest posterior density (HPD) intervals for nodal ages.
Divergence time estimation forS. mikadoi
The maximum clade credibility trees based on ITS and cpDNA are shown with clade posterior probabilities above branches (Fig. 5). Nodes with identical sequences are collapsed. Clade depth indicates mean nodal age (years) and nodes with PP ≥ 0.5 are annotated with the 95% HPD intervals for node ages by bars. The estimated age of the MRCA of S. mikadoi was 0.33 Mya (95% HPD interval = 0.03–0.75 Mya) based on ITS, and 0.13 Mya (95% HPD interval = 0.003–0.330 Mya) based on cpDNA. The ages of the MRCAs of terminal clades ranged 0.03–0.07 Mya (95% HPD interval = 0.00035–0.190 Mya) based on ITS, and 0.01–0.02 Mya (95% HPD interval = 0.00008–0.070 Mya) based on cpDNA.
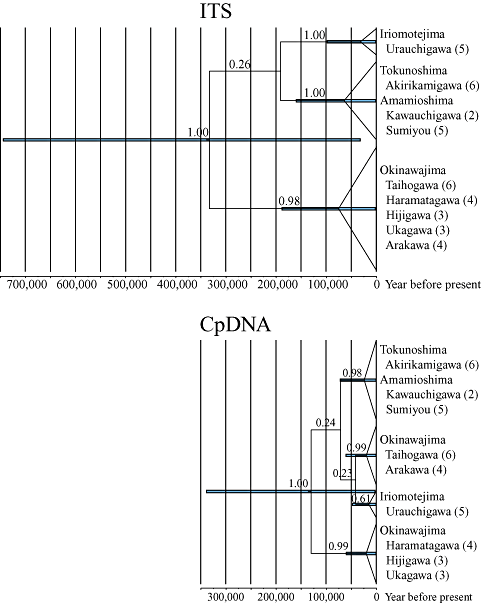
Maximum clade credibility trees based on internal transcribed spacer (ITS) and chloroplast DNA (cpDNA). Nodes with identical sequences are collapsed. Clade posterior probabilities (PP) are indicated above branches. Clade depth indicates mean nodal age (year) and nodes with PP ≥ 0.5 are annotated with the 95% highest posterior density (HPD) intervals for node ages by bars.
Phylogeography based on cp DNA
The geographical distribution of the ITS types and cpDNA haplotypes is shown in Figure 2, with their statistical parsimony networks. ITS type A was found in Iriomotejima Island, ITS type B was found in Okinawajima Island, and ITS type C was found in Tokunoshima and Amamioshima islands. In the statistical parsimony network, ITS type A and the other types B and C were connected with three mutational steps; ITS types B and C were distinguished from each other by two mutational steps. The ITS types of the Australian species S. bellioides, S. dominii, and S. gunnii (D–F, not shown) were not connected to the network of S. mikadoi by the 95% parsimony criterion. CpDNA haplotype A was found in Iriomotejima Island, haplotypes B and C were found in Okinawajima Island, and haplotype D was found in Tokunoshima and Amamioshima islands. In the statistical parsimony network, the four haplotypes of S. mikadoi were distinguished from each other by one mutational step. Haplotype A from Iriomotejima Island was found in a central position with the other three haplotypes B, C, and D radiating from haplotype A. The haplotypes of S. bellioides (E) and S. gunnii (G) were connected to the haplotype of S. dominii (F), which was connected to haplotype A of S. mikadoi separated by two mutational steps.
DISCUSSION
Systematic considerations
In the Bayesian tree (Fig. 3), Solenogyne and Lagenophora formed a well supported monophyletic clade, except for L. panamensis from Panama, that clustered with three other Astereae genera from Central America. Within Lagenophora, two sections, namely Pseudomyriactis and Lagenophora are recognized based on the morphological differences in the beak of the achenes and stem leaves; L. panamensis belongs to the former, whereas the other congeners analyzed belong to the latter (Cabrera, 1966; Cuatrecasas, 1986). The Bayesian tree is consistent with this split. Within the Solenogyne–Lagenophora clade, the first split separates four Lagenophora species (L. stipitata, L. strangulata, L. cuneata, and L. pumila) from other species of Lagenophora (L. gracilis, L. lanata, and L. huegelii) plus all Solenogyne samples. The latter three Lagenophora species split off in two grades from the Solenogyne samples. This supports the hypothesis that Solenogyne has arisen from a Lagenophora ancestor and has a more recent origin than Lagenophora (Davis, 1950). The clade of Solenogyne plus the three Lagenophora species shares a monopodial habit, whereas the other Lagenophora species are sympodial in growth (Drury, 1974; Adams, 1979). The results from our phylogenetic analyses demonstrate that Lagenophora as currently defined is polyphyletic. Based on this result, one taxonomic option is synonymizing Solenogyne with Lagenophora (Bentham, 1867; Maiden & Betche, 1916; Drury, 1974; Koyama, 1995). Transferring the three Lagenophora species (L. gracilis, L. lanata, and L. huegelii) into Solenogyne is another option (Adams, 1979) because they share similar habit with Solenogyne species. However, the type species of Lagenophora, L. nudicaulis (Comm. ex Lam.) Dusén, was not collected and examined in the present study. Thus, a taxonomic revision is beyond the scope of the present study, but it is a topic for future investigation.
Ryukyu–Australia antitropical distribution
Solenogyne mikadoi formed a clade with the three Australian congeners and this clade was sister to L. huegelii, which is also endemic to southern Australia (Fig. 3). In the present study, L. pinnatifida, which is endemic to New Zealand, was not analyzed, and therefore we cannot rule out the possibility that L. pinnatifida is included in the Solenogyne–L. huegelii clade or Solenogyne clade. The four Solenogyne species and L. huegelii share a monopodial habit (Drury, 1974) and the four Solenogyne species share tubular ray florets and achenes with neither beak nor gland (Davis, 1950; Cabrera, 1966; Adams, 1979), whereas L. pinnatifida does not have these character states. The ITS phylogeny (Fig. 3) indicated that these morphological characters have phylogenetic signals. Furthermore, morphologically, L. pinnatifida is regarded to form a species complex with L. stipitata (Drury, 1974), which formed a distinct clade with L. strangulata, L. cuneata, and L. pumila. Therefore, the possibility raised above appears to be low. The reconstructed phylogeny indicates a direction of dispersal from Australia to Japan. Overall, it can be safely stated that S. mikadoi is disjunctly distributed from its sister species more than 7000 km away, and has derived from an Australian congener. To our knowledge, this is the first molecular phylogeographical evidence for an antitropical distribution of a vascular plant between East Asia and Australia.
The age of the MRCA of Solenogyne was 2.38 Mya (0.93–4.16 Mya), when the lineage of S. mikadoi and the Australian lineage diverged (Fig. 4). This is much more recent than the collision of the Australian continent with the Philippine Sea plate arc which started by approximately 25 Mya (Hall, 2001). Thus, it was not the collision which facilitated the range expansion of Solenogyne. In plant species pairs showing antitropical distributions in North and South America, divergence times based on molecular data fall generally in the late Neogene to the Holocene (i.e. from 8.4 Mya to very recent) (Bell & Patterson, 2000; Fukuda, Yokoyama & Ohashi, 2001; Lia et al., 2001; Wen et al., 2002; Beier et al., 2004; Hughes & Eastwood, 2006; Moore, Tye & Jansen, 2006; Spalik et al., 2010; Popp et al., 2011). If we take other types of intercontinental disjunctions, divergence times for plant species pairs in Eastern Asia and Eastern America occurred in the late Miocene or later (0.31–16.77 Mya with average 4.98 Mya; Xiang et al., 2000), and for pairs in Eurasian and western North American deserts with the Mediterranean climate, fall in the Miocene to the Holocene (0.021–21.90 Mya; Wen & Ickert-Bond, 2009). Therefore, the oldest estimates for the antitropical distributions are younger than the oldest estimates for other intercontinental disjunctions, although it is certainly premature for generalizations because not enough lineages have been analyzed with antitropical distributions. In Miocene, positions of the North and South American, African, Australian, and Eurasian continents have already been similar to the modern positions (Torsvik et al., 2010). Thus, we consider that the timing of continental drift does not explain the different oldest estimates. Generally speaking, long-distance dispersals are more likely to occur within the same latitudinal climatic zone (e.g. between East Asia and North America) than between climatic zones (i.e. antitropical pairs) because dispersals are not successful unless the climates in a new land or stopovers are appropriate (Morley, 2003). Climatic oscillations during the Plio-Pleistocene (Hall, 2001) may have provided a corridor of suitable climate at some time and enabled latitudinal dispersal through the tropics. In Australia, dense closed forest cover rapidly changed to dry, open vegetation in the Pliocene (approximately 1.8–5.0 Mya) in response to climatic oscillations (Hill, 2004). In the late Pliocene, temperate rainforests in south-eastern Australia dramatically shifted to a landscape dominated by plants of Asteraceae and Poaceae and with very few sclerophyll trees (Hill, 2004; Martin, 2006). This landscape is very similar to the present-day habitats Solenogyne occupies in Australia. Population expansion and large production of fruits in such landscapes may have increased the chance of the range expansion from Australia to the Ryukyu Archipelago.
The age of the MRCAs of three Australian Solenogyne species was 1.15 Mya (0.30–2.21 Mya) and, of S. bellioides and S. dominii, was 0.63 Mya (0.15–1.22 Mya) (Fig. 4). In the Pleistocene (approximately 0.01–1.80 Mya), there was very little glaciation in Australia and it was restricted to the south-eastern highlands and Tasmania (Martin, 2006). In south-eastern Australia, the alternation of more open or steppe vegetation in the drier glacial periods and more wooded vegetation in the wetter interglacial periods occurred through the Pleistocene (Martin, 2006). Fragmentation of open vegetation habitats as a result of local recovery of forests during the interglacial periods and genetic isolation may explain the divergences of the Australian Solenogyne species. Their present distribution ranges are overlapping and their habitat segregation is not evident, and this may be explained by secondary contacts of the species.
Because Australia and the Ryukyu Archipelago have never been connected by land, even via the Sundaland and East Asian continent (Hall, 2001), media for an over-sea dispersal need to be considered, although the fruits of Solenogyne have no special mechanism for long-distance dispersal. Sea-current dispersal is unlikely because extant species of Solenogyne grow inland. Achenes of the four Solenogyne species are approximately 1–2.5 mm at the long axis (K. Nakamura, unpubl. data) and are small enough to be accidentally dispersed by migratory birds with exozoochory and/or by strong winds. Dispersal by migratory birds, which head to terrestrial habitats, appears more realistic than haphazard dispersal by strong winds. Eastern Australia and the Ryukyu Archipelago are situated on the flyway of many migratory birds called the East Asian–Australasian Flyway (Wilson & Barter, 1998; Amami Ornithologist Club, 2009). Thus, it would be possible to assume indirect or direct dispersal of achenes by these migratory birds, although it is not clear whether the East Asian–Australasian Flyway has existed for a geohistorical time scale. Solenogyne has an autogamous breeding system (K. Nakamura, unpubl. data), which is characteristic to antitropical distributions (Raven, 1963; Wen & Ickert-Bond, 2009) and likely facilitates long-distance migrations because new populations can be established even when a single plant colonizes and/or when suitable pollinators are absent in colonized areas (Donoghue, 2011).
It is intriguing that Solenogyne is absent from intervening areas such as New Guinea, Indonesia, Philippines, and Taiwan (Fig. 1). The genus may have been overlooked in these areas because some are botanically very poorly known (e.g. New Guinea). Aside from this possibility, antitropical distributions may arise from long-distance dispersal with no stopover at the tropics or, alternatively, are the result of extinctions of intervening tropical populations of previously widespread lineages (Hilbish et al., 2000; Burridge, 2002; Mabuchi et al., 2004). Some migratory birds are known to fly nonstop between Australia and East Asia (Wilson & Barter, 1998). Such ‘long jump’ migration may explain the absence of Solenogyne in the intervening areas. The extinction hypothesis cannot be proven without fossil records from the tropics, as is the current case for Solenogyne. However, it cannot be flatly discounted either. Extinctions in the tropics can largely be attributed to two factors, namely unsuitably high temperatures during climate oscillation and/or competition with tropical species (Briggs, 1987; Hilbish et al., 2000). The climate–extinction hypothesis has been applied to antitropical distributions of many marine organisms (Parrish, Serra & Grant, 1989; Hilbish et al., 2000; Mabuchi et al., 2004) and also to land plants in North and South America (Raven, 1963; Wen & Ickert-Bond, 2009). This is a possible explanation for Solenogyne, considering that the three Australian species are distributed in temperate areas (Adams, 1979; Brown & Porteners, 1992) and S. mikadoi, although distributed in the subtropical Ryukyu Archipelago, grows in cool environments such as rocky beds of mountain streams (Yokota & Hiraiwa, 2006). Although in South-east Asian islands there are cooler environments at high altitudinal areas (Barlow, 1981), such niches might have been occupied by other species, as discussed below. On the other hand, the competition–extinction hypothesis appears more likely because competition with tropical species would have been severe on South-east Asian islands. These have an extremely rich flora characterized by exceptionally large numbers of relictual, archaic forms of flowering plants, suggesting persistence of tropical rain forests since the early Tertiary (Takhtajan, 1986; Morley, 2001, 2003). However, the Ryukyu Archipelago is younger and did not attain its modern form until 5–6 Mya (Chiang & Schaal, 2006), with less competition from the comparatively depauperate flora thus enabling colonization of Solenogyne. Many of the small islets of Wallacea have emerged from the sea by tectonic movements and been populated by plants and animals since approximately 5 Mya (Hall, 2001). During glacial periods, the huge continental shelf of Australia–New Guinea and shallow areas of the South China Sea and Java Sea were exposed as dry land, and the resulting decrease in moisture from summer and winter monsoons to the Lesser Sunda Islands, Java, Borneo, Sumatra, and the Philippines likely increased the areas of seasonal forests and grasslands in lowlands (Heaney, 1991). These newly-emerged open vegetation communities could have enabled Solenogyne to establish populations there, and the decrease or disappearance of the habitats in interglacial periods due to sea level rises and vegetation changes may explain the present-day absence of Solenogyne in South-east Asia. The long branch leading to the S. mikadoi clade may indicate these extinction events (Fig. 3).
Directions of intercontinental dispersals have been extensively discussed in the literature. Molecular phylogenetic studies on land plants distributed in North and South America reported more examples of southward migrations (Bell & Patterson, 2000; Fukuda et al., 2001; Li et al., 2002; Wen et al., 2002; Beier et al., 2004; Hughes & Eastwood, 2006; Moore & Jansen, 2006; Moore et al., 2006; Ickert-Bond, Rydin & Renner, 2009; Spalik et al., 2010; Popp et al., 2011) than of northward migrations (Fukuda et al., 2001; Lia et al., 2001; Bell & Donoghue, 2005; Simpson, Tate & Weeks, 2005). Dominance of southward dispersal was indicated for land plants showing antitropical amphiantarctic disjunctions (Spalik et al., 2010). To determine whether a predominant direction of dispersal exists in East Asia–Australia antitropical distributions, more studies are needed, while the Ryukyu Archipelago, with a much younger geohistory, might have provided suitable open habitats and acted as a sink for chance migrants from source Australian populations.
Migratory process in the ryukyu archipelago
In the statistical parsimony network (Fig. 2), the cpDNA haplotype A of S. mikadoi from Iriomotejima Island connected with the haplotype F of S. dominii. This indicates that an ancestral lineage of S. mikadoi first colonized Iriomotejima Island. The other three haplotypes of S. mikadoi radiated from haplotype A. This indicates that haplotype A migrated northward throughout the distribution range of the species. The northward migration was followed by local differentiations (i.e. haplotypes B and C were derived from haplotype A in Okinawajima Island, and haplotype D was derived from haplotype A in Amamioshima or Tokunoshima islands). In the statistical parsimony network of ITS, the three Australian species (ITS types D–F) were not connected to the network of S. mikadoi by the 95% parsimony criterion, and thus migratory direction cannot be inferred. Assuming that the ITS network is rooted at the ITS type of Iriomotejima Island, with reference to the cpDNA network, the topology of the ITS types A–C is compatible with the above migratory process deduced based on the cpDNA network.
Palaeogeographically, during the Neogene, the Ryukyu Archipelago formed a land-bridge and connected to the surrounding landmasses more than once, to Kyushu of the main Japanese islands in the north and to south-eastern China via Taiwan in the south (Ota, 1998). These land connections allowed various lineages of terrestrial organisms to expand their ranges among the islands (Ota, 1998; Nakamura et al., 2009). During the Pliocene (approximately 1.8–5.0 Mya), however, subsidence created two deep-water passages in the island arc: the Tokara Gap (the Tokara tectonic strait) to the north of Amamioshima Island and the Kerama Gap to the south of Okinawajima Island (Fig. 2) (Ota, 1998). Subsequently, any land connection across these gaps was unlikely even during Quaternary glacial sea-level minima (Ota, 1998) because the sea is currently more than 1000 m deep at these two gaps (Kawana, 2002). In the early Pleistocene, present-day Amamioshima, Tokunoshima, and Okinawajima islands formed a large island (Fig. 2) (Ota, 1998). Subsequently, the islands were separated during the mid Pleistocene (Kimura, 2002b).
The age of the MRCA of S. mikadoi was estimated here as 0.33 Mya (0.03–0.75 Mya) based on ITS and 0.13 Mya (0.003–0.330 Mya) based on cpDNA (i.e. the mid Pleistocene or later). Considering the long isolation of Iriomotejima from the northern islands since the Pliocene, this result suggests that northward migration and/or gene flow occurred crossing the Kerama Gap via over-sea dispersal by wind and/or birds. Migrants from Iriomotejima Island might have colonized Okinawajima, Tokunoshima, and Amamioshima islands individually, or might have first colonized one island and then the others in a stepwise manner. The distribution patterns of the ITS types and cpDNA haplotypes showed genetic discontinuity between Okinawajima Island and Tokunoshima plus Amamioshima islands. This genetic differentiation pattern has been reported for multiple organisms (Japanese newt Cynops: Hayashi & Matsui, 1988; Ceratopteris: Watano & Masuyama, 1994; wood-feeding cockroaches Salganea: Maekawa et al., 1999; pit vipers Trimeresurus: Toda et al., 1999; Lilium: Hiramatsu et al., 2001), with a rare exception that showed a genetic discontinuity between Tokunoshima and Amamioshima islands (Aster: Maki, 2001). This general pattern may suggest vicariance events between Okinawajima Island and Tokunoshima plus Amamioshima islands (Hiramatsu et al., 2001), which is consistent with the palaeogeography in the mid to late Pleistocene (Kimura, 2002b). Alternatively, this pattern can be simply explained by a geographical distance effect; the genetic discontinuity was caused by the larger geographical distance between Okinawajima Island and the other two islands than between Tokunoshima and Amamioshima islands (Fig. 2).
Conclusions
A sister relationship between S. mikadoi and the Australian congeners was revealed and the antitropical distribution of Solenogyne was proven. A sister relationship between Solenogyne and Australia endemic L. huegelii indicated an Australian origin of Solenogyne. Solenogyne mikadoi and the Australian congeners diverged during the Plio-Pleistocene. The ancestral lineage of S. mikadoi has likely first colonized the southernmost island in the Ryukyu Archipelago, and from there northwards, most likely by over-sea dispersals. The antitropical distribution of Solenogyne might have arisen through long-distance dispersal across the tropics or, alternatively, through extinction in the tropics as a result of unsuitably high temperatures during climate oscillation and/or competition from the tropical flora that survived there since the early Tertiary. The range expansion from Australia to the Ryukyu Archipelago likely followed the flourishing of Solenogyne in open vegetation communities radiating in south-eastern Australia during the late Pliocene. Climatic oscillations during the Plio-Pleistocene may have provided a corridor of suitable climate. Open vegetation in lowland areas, which emerged above sea in the Plio-Pleistocene in South-east Asia via tectonic movements and eustatic sea level drop could have enabled Solenogyne to pass through the area. This is the first molecular phylogeographical evidence for an antitropical distribution of land plants between East Asia and Australia, and contributes to a first insight into our understanding of this intriguing global phenomenon.
ACKNOWLEDGEMENTS
We thank Kuniaki Watanabe and Ron Booth for plant materials and help in the fieldwork. This work was supported in part by a postdoctoral fellowship from Academia Sinica to K.N., a research grant from Academia Sinica to C.I.P., and Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to G.K. (C-20510220).




