Developmental plasticity varied with sex and position in hatching hierarchy in nestlings of the asynchronous European roller, Coracias garrulus
Abstract
Allocation rules between ornamental and other functional traits of birds may differ among individuals and vary with environmental conditions. We supplemented roller (Coracias garrulus) nestlings with methionine in a between-nest design to investigate the way in which the sex and position in the hatching hierarchy affect the allocation of resources among growth, immunity, and plumage coloration. Methionine induces the production of lymphocytes at expense of growth; thus, we used it to manipulate growth and immunity, which are two traits likely to compromise plumage coloration. We predicted that late-hatched chicks within a brood (juniors) compared to early-hatched chicks (seniors) should allocate more to traits directly providing fitness than to ornamental traits because juniors are more affected than seniors by sibling competition. The methionine treatment effectively enhanced the production of lymphocytes in experimental broods. This appeared to be at the expense of plumage coloration in junior nestlings because, in supplemented nests, junior males showed a trend to display less greenish bellies than junior males from control nests. However, juniors from supplemented nests maintained wing growth as in control juniors. The plumage coloration of seniors was unaffected by the methionine supplementation, although they paid the costs of lymphocyte production at a level of growth that was reduced compared to senior nestlings in control nests. Hence, sex, and hatching order affected resource allocation among growth, immunity, and plumage coloration of roller nestlings. © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 99, 500–511.
INTRODUCTION
The study of how the environment may alter the relationships among traits within individuals is a promising field in phenotypic plasticity research and may help to elucidate how natural selection shapes individual phenotypes (Fox, Roff & Fairbairn, 2001). Traits of an organism are not independent but are linked to each other to varying degrees; thus, the environment may affect the investment made by the organism in each trait, leading to the plasticity of character relationships. Selection pressures on any of the traits are expected to modify allocation rules, which should favour a set of traits with the highest expected fitness returns (Lindström, Metcalfe & Royle, 2005).
Ornamental and other functional traits can contribute differently to overall fitness. Therefore, natural selection will adjust the degree of plasticity of each of these traits to its proportionate contribution to fitness under each ecological circumstance (Badyaev & Duckworth, 2003). For example, Hunt et al. (2004) found that male field crickets (Teleogryllus commodus) reared on a rich diet invested more energy in sexual displays but died sooner than those reared on poor diets. Also, Sadd et al. (2006) demonstrated that male mealworm beetles (Tenebrio mollitor) increased their investment in attractiveness in response to a survival threat. Comparatively less attention has been paid to the fact that different individuals may respond with different allocation rules among functional and ornamental traits when faced with the same environmental conditions. Indeed, allocation rules may differ from one individual to another within a population or a brood as a function of their conditions and characteristics (e.g. sex, social position; Lindström et al., 2005). Indeed, Muñoz, Aparicio & Bonal (2008) have shown that male barn swallows (Hirundo rustica) differed in their allocation of resources to ornamental versus functional feathers. However, how environmental variation may induce variation in each male's rule remains to be elucidated. Siefferman & Hill (2007) manipulated rearing environmental conditions in eastern bluebird (Sialia sialis) nests to show that nestling males showed a greater plasticity than females: males were globally larger and more ornamented at the best rearing conditions. In the present study, we go a step further by investigating, in a bird species, the European roller (Coracias garrulus), the sex- and hatching order-specific modulation of the trade-offs between ornamental and other functional traits under different developmental conditions.
The Roller is a secondary hole-nesting bird (Cramp & Simmons, 1988) sexually dichromatic in their structural ultraviolet (UV)–blue coloration of the head and scapulars, with adult males having more pure UV-blue colour than adult females (Avilés, 2006; Silva et al., 2008). Individuals mate assortatively in relation to green–yellow chroma of the head and brightness of the brown back. Moreover, brightness of different traits of adults is related to individual variation in condition and parental contribution (Silva et al., 2008), which might indicate the action of sexual selection on structural coloration. Rollers only make a partial post-juvenile moult that involves the head, body, lesser and median upper wing-covers, and part of the tail (Cramp & Simmons, 1988). Furthermore, the first prebreeding moult is rather limited, and occasionally absent (Cramp & Simmons, 1988). Therefore, first-breeders may still retain feathers grown as nestlings. The roller is a socially monogamous species in which both sexes incubate the eggs, brood, and feed the young. Modal clutch size in the population the year of the study was six eggs (N = 27 nests). Incubation begins before clutch completion, usually after the third egg (Cramp & Simmons, 1988), which results in patent size hierarchies within broods (Sosnowski & Chmielewski, 1996; Parejo, Silva & Avilés, 2007).
In the present study, we experimentally investigate whether variation in rearing environment leads to different resource allocation in growth, immunity, and structural coloration, and whether these priorities depend on sex and position in the hatching hierarchy in nestling rollers. The rearing environment has been shown to markedly affect development, behaviour, reproduction, life history, and survival of individuals (Lindström, 1999; Metcalfe & Monaghan, 2001; Bize et al., 2003). To modify the rearing environment, we used dl-methionine that was supplemented to all nestlings in half the nests in a population. We then measured the growth of wing length, the increase in body mass, immunity, and colour of nestling rollers just before fledging. Methionine is routinely used in poultry research to increase general performance and immunocompetence (Tsiagbe et al., 1987; Grimble & Grimble, 1998). Experimental work with wild birds shows that supplemental methionine induces the production of lymphocytes at the expense of growth (Soler et al., 2003; Brommer, 2004; Tschirren & Richner, 2006). Hence, methionine supplementation changes the priority rules of resource allocation (Soler et al., 2003). Manipulations of the rearing environment usually have been achieved by changing food availability for nestlings, which leads, as in natural conditions, to some kind of co-variation between growth and immunity. However, we chose methionine-supplementation because methionine often triggers a trade-off between immunity and growth by the activation of the immune system at the expense of growth (see above) whenever resources are limiting. Consequently, this manipulation, by setting the trade-off between immunity and growth, allowed us to better understand the trade-offs between ornamental and other functional traits. Therefore, by providing methionine, we expected to manipulate nestling growth and immunity, aiming to investigate the effect of rearing conditions in resource allocation among growth, immunocompetence, and coloration. Immunocompetence may be signalled through ornaments (Hamilton & Zuk, 1982); however, allocating resources to immunity may compromise ornament production whenever there exists a resource limitation because both types of traits are costly to produce and maintain (Wedekind & Folstad, 1994).
Because it is frequently subject to sexual selection, ornamental plumage coloration tends to be phenotypically plastic (Andersson, 1994; Badyaev & Duckworth, 2003). In addition, because nestling plumage colour contributes presumably less to overall nestling fitness during development than growth and immunity, we expect (1) that the treatment affects more negatively plumage colour of nestlings than growth and immunity. Sexual selection theory predicts more exaggerated ornaments to be more sensitive to environmental conditions (Cotton, Fowler & Pomiankowski, 2004) and male rollers are more ornamented than females (Silva et al., 2008). We, thus, expect (2) that the plumage colour of males is more plastic than that of females. In addition, we considered the position of the chick in the within-clutch hierarchy because hatching asynchrony creates size differences within broods, and chicks of different sizes may show different resource allocation priorities (Werschkul & Jackson, 1979; Ricklefs, 1982; Nilsson & Svensson, 1996; Parejo et al., 2007). Late-hatched chicks within a brood are under stronger competition than early-hatched chicks; thus, juniors (i.e. all nestlings within a brood hatched from the second day of the hatching period) compared to seniors (i.e. three first nestlings in the hierarchy within each brood that usually hatch the first day of the hatching period) should allocate more to traits directly providing fitness than to ornamental traits. We therefore predict that (3) methionine supplementation should make juniors within the broods pay the costs of the activation of the immune system more at the level of plumage coloration (ornamental trait) than at the level of growth (more directly fitness related trait). At the same time, seniors might pay the cost of lymphocyte production at the level of growth because their growth is not so limiting for survival.
MATERIAL AND METHODS
Study population
The study was performed during the 2007 breeding season in a roller population breeding in nestboxes in the Cáceres province, in western Spain (39°27′N, 6°20′E). Nestboxes were placed on electric poles crossing the area. The study area is characterized by the predominance of dry pastures with a general lack of trees and thus of nesting opportunities for the species (Avilés et al., 1999; Parejo et al., 2007).
Experimental protocol
In birds, methionine demands are high during immune defence. Methionine requirements are covered by both endogenous production and dietary ingestion. An insufficiency of methionine, as for other sulphur amino acids, leads to growth retardation and compromises the synthesis of glutathione, a substance that is required for the activation of T cells (Grimble & Grimble, 1998).
Roller nests were randomly assigned to the methionine-supplemented (N = 14 nests) or control (N = 12 nests) group. The treatment began when the youngest chick of the brood was 3 days old. Nestlings in methionine nests were supplemented with an age-dependent dose of dl-methionine (Sigma Chemicals) suspended in tap water over 4 days. The same amount of tap water was administered to nestlings in control nests through the same period. Therefore, experimental and control nests were disturbed equally. Dosage was 0.02 mL of solution per 1 g of chick body weight. The solution was made suspending 0.1 g methionine in 1 mL of tap water for methionine-supplemented nests and using tap water for control nestlings (Brommer, 2004). Chicks were individually identified from the beginning of the treatment by colouring one of their tarsi with felt-tip waterproof markers and by weighing daily before the administration of the dosage.
Previous field studies have used methionine as an activator of the immune system to assess the existence of a trade-off between immunity and growth in within-nest designs (Soler et al., 2003; Brommer, 2004). That design allows for controlling parental quality or assessing the genetic component of resource allocation. In the present study, we used a between-nest design to investigate variation in resource allocation within broods in relation to individual characteristics such as sex and hatching order, which is not easily achieved in a within-nest design. Within-subjects designs are more powerful to detect effects than between-subjects designs (Thompson & Campbell, 2004). However, in the present study, a between-subject design has the advantage of better allowing an identification of the effects of sex and position in the hatching hierarchy on nestling characteristics. Furthermore, by using this type of design, we avoided the carryover effects of within-nest designs (Thompson & Campbell, 2004). These carryover effects comprise any effects that are transferred from one experimental condition to another and that might, for example, cause different behaviour in control and experimental siblings, thus creating a confounding extraneous variable that varies with the treatment. However, because we were also interested in controlling for parental quality and the genetic component of resource allocation in our design, we also controlled all our analyses for the nest effect (nested within the treatment) fitted as a random factor in our analyses (see below).
Experimental and control nests did not differ in laying dates [analysis of variance (ANOVA) model: F1,24 = 0.37, P = 0.55], clutch size (ANOVA model: F1,24 = 0.59, P = 0.45), initial brood size (ANOVA model: F1,24 = 0.19, P = 0.66), degree of hatching asynchrony measured as the number of days between the dates in which the first and the last nestling hatched in each nest (ANOVA model: F1,24 = 0.91, P = 0.35) or secondary sex ratio (generalized linear model: χ2 = 1.15, d.f. = 1,22; P = 0.28), which suggests a proper treatment randomization.
Hatching order
Nestboxes were visited regularly to determine laying and hatching dates. During the hatching period (22 days after the laying of the third egg), nests were visited daily. Hatching order for nestlings hatched in different days was thus known. As a consequence of the incubation pattern of the species, which usually begins incubation after the laying of the third egg, the three earlier chicks generally hatch the same day and, consequently, it was not always possible to establish the rank order of these three earlier chicks. Therefore, the three earlier chicks of each brood were assigned to the same hatching rank. The other nestlings of a brood usually each hatch on a different day.
Therefore, for comparison between nestlings occupying high and low positions in the hatching hierarchy, we distinguished two groups within each brood. The first group included the three first nestlings in the hierarchy (seniors). The second group included all the remaining nestlings of a brood (juniors). The number of nestlings assigned to each group is related to brood size; however, the most common brood size at hatching is six nestlings (mode = 6, mean ± SE = 5.27 ± 0.21, N = 26), and then most broods were divided in half. Nevertheless, brood size was introduced as a continuous effect in all statistical analyses to account for non-independence between brood size and sample size in each group.
Nestling growth
Subsequently, we measured the increase in wing length and body mass of chicks (1) between the beginning and the end of the treatment administration to measure its immediate effect on growth and (2) between the beginning of the treatment and day 15 of the nestling period to measure a more long-term response of individuals to methionine. Therefore, nestling measurements were taken at the day of the start of the treatment, at the day of the end of the treatment, and a third time at day 15 post-hatching. For that purpose, we used a rule to measure wing length to the nearest 1 mm, and an electronic balance for body weight to the nearest 0.01 g. Growth during the treatment and during the nestling period were calculated from the difference between pairs of measures taken at the end and the beginning of the treatment, and at day 15 post-hatching and the beginning of the treatment, respectively.
Sex identification and nestling immunity
Fifteen days after hatching, blood was extracted from nestlings by brachial venipuncture. A drop of blood was stored in ethanol for later molecular sexing and another drop was smeared on a slide, air-dried, and fixed in ethanol until examination. Nestlings were sexed by molecular methods (sensuFridolfsson & Ellegren, 1999) using blood samples stored in ethanol. The detailed protocol is described by Parejo et al. (2007). Blood smears were then stained with azure-eosin and examined to estimate the number of lymphocytes per 10000 erythrocytes. Because methionine increases the production of immune cells from the lymphocyte repertoire, we used the lymphocyte count to measure the effect of this sulphur aminoacid on the immune system.
Colour measurements
Twenty days after hatching, we plucked three to five feathers from the same location of the belly and rump of nestlings. Belly and rump colorations strongly correlate with head and scapular colorations, respectively (D. Parejo, N. Silva, J. M. Avilés & E. Danchin, unpubl. data), which are structural-based colours in rollers (Silva et al., 2008). Feathers from the belly and the rump, however, are larger and wider than those from the head and scapulars, which makes them more suitable for achieving repeatable reflectance measures. Feathers were carefully placed on black paper in a fashion that mimicked the way that the feathers naturally lay on the bird for colour measurements. Spectral data were always recorded by the same individual (N.S.) in total darkness with an Ocean Optics DH 2000 spectroradiometer. Plumage reflectance was quantified in the range 300–700 nm with a deuterium and a halogen light source using a bifurcated micron fibre optic probe at a 45° angle from the feather surface (Cuthill et al., 1999) and illuminating an area of 1 mm2. Using the spectra acquisition software package OOIBase, we sequentially recorded ten spectra relative to a standard white reference (WS-2) and then averaged the spectra to reduce electrical noise from the collection array within the spectrometer. This process was repeated three times, and the probe lifted and replaced on the feather sample between each scan. We then averaged the three spectra for each body region and individual. Reflectance data were summarized using the three standard descriptors of reflectance spectra: brightness, chroma, and hue. Brightness was calculated as the summed reflectance in the range 300–700 nm. Chroma was, in the case of the rump coloration, the ratio of the total reflectance in the range of interest (300–550 nm; Fig. 1, UV–blue–green chroma) and the total reflectance of the entire spectrum (300–700 nm). When the spectra showed a bimodal pattern, as in the belly (Fig. 1), two measures of chroma were calculated, with each one corresponding to each peak of reflectance (chroma UV–blue: 300–475 nm; chroma green–yellow: 475–625 nm). Hue referred to the wavelength at which the maximum peak of reflectance is reached. As for the chroma, when the spectra showed a bimodal pattern, one hue value per each of the two peaks was calculated.
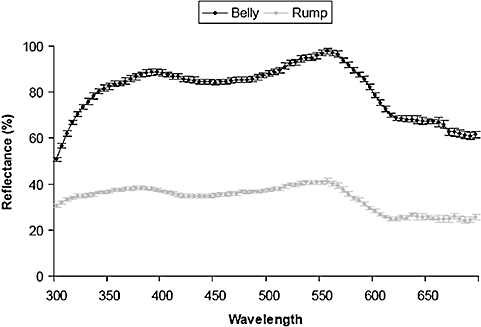
Mean ± SE reflectance spectra of the belly and rump of fledgling European rollers (males and females pooled) (N = 87 individuals).
Statistical analysis
Colour variables were entered into a principal component analysis (PCA) for each body region separately (Doucet & Montgomerie, 2003). PC scores originated from the different PCAs were then used to define inter-individual differences in nestling structural coloration. Belly coloration was reduced to two PCs: (1) the first one explained 43% of the variation and achieved strong loadings for green–yellow chroma (0.54) and for UV–blue hue (0.56); (2) the second one explained 22% of the variation and achieved strong loadings for brightness (−0.71) and UV–blue chroma (0.51). An individual with a high positive belly PC1 score showed more saturated green–yellow and longer wavelength ultraviolet–blue plumage in the belly. By contrast, individuals with high positive PC2 scores were less brilliant and more pure UV–blue coloured, with longer wavelength green-yellow bellies. Rump coloration was reduced to two PCs: (1) the first one explained 60% of the variation of colour variables and achieved strong loadings for UV–blue–green chroma (−0.66) (chroma UV–blue green: 300–550 nm) and hue (0.60); (2) the second PC explained 27% of the variation and achieved strong loadings for brightness (0.85). An individual with a high positive PC1 score for rump colour was less pure UV–blue–green in the rump, with a more right–shifted hue. However, individuals with high positive PC2 scores for rump display brighter rump plumage.
We performed linear mixed models (MIXED SAS procedure) to test for differences in growth during the treatment and through the nestling period until day 15 post-hatching, lymphocyte counts and PC colour scores between nestlings from methionine-supplemented and nestlings from control nests. In all these analyses, nestlings were used as statistical units and thus the nest (nested inside the treatment) was introduced as a random factor to account for the non-independence of nestlings from the same nests. The treatment, the sex and the hatching order (seniors versus juniors) were introduced as fixed factors in analyses. In addition, brood size was introduced in analyses as a continuous effect to take into account the non-independence between hatching order and brood size and also because allocation rules may be likely to differ between large- and small-size broods. Because we aimed to study whether sex and position in the hatching hierarchy (i.e. individual variation) mediated nestling allocation between ornamental (i.e. colour) and other functional (i.e. condition, growth and immunology) traits, two and three-way interactions of fixed terms were also entered in our models. Nevertheless, whenever we found a significant interaction that included the treatment effect, we analysed the effect of the treatment on the split dataset (e.g. male versus female nestlings and/or seniors versus juniors) to explore the significance of this factor as a main effect within each group (Engqvist, 2005).
We compared the mortality rate (arcsin transformed) between treatment and control nests by performing an ANOVA model (general linear models SAS procedure). Mortality rate was measured as the proportion of chicks present in a given nest the day before the beginning of the treatment that died before fledging. This measure of mortality rate aimed to take into account a possible harmful effect of the treatment on nestling health.
Analyses were performed using SAS, version 9.1 (SAS Institute). Nonsignificant covariate interaction terms (P ≥ 0.8) were removed starting with the highest-order interactions down to the main effects sensuEngqvist (2005). The significance level was set at α = 0.05.
Data on nestlings used in the analyses are from individuals for which information on all the parameters measured is available (i.e. nestlings that fledge). Hence, nestling mortality just before treatment administration or later explains why sample sizes are reduced in some analyses compared to the initial brood sizes.
RESULTS
Chick growth
Wing growth of nestlings during the methionine administration (i.e. between days 3 and 6) was affected by the treatment in interaction with the position in the hatching hierarchy (Table 1). During treatment administration, the methionine induced a reduction of wing growth in senior but not in junior nestlings (Fig. 2, Table 2). During its administration, the experimental treatment did not affect the increase in body mass of nestlings either as a main effect or in interaction with position in the hatching hierarchy or sex (Tables 1, 2).
| Variable | Growth during the treatment | Growth through the nestling period until day 15 post-hatching | ||
|---|---|---|---|---|
| Test statistic | P | Test statistic | P | |
| (1) Wing | ||||
| Sex | F 1,61 = 1.31 | 0.26 | F 1,62 = 0.73 | 0.40 |
| Hatching order | F 1,61 = 45.85 | < 0.0001 | F 1,63 = 26.84 | < 0.0001 |
| Treatment | F 1,21 = 0.85 | 0.37 | F 1,21 = 0.89 | 0.36 |
| Sex × Hatching order | F 1,59 = 1.12 | 0.29 | F 1,59 = 0.03 | 0.87 |
| Sex × Treatment | F 1,60 = 1.09 | 0.30 | F 1,61 = 1.71 | 0.20 |
| Hatching order × Treatment | F 1,61 = 4.69 | 0.034 | F 1,60 = 0.70 | 0.41 |
| Sex × Hatching order × Treatment | F 1,58 = 1.82 | 0.18 | F 1,58 = 0.95 | 0.33 |
| Nest | Z = 2.4 | 0.008 | Z = 2.86 | 0.002 |
| Brood size | F 1,61 = 15.8 | 0.0002 | F 1,63 = 2.71 | 0.10 |
| (2) Weight | ||||
| Sex | F 1,62 = 7.87 | 0.007 | F 1,62 = 7.36 | 0.0086 |
| Hatching order | F 1,62 = 7.23 | 0.009 | F 1,62 = 6.52 | 0.01 |
| Treatment | F 1,21 = 0.01 | 0.92 | F 1,21 = 0.13 | 0.72 |
| Sex × Hatching order | F 1,60 = 0.55 | 0.46 | F 1,61 = 0.87 | 0.35 |
| Sex × Treatment | F 1,59 = 0.02 | 0.89 | F 1,60 = 0.46 | 0.50 |
| Hatching order × Treatment | F 1,61 = 1.09 | 0.30 | F 1,59 = 0.03 | 0.87 |
| Sex × Hatching order × Treatment | F 1,58 = 0.42 | 0.52 | F 1,58 = 0.0 | 0.97 |
| Nest | Z = 2.17 | 0.01 | Z = 3.02 | 0.001 |
| Brood size | F 1,62 = 0.21 | 0.64 | F 1,62 = 3.04 | 0.086 |
- All the interactions among independent variables were included in the models. Brood size was introduced in analyses as a continuous effect. Dependent variables were wing growth (1) and weight gain (2). The nest was introduced as a random effect (nested within the treatment) in all statistical models. Retained effects are shown in bold.
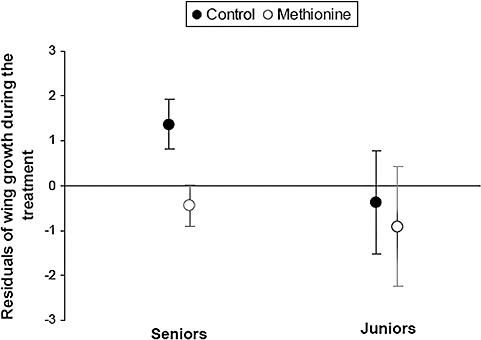
Effects of methionine-supplementation and nestling position in the hatching hierarchy on wing growth (mm) (mean ± SE) (interaction treatment × hatching order: F1,61 = 4.69, P = 0.034) during the supplementation period (between days 3 and 6) of roller nestlings. Senior (linear mixed model performed only on seniors: treatment effect, F1,21 = 3.86, P = 0.06; brood size effect, F1,39 = 13.97, P = 0.0006; nest effect, Z = 2.67, P = 0.0038) but not junior nestlings (linear mixed model performed only on juniors: treatment effect, F1,10 = 0.01, P = 0.93; brood size effect, F1,12 = 8.16, P = 0.01; nest effect, Z = 2.10, P = 0.01) showed a trend to reduce their wing growth in response to methionine administration. Position in the hatching hierarchy was established by classifying nestlings either as seniors if they were born in the three first positions of the hatching hierarchy or as juniors if they were born later on. The y-axis represents the residuals from the general linear mixed model performed controlling for the effect of brood size as a covariable and of nest (nested inside treatment) as a random factor (Table 1).
| Dependent variables | Control nests | Methionine nests | ||||||
|---|---|---|---|---|---|---|---|---|
| Seniors | Juniors | Seniors | Juniors | |||||
| Males | Females | Males | Females | Males | Females | Males | Females | |
| Wing growth 1a | 15.6 ± 1.2 (12) | 14.1 ± 1.4 (13) | 13.8 ± 1.5 (5) | 10.9 ± 2.0 (8) | 17.5 ± 1.4 (22) | 14.4 ± 1.5 (16) | 11.0 ± 2.8 (6) | 12.3 ± 2.8 (6) |
| Wing growth 2c | 61.4 ± 2.1 (12) | 61.6 ± 4.9 (13) | 58.6 ± 2.7 (5) | 51.6 ± 5.7 (8) | 65.0 ± 2.7 (22) | 58.5 ± 2.8 (16) | 57.0 ± 4.1 (6) | 58.5 ± 5.8 (6) |
| Weight gain 1b | 30.7 ± 2.1 (12) | 27.5 ± 2.2 (13) | 34.2 ± 4.1 (5) | 21.9 ± 3.3 (8) | 31.2 ± 1.9 (22) | 26.8 ± 2.9 (16) | 23.5 ± 4.1 (6) | 16.5 ± 9.1 (6) |
| Weight gain 2d | 79.7 ± 4.2 (12) | 78.9 ± 3.8 (13) | 92.8 ± 8.1 (5) | 75.2 ± 4.9 (8) | 84.9 ± 4.2 (22) | 84.0 ± 5.0 (16) | 89.0 ± 3.6 (6) | 82.0 ± 4.4 (6) |
| Lymphocytes | 42.5 ± 5.7 (11) | 42.1 ± 5.3 (12) | 47.6 ± 3.7 (5) | 50.4 ± 8.7 (8) | 50.7 ± 4.4 (19) | 48.6 ± 4.7 (13) | 50.3 ± 7.6 (6) | 51.7 ± 9.3 (6) |
| Belly PC1 | −0.08 ± 1.4 (12) | 0.7 ± 1.5 (12) | −0.4 ± 1.6 (5) | 0.4 ± 1.3 (9) | −0.5 ± 1.5 (21) | 0.3 ± 1.4 (16) | 1.1 ± 1.6 (6) | −0.7 ± 1.3 (6) |
| Belly PC2 | −0.2 ± 1.1 (12) | 0.1 ± 0.8 (12) | −0.8 ± 0.9 (5) | 0.2 ± 0.9 (9) | 0.01 ± 1.0 (21) | 0.1 ± 1.3 (16) | −0.01 ± 1.7 (6) | 0.4 ± 0.7 (6) |
| Rump PC1 | −0.8 ± 1.8 (12) | 0.5 ± 0.6 (12) | 0.6 ± 0.7 (5) | −0.1 ± 1.3 (9) | −0.5 ± 1.6 (21) | 0.2 ± 1.4 (16) | 0.5 ± 1.1 (6) | 0.3 ± 1.2 (6) |
| Rump PC2 | −0.08 ± 0.7 (12) | −0.3 ± 0.5 (12) | −0.6 ± 0.6 (5) | −0.4 ± 0.7 (9) | 0.5 ± 0.9 (21) | −0.1 ± 1.2 (16) | 0.3 ± 0.6 (6) | 0.2 ± 0.7 (6) |
- Values are mean ± SEM (number of nestlings). Superscript ‘a’ and ‘b’, respectively, show wing growth and mass increase of roller nestlings during the supplementation period (between day 3 and 6). Superscript ‘c’ and ‘d’, respectively, show wing growth and mass increase through the nesting period until day 15 post-hatching.
Neither wing growth, nor increase in body mass of nestlings through the nestling period until day 15 post-hatching were affected by the experimental treatment as a main effect or in interaction with position in the hatching hierarchy or sex (Tables 1, 2).
Chick survival
Mortality rate through the nesting period was higher in control (41.9%) than in methionine-supplemented nests (12.38%) (general linear models: treatment effect, F1,24 = 6.4, P = 0.02). Mortality mainly occurred during the first 15 days of nestling stage. Nestlings occupying different hatch ranks were not differently affected by mortality [ten out of 20 dead nestlings (16 control and four methionine-supplemented chicks) were seniors]. Sources of chick mortality, although unknown, appeared to be natural factors, such as starvation or predation.
Lymphocyte counts
Chicks raised in methionine-supplemented nests (mean ± SE, N = 50.18 ± 2.76, 44) showed higher, although not significantly, lymphocyte counts than chicks from control nests (mean ± SE, N = 44.83 ± 3.12, 36) (Table 3). This pattern, however, was unaffected by sex, position in hatching hierarchy, and the interaction terms (Tables 1, 2).
| Variable | Effect | Test statistic | P |
|---|---|---|---|
| Number of lymphocytes | |||
| Sex | F | F 1,56 = 0.13 | 0.72 |
| Hatching order | F | F 1,55 = 0.02 | 0.89 |
| Treatment | F | F 1,77 = 3.74 | 0.057 |
| Sex × Hatching order | F | F 1,52 = 0.17 | 0.68 |
| Sex × Treatment | F | F 1,53 = 0.26 | 0.61 |
| Hatching order × Treatment | F | F 1,54 = 1.32 | 0.25 |
| Sex × Hatching order × Treatment | F | F 1,51 = 0.10 | 0.75 |
| Nest | R | Z = 0.92 | 0.18 |
| Brood size | F | F 1,77 = 5.88 | 0.02 |
- All the interactions among independent variables were included in the model. Brood size was introduced as a continuous effect and the nest as a random effect (nested within the treatment). Retained effects are shown in bold.
Plumage colour
Nestling belly coloration was influenced by the interaction between the treatment, position in the hatching hierarchy, and the sex of nestlings (Tables 2, 4). Senior males of methionine-supplemented nests, and to lesser extent senior females, showed higher values of belly PC1 than seniors of control nests. Juniors from methionine-supplemented nests, however, showed lower values of belly PC1 than juniors from control nests (Fig. 3). Moreover, analyses performed on seniors and juniors separately showed that the effect of the treatment and the sex on belly coloration differed between senior and junior nestlings (Fig. 3). Indeed, belly coloration of senior nestlings was only affected by the sex, and males were consistently more colourful than females. In junior nestlings, belly coloration was affected by the treatment in interaction with the sex. In junior males, belly coloration was reduced in response to the treatment more than in junior females. The treatment did not affect belly PC2 either as a main effect or in interaction with the hatching position or sex (P > 0.1 in all cases) (Table 2).
| Variable | Effect | Test statistic | P |
|---|---|---|---|
| Belly PC1 | |||
| Sex | F | F 1,78 = 0.33 | 0.56 |
| Hatching order | F | F 1,78 = 0.60 | 0.44 |
| Treatment | F | F 1,78 = 0.19 | 0.66 |
| Sex × Hatching order | F | F 1,78 = 4.25 | 0.04 |
| Sex × Treatment | F | F 1,78 = 3.72 | 0.06 |
| Hatching order × Treatment | F | F 1,78 = 0.37 | 0.55 |
| Sex × Hatching order × Treatment | F | F 1,78 = 4.03 | 0.048 |
| Nest | R | Z = 0.61 | 0.27 |
| Brood size | F | F 1,78 = 5.13 | 0.03 |
| Rump PC1 | |||
| Sex | F | F 1,60 = 0.48 | 0.49 |
| Hatching order | F | F 1,60 = 3.68 | 0.06 |
| Treatment | F | F 1,21 = 0.09 | 0.77 |
| Sex × Hatching order | F | F 1,60 = 4.85 | 0.03 |
| Sex × Treatment | F | F 1,59 = 1.08 | 0.30 |
| Hatching order × Treatment | F | F 1,58 = 0.48 | 0.49 |
| Sex × Hatching order × Treatment | F | F 1,57 = 1.11 | 0.30 |
| Nest | R | Z = 1.80 | 0.036 |
| Brood size | F | F 1,60 = 0.60 | 0.44 |
- All the interactions among independent variables were included in the models. Dependent variables were belly PC1 and rump PC1. Analyses with belly and rump PC2 were also performed (see text) but they were not included in the table because of their lack of significance. Brood size was introduced as a continuous effect and the nest as a random effect (nested within the treatment) in all statistical models. Retained effects are shown in bold.
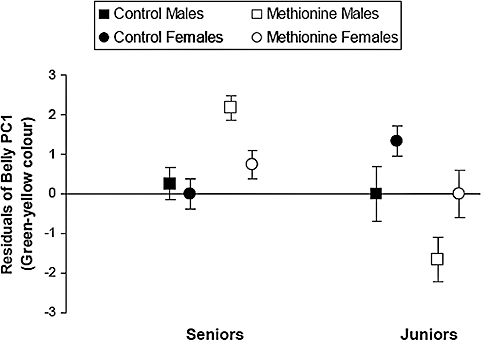
Effects of methionine-supplementation, nestling position in the hatching hierarchy and sex on values of the belly PC1 (mean ± SE) (Interaction treatment × hatching order × sex: F1,78 = 4.03, P = 0.048). Analyses performed on seniors and juniors separately show that the effect of the treatment and the sex on belly coloration differed between senior and junior nestlings. Indeed, belly coloration of senior nestlings was only affected by the sex and males were consistently more colourful than females (linear mixed model performed only on seniors: treatment × sex effect, F1,56 = 0.01, P = 0.94; treatment effect, F1,57 = 0.10, P = 0.75; sex effect, F1,59 = 4.77, P = 0.03; brood size effect, F1,58 = 3.39, P = 0.071). In junior nestlings, belly coloration was affected by the treatment in interaction with the sex (linear mixed model performed only on juniors: treatment × sex effect, F1,21 = 6.07, P = 0.02; treatment effect, F1,21 = 0.61, P = 0.44; sex effect, F1,21 = 0.90, P = 0.35; brood size effect, F1,21 = 3.82, P = 0.06). Belly coloration was reduced in response to the treatment more in junior males (linear mixed model performed only on junior males: treatment effect, F1,8 = 4.01, P = 0.08; brood size effect, F1,8 = 2.65, P = 0.14) than in junior females (linear mixed model performed only on junior females: treatment effect, F1,12 = 1.84, P = 0.20; brood size effect, F1,12 = 0.96, P = 0.35). Nestlings were considered either as seniors if they were born in the three first positions of the hatching hierarchy or as juniors if they were born later on. The y-axis represents the residuals from the general linear mixed model performed controlling for the effect of brood size as a covariable (Table 4).
Nestling rump PC1 was not affected by the treatment, either as a main effect or in interaction with the nestling position in the hatching hierarchy or sex (Tables 2, 4). Values of rump PC1 were only affected by the nest of origin and the interaction between nestling position in hatching hierarchy and sex (Table 4). The treatment did not affect rump PC2 either as a main effect or in interaction with position in hatching hierarchy or sex (P > 0.1 in all cases) (Table 2).
DISCUSSION
In the present study, we investigated the way in which the sex and position in the hatching hierarchy affect the allocation of resources among growth, immunity, and plumage coloration in nestling rollers in two contrasted environmental conditions (natural versus methionine-supplemented nests). The results obtained reveal that the allocation between ornamental and other functional traits during nestling development in the species is phenotypically plastic. Furthermore, as predicted, this plasticity is affected by nestling sex and position in the hatching hierarchy.
Overall, the methionine supplementation appeared to enhance performance of roller nestlings because the mortality rate was lower in methionine than in control nests. Additionally, lymphocyte production showed a trend to be activated in methionine compared to control nestlings. These results provide more evidence of the performance benefits (Tsiagbe et al., 1987) and immunoenhacing properties (Soler et al., 2003; Brommer, 2004) of methionine. Previous studies with wild birds found a positive effect of this sulphur aminoacid on immunocompetence, but a negative effect in growth shortly after the initiation of methionine administration (Soler et al., 2003; Brommer, 2004; Tschirren & Richner, 2006). In accordance with this, the results obtained in the present study could also show a trade-off between immunity and nestling growth during methionine administration, and this effect was mainly apparent in the senior nestlings of each brood. Furthermore, we found that senior nestlings in methionine-supplemented nests who reduced their growth in response to the methionine supplementation did not show less greenish structural plumage in the belly than seniors in control nests (2, 3). However, juniors from methionine-supplemented nests, who did not reduce their growth, paid the costs of maintaining this growth by displaying duller structural plumage in the belly than juniors in control nests (2, 3). This trade-off between ornaments and other functional traits is assumed to occur whenever resources are limited (Roff, 1992; Stearns, 1992). Many studies have shown a trade-off between allocating resources toward ornaments and immunity (Folstad & Karter, 1992; Verhulst, Dieleman & Parmentier, 1999; Peters et al., 2004) or survival prospects (Møller & de Lope, 1994; Veiga, 1995; Hunt et al., 2004; Muñoz et al., 2008). The compromise between growth and plumage coloration provides support for an environmental determination of structural nestling colorations in European rollers, which has been previously demonstrated for carotenoid-based nestling colorations in the great tit (Parus major) (Fitze, Kolliker & Richner, 2003) and the blue tit (Cyanistes caeruleus) (Hadfield & Owens, 2006), and structural nestling coloration in the eastern bluebird (Siefferman & Hill, 2007). The trade-off that we identified between body growth and plumage colour is consistent with the idea that structural colours are costly signals that require the allocation of resources that cannot be used in other functional traits (handicap principle) (Zahavi, 1975).
One alternative interpretation to our results is that methionine was responsible for improved conditions at supplemented-nests, leading to differences in mortality and immunity between the two groups. This explanation could easily explain the results obtained on plumage coloration if there are more senior chicks in methionine nests (as appears to be the case; Table 2), and they monopolize food, prejudicing juniors, and hence induce the reduction in their plumage coloration. However, the results obtained on wing growth cannot be explained within this framework, which reduces the plausibility of the explanation. In any case, our data show that some of the variation in structural plumage coloration of rollers is environmentally determined because different rearing environments cause different nestling phenotypes.
Nestling sex also had a role in the determination of resource allocation among traits under different environmental conditions. Adult male rollers display more exaggerated structural coloration than females (Silva et al., 2008), which may indicate that plumage colour has higher importance for males than for females. This may explain why, within a brood, senior males are more sensitive than senior females to the trade-off between growth and belly coloration. It may also explain why junior males were more sensitive than junior females to the trade-off between immunity and belly coloration. Therefore, plumage colour of males appears to be more plastic than that of females, which is expected because more exaggerated ornaments are likely to be more sensitive to environmental conditions. Some of this structural plumage is retained by juveniles until their first reproduction; thus, plumage colour at the nestling stage may be important in future fitness. As in the present study, Siefferman & Hill (2007) showed greater environmental sensitivity of blue structural coloration in male than in female eastern bluebird nestlings. Furthermore, Tschirren, Fitze & Richner (2003) showed that male great tit nestlings had higher susceptibility to parasites than females.
Resource allocation among traits under different environmental conditions (control versus methionine-supplemented nests) was affected by nestling position in the hatching hierarchy. This effect may be a result of the position of a nestling in the within-brood hierarchy or, alternatively, to nestling age at the moment methionine was administered. Methionine supplementation began the day the youngest chick was 3 days old, which, in highly asynchronous broods, may involve a difference of as much as 5 days between first-hatched seniors and last-hatched juniors with respect to the day that the treatment began. Therefore, nestling age during the experiment could also explain the effect of treatment on morphology. Nevertheless, the data obtained in the present study show that seniors and juniors allocated resources differently under different environmental conditions, either as a result of their position in the hatching hierarchy or their different age. The effect of the treatment on both wing growth during the treatment administration and belly colour just before fledging was modulated by nestling position in the hatching hierarchy or by nestling age. Methionine-supplemented seniors showed a trend to reduce wing growth compared to control seniors, which might be the factor that allowed them to allocate the same (females) or even more (males) than control seniors to plumage coloration (2, 3). At the same time, methionine-supplemented junior nestlings, which maintained their growth rates as control juniors, could not allocate resources to plumage coloration and showed a trend to reduce their belly coloration (2, 3). The importance of hatching position or nestling age determining the rules of allocation among different traits is interesting. Sibling competition is a key factor in the evolution of life history, behaviour, and physiology in a wide variety of organisms in which siblings compete for resources (Trivers, 1974; Mock & Parker, 1997). The order of hatching within an asynchronous brood and nestling age may determine nestling demands and may thus modulate their investment in life-history traits (Nilsson & Svensson, 1996). The question then is why nestlings allocate resources to plumage coloration if this is a costly trait. The function of conspicuous plumage coloration in altricial birds is poorly known (Tschirren, Fitze & Richner, 2005; Kilner, 2006). In this species, because first-breeders may still retain feathers grown as nestlings, one advantage of being colourful during the nesting period may be an increased probability of mating. Indeed, seniors, who grow more at early stages of the nesting period, may afford to pay the costs of the methionine supplementation at the level of growth and allocate more resources to structural plumage coloration. This resource allocation may provide these nestlings with advantages at the level of mating. In this case, the plumage colour of a nestling and its coloration as a breeding adult should be correlated. Unfortunately, we have no data available to test this prediction. Conversely, junior nestlings pay the effects of methionine at the level of plumage coloration and maintain their already reduced growth compared to seniors. In this case, the advantages of this resource allocation are likely to be obtained at the level of survival. Alternatively, plumage coloration of fledglings might have a function in parent–offspring communication and mediate parental favouritism either at the nest (Tschirren et al., 2005; Galván, Amo & Sanz, 2008) or later during the postfledging dependence period (Tanner & Richner, 2008). Also, the plumage coloration of nestlings might be used as an intra-age class indicator of dominance in the postfledging dependence period, as has been shown in the Eurasian kestrel (Falco tinnunculus) (Vergara & Fargallo, 2008).
Taken together, these results demonstrate the existence of phenotypically plastic trade-offs among growth, immunity, and plumage coloration in nestling rollers. Phenotypic plasticity indicates that priority rules may change under varying environmental conditions. Ornamental traits are likely to be disfavoured compared to other functional traits when energy is scarce. Furthermore, developmental plasticity is affected by sex and position in the hatching hierarchy in asynchronous nestling rollers, which suggests that individuals with different qualities and under different environmental conditions may develop the same phenotype. Finally, these sex- and hatching order-specific plasticity indicates that priority rules are likely to be affected by optimality at the level of the individual.
ACKNOWLEDGEMENTS
We thank all individuals who collaborated in data collection either in the field (C. Landsmann, V. Lartigot, and X. Mandine) or in the laboratory (J. M. Gasent). J. J. Soler, J. E. Brommer, and B. Tschirren kindly provided advice on the use of methionine. J. J. Soler also provided many interesting suggestions for the manuscript. Fieldwork was carried out by permission of the Junta de Extremadura and complied with the Spanish laws. This research work was partially supported by a doctoral grant to N.S. by the European Social Fund, an I3P contract to D.P. funded by the European Social Fund and by the Spanish Ministerio de Educación y Ciencia-FEDER, Secretaría de Estado de Universidades e Investigación (project ref. CGL2005-04654/BOS).




