Offspring performance is linked to parental identity and male breeding ornamentation in whitefish
Abstract
The ‘good genes’ hypothesis predicts that males advertise their quality with different sexual ornaments and that females are able to recognize the genetic quality of males by evaluating these characteristics. In the present study, we investigated the parental effects on offspring performance (feeding and swimming ability of newly-hatched larvae) and examined whether male ornamentation indicates offspring success in performance trials of whitefish (Coregonus lavaretus Linnaeus). Offspring first-feeding success had a strong paternal effect and it was also positively correlated with the size of male breeding tubercles, indicating that breeding ornamentation of males can function as an honest indicator of their genetic quality. In addition, the observed positive correlation between male tubercle size and condition factor suggests that highly ornamented males are efficient foragers and that this trait may have a heritable basis. By contrast to feeding success, only a maternal effect was found in the swimming ability of the larvae. Clear family-specific differences observed in both measures of performance strongly suggest that parental identity may have important effects on larval survival in the wild. © 2009 The Linnean Society of London, Biological Journal of the Linnean Society, 2009, 98, 532–539.
INTRODUCTION
According to sexual selection theory, female choosiness for males is based either on direct benefits such as resources and parental care or indirect benefits (good genes) (Hamilton & Zuk, 1982; Andersson, 1994). In the latter, nonresource-based mating systems males provide only genes to their mates (Neff & Pitcher, 2005), and thus females should select male genes that maximize the survivorship or mating success of the offspring. The ‘good genes’ hypothesis predicts that males advertise their quality with different sexual ornaments and females are able to recognize the genetic quality of males on the basis of these ornaments. Sexual ornaments are often costly to produce or maintain (Maynard Smith & Harper, 2003) and elaborate ornaments are believed to be handicaps that reduce the survival probability of the bearer (Zahavi, 1975). However, it has been demonstrated that signals can sometimes be both reliable and cost-free (Hurd, 1997; Taylor, Hasson & Clark, 2000; Maynard Smith & Harper, 2003; Byers & Waits, 2006).
Despite the strong theoretical basis of the good genes hypothesis, only a few studies have presented direct genetic evidence supporting the hypothesis (Welch, Semlitsch & Gerhardt, 1998; Eilertsen et al., 2009). Earliest empirical tests of the hypothesis had a strong bias towards a few vertebrate taxa (Møller & Alatalo, 1999) and resource-based mating systems (Milinski & Bakker, 1990; Hill, 1991; Iyengar & Eisner, 1999), in which the confounding effect of nongenetic (i.e. direct) benefits, such as the effect of male care could not often be ruled out. Because the selection for direct benefits may overwhelm the selection for genetic benefits (Kirkpatrick & Barton, 1997), the genetic effects of sexual selection are ideally tested in species with nonresource-based mating systems. Accordingly, more recent studies, for example, as conducted in frogs (Welch et al., 1998) and fish (Wedekind, Müller & Spicher, 2001; Kortet et al., 2004b; Rudolfsen et al., 2005), have concentrated on mating systems in which females receive no resources from males. Because whitefish (Coregonus lavaretus) males do not provide paternal care to offspring and because females produce large number of eggs that are externally fertilized, the species is an ideal model species for the test of genetic effects of sexual selection. Whitefishes (Coregonus spp.) are freshwater fish species in which both sexes develop a distinct breeding ornamentation (i.e. keratinized epidermal tubercles prior to the spawning period) (Wiley & Collette, 1970; Wedekind et al., 2001, 2008). It has been suggested that the primary function of breeding tubercles in whitefish is to maintain contact between individuals during spawning, although the conspicuous white colour and hardness of these ornaments may enable tubercles to be used also as visual and tactile signals (Wiley & Collette, 1970). Even though it is not known whether these tubercles are costly to produce (Wedekind et al., 2008; but see also Kortet et al., 2003), previous studies have demonstrated that the size and/or number of the breeding tubercles are linked to individual condition (Wedekind, 1992; Rudolfsen et al., 2008), parasite resistance (Kortet et al., 2004b) and male dominance (Kortet et al., 2004a). In addition, Wedekind et al. (2001) demonstrated strong maternal and paternal effects in egg mortality of whitefish, and reported a negative association between the size of male breeding tubercles and embryonic mortality (i.e. mortality caused by bacterial infection could be predicted by male ornamentation). Because of the inconsistent results obtained with respect to the embryonic mortality of whitefish, however, Wedekind et al. (2008) recently suggested that only some aspects of genetic quality are revealed by breeding tubercles or, alternatively, individuals vary in their sexual signalling strategies regarding ornamentation.
The association between secondary sexual traits and survival of the offspring has been demonstrated in numerous studies (Møller & Alatalo, 1999; Wedekind et al., 2001, 2008) but fitness-related offspring performance is much less studied. There are, however, indications that predator avoidance of the offspring is positively linked to male ornamentation (Sheldon et al., 2003; Evans et al., 2004). Whole organism performance integrates numerous morphological, physiological, and behavioural characteristics of individuals (Ghalambor, Walker & Reznick, 2003). Because natural selection rarely acts on a single trait alone (Calsbeek & Irschick, 2007), performance measures are especially suitable for examination of genetic variation in fitness components. For example, feeding and swimming ability of newly-hatched fish larvae are crucial factors determining subsequent growth and survival of the larvae (Braum, 1978; Fuiman & Magurran, 1994; Fuiman & Higgs, 1997; Mahjoub et al., 2008). Similarly, poor foraging skills of inexperienced juveniles have been suggested to result in starvation in birds (Sullivan, 1989; Ward & Kennedy, 1996). In the present study, we examined the parental effects on two offspring fitness performance measures (feeding and swimming ability of newly-hatched larvae) and studied whether male ornamentation indicates offspring success in these performance tests. For this purpose, we created sib groups by crossing randomly selected parents from a wild whitefish population.
MATERIAL AND METHODS
Parental fish and creation of sib groups
Parental fish originated from the Koitajoki River, eastern Finland. The fish were caught by seining in the early November 2007 and kept in submerged holding cages. Fish sizes are given in Table 1. On 9 November 2007, we stripped eggs from two ready-to-spawn females and distributed them into ten batches on Petri dishes. We collected sperm from ten males and used 10 µL to fertilize egg batches in all possible combinations resulting in 20 sib groups. Activation of sperm was performed using water from the Koitajoki River. On 12 November 2007, the fertilization rate of each sib group was determined from a random sample of approximately 100–150 eggs using microscopic examination. Subsequently, each group was divided into three replicates and the eggs were incubated in nonchlorinated tap water at 4 °C until hatching in March 2008. Dead eggs were removed weekly during the incubation period. To measure the size of the breeding tubercles of the parental fish, we made plaster casts immediately after fertilization, as described by Wedekind et al. (2001), and the average cast depth (with 0.01 mm accuracy) of ten tubercles in the middle row anterior to the anal fin was measured by a dial indicator (Mitutoyo; Table 1). To estimate the precision of these measurements, 80 tubercles in eight fish were measured twice. The coefficient of determination (r2) of the linear regression between the measurements was 0.681, indicating high repeatability of the measurement method. The condition factor (K) of the males was calculated using the equation: K = 100 × fresh mass (g)/[body total length (cm)]b, where b is the slope of a regression of log10 (mass) on log10 (length) of the population (Bolger & Connolly, 1989).
| Fish number | Total length (cm) | Fresh mass (g) | Mean ± SD tubercle size (mm) |
|---|---|---|---|
| Females | |||
| 1 | 32.0 | 285 | 0.07 ± 0.02 |
| 2 | 32.8 | 293 | 0.16 ± 0.04 |
| Males | |||
| 1 | 34.5 | 339 | 0.35 ± 0.05 |
| 2 | 32.7 | 298 | 0.20 ± 0.06 |
| 3 | 33.1 | 293 | 0.31 ± 0.07 |
| 4 | 35.6 | 355 | 0.39 ± 0.12 |
| 5 | 33.6 | 274 | 0.08 ± 0.02 |
| 6 | 35.8 | 352 | 0.27 ± 0.06 |
| 7 | 33.0 | 277 | 0.14 ± 0.04 |
| 8 | 35.3 | 333 | 0.24 ± 0.16 |
| 9 | 35.7 | 330 | 0.17 ± 0.10 |
| 10 | 34.9 | 334 | 0.16 ± 0.10 |
Feeding experiment
After hatching, we investigated the first-feeding performance of yolk-sac larvae by feeding experiments. Individual food-naïve larvae were allowed to feed on Artemia nauplii in 500-mL plastic containers for 5 min. Water temperature was 7 °C, with a water volume of 100 mL and an Artemia density of 2750 nauplii 100 mL−1. The number of larvae in each sib group varied in the range 7–21. After the experiments, the larvae were anaesthetized in an overdose of MS-222 and preserved in a solution of 70% ethanol and 1% neutralized formalin. The larvae were measured for total length (TL), and the length (l) and height (h) of yolk were also measured for the calculation of yolk volume (V) by the equation for a prolate spheroid: V = 0.5236 × l × h2 (Blaxter & Hempel, 1963; Huuskonen, Penttinen & Piironen, 2003). The number of Artemia ingested by each larva was counted by dissecting the alimentary tract under microscope.
Swimming experiment
To determine the swimming ability of yolk-sac larvae, we constructed a simple swimming tube system with gravity-driven flow. Briefly, the system consisted of aquarium pump which lifted water into 10-L plastic container at a height of 60 cm. Water flowed from the container into a tube (diameter 9 mm) at a constant velocity of 6.2 cm s−1. This corresponds to 5.3 body lengths s−1 in the tested larvae with mean TL of 11.6 mm. In the experiments, individual larvae were forced to swim against a current at 7 °C, and their fatigue time was recorded when they drifted against a net placed at the rear end of the tube and could not continue swimming within 5 s. Because of unfortunate incidents during the egg incubation period, the number of larvae in each sib group varied in the range 2–21 and, in four groups, no larvae were left to be tested. After the experiments, the larvae were preserved and measured as described above. In addition, the number of preanal myomeres was counted because locomotory performance may be functionally related to vertebral number (Swain & Lindsey, 1984).
Statistical analysis
The effects of male and female identity, as well as their interaction on the offspring feeding performance, were analysed in two different ways. First, we scored the ingestion ability of the larvae on the basis of whether they had fed or not (1/0), and tested the effects of parental identity on this binary response variable by a generalized linear model with binomial error distribution and logit link function. Second, we analysed the number of Artemia eaten (termed feeding success) by a generalized linear model with negative binomial error distribution and log link function. This approach was chosen because the count data were strongly skewed and overdispersed. The swimming experiment data were also skewed but log-transformation normalized the distributions and enabled the use of analysis of covariance (ANCOVA). The effects of male and female identity, as well as their interaction on the offspring swimming time, were tested by two-way random effects Ancova using TL of the larvae as a covariate. The relationship between larval swimming time and their myomere count was examined by Pearson's correlation after log-transformation of both variables. The correlation between male breeding tubercle size and offspring feeding success was analysed by a semi-partial correlation analysis (effect of yolk volume partialled out) after log-transformation of the initial variables. All analyses were carried out using SPSS, version 15.0 (SPSS Inc.).
RESULTS
There was large family-specific variation in the first-feeding performance of whitefish larvae. The mean ingestion ability varied in the range 0–80% (Fig. 1A) and, in the most successful sib group, the mean feeding success was over five nauplii per larva, whereas, in the poorest group, none of the larvae had ingested nauplii (Fig. 1B). Individual variation in the feeding success also was large, in the range 0–24 nauplii per larva. The effect of male on the offspring ingestion ability was statistically significant, whereas the effects of female as well as female–male interaction were not significant (generalized linear model; male: χ2 = 21.12, d.f. = 9, P = 0.012; female: χ2 = 0.00, d.f. = 1, P = 0.999; female × male: χ2 = 8.52, d.f. = 9, P = 0.482). In the case of offspring feeding success, the effect of male was statistically highly significant, whereas the effects of female as well as female–male interaction were not significant (generalized linear model; male: χ2 = 1752.90, d.f. = 9, P < 0.001; female: χ2 = 0.44, d.f. = 1, P = 0.505; female × male: χ2 = 1.90, d.f. = 8, P = 0.984). This paternal effect is clearly visible in Figure 1B: offspring feeding success of different males tends to be at similar level regardless of the female. Because there was a weak but statistically significant negative correlation between the number of Artemia ingested and yolk volume of the larvae (r = −0.144, N = 250, P = 0.023), we used a semi-partial correlation analysis to examine the relationship between the size of male breeding tubercles and offspring feeding success. In the analysis, yolk volume was used as a control variable and the data of both females were pooled because there was no maternal effect in the feeding success. There was a significant positive correlation between the offspring feeding success and size of male breeding tubercles (semi-partial correlation, with the effect of yolk volume partialled out, r = 0.663, N = 10, P = 0.030). A significant positive correlation existed also between the condition factor of males and size of their breeding tubercles (r = 0.777, N = 10, P = 0.008), whereas the latter did not correlate with fresh mass or total length of the males (P > 0.05).
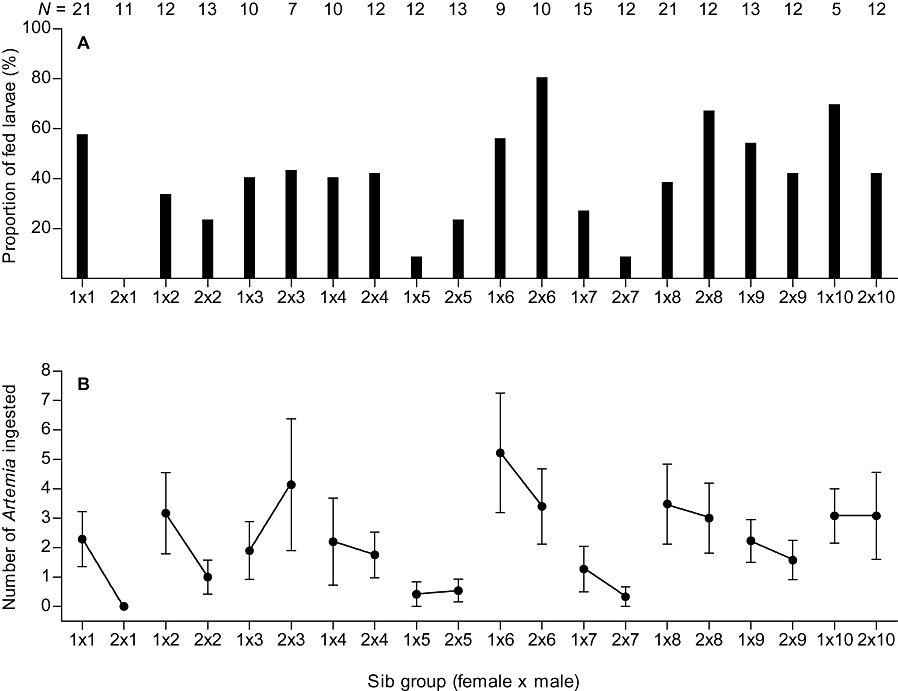
Larval ingestion ability (A) and feeding success (B) of different sib groups (groups sired by the same male have been connected by a line). Numbers of larvae (N) tested are also given.
Family-specific variation was high also in the swimming ability of the larvae. In most sib groups, the mean fatigue time was approximately 20–40 s, but there were two groups in which the time exceeded 60 s (Fig. 2). As a result of missing observations in four sib groups (Fig. 2), we performed Ancova using a subset of the data with observation in all cells. This left out of the analysis offspring sired by males number 1, 6, and 7. The effect of female on the offspring swimming ability was statistically significant, whereas the effects of male, female–male interaction and TL of the larvae were not (two-way random effect Ancova with TL as a covariate; female: F1,10 = 10.7, P = 0.008; male: F6,5 = 1.0, P = 0.468; female × male: F6,147 = 0.5, P = 0.739; TL: F1,147 = 1.2, P = 0.269). Female number 2 had larger breeding tubercles (Table 1) and produced, with all males, better swimming larvae than female number 1 (Fig. 2), although there was no difference in TL of the offspring between the females (random effect analysis of variance, F1,199 = 1.4, P = 0.226). There was a weak but statistically significant positive correlation between swimming ability of the larvae and their myomere number (r = 0.183, N = 187, P = 0.012).
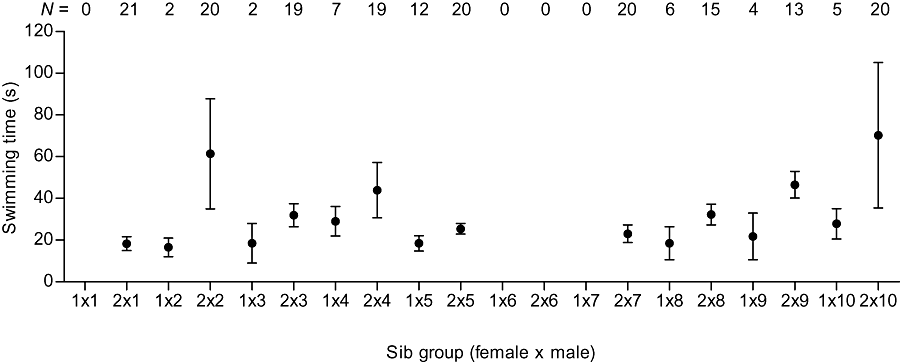
Larval swimming ability of different sib groups. Numbers of larvae tested (N) are also given.
DISCUSSION
Previous studies have demonstrated both maternal and paternal effects on embryonic mortality in whitefish (Wedekind et al., 2001, 2008), and some aspects of survival were positively linked to male tubercle size. In the present study, offspring first-feeding success was affected by the male and it was also positively correlated to the size of male breeding tubercles. Hence, the results obtained in the present study suggest that male breeding ornamentation may function as an honest indicator of genetic quality of males. Coregonids start external feeding soon after hatching when they still have considerable yolk resources left (Braum, 1978; Koho, Karjalainen & Viljanen, 1991; Kamler, 1992). Active feeding during the yolk sac stage increases body mass and improves resistance to starvation (Koho et al., 1991). Larvae with high feeding success are not only resistant to starvation, but also are likely to grow rapidly and suffer lower predation rates (Fuiman & Magurran, 1994), as well as gain competitive advantage over conspecifics (Abbott & Dill, 1989). The observed positive correlation between male tubercle size and condition factor suggests that highly ornamented males are efficient foragers and, according to the results of the present study, this trait may have a heritable basis. Fitness benefits for females mating with highly ornamented males have previously been shown to include lower offspring mortality (Møller & Alatalo, 1999; Wedekind et al., 2001, 2008), higher offspring parasite resistance (Barber et al., 2001), higher offspring growth rate (Eilertsen et al., 2009), as well as better offspring condition (Sheldon et al., 1997). In a study conducted by Barber et al. (2001), a trade-off between growth rate and parasite resistance was observed because the resistant individuals grew less quickly than susceptible conspecifics. It is likely that trade-offs between different fitness components explain the results in which negative correlations between viability and secondary sexual characters have been observed (Møller & Alatalo, 1999).
By contrast to the feeding success, no paternal effect was found in the swimming ability of the larvae. This is in accordance with a study conducted by Evans et al. (2004) who found no effect of male ornamental coloration on offspring swimming speed under simulated predation risk. In the adult guppy (Poecilia reticulata), however, a positive correlation between the intensity of male carotenoid ornamentation and swimming performance was detected (Nicoletto, 1991). The swimming ability is dependent on the size of the fish (Hammer, 1995). Hence, it is essential to control for fish size in fatigue tests. In the present study, however, larval length did not have an effect on swimming ability, most likely as a result of the small size range (10.6–12.5 mm) of the newly-hatched larvae. The strong size-dependency of swimming ability makes it more likely to be influenced by maternal than paternal effects (Evans et al., 2004). Indeed, the present study revealed a maternal effect, although the study did not originally aim to examine female influence because of the small number of females. Female effects on offspring traits can be divided into genetic and nongenetic effects. The latter represent inherited environmental effects that reflect female phenotypic investment on the offspring (Bernardo, 1996). In fish, this is commonly related to female size: large females produce large eggs which, in turn, generally result in large larvae (Kamler, 1992; Chambers & Leggett, 1996). In the present study, the females were of very similar size (Table 1) and there was no difference in the size of their offspring either. Thus, the female effect appeared to be of a qualitative rather than quantitative nature. Several studies have, for example, demonstrated a positive relationship between offspring viability and egg nutrient content (Kamler, 1992).
Many studies have found strong associations between morphology and performance in vertebrates (Billerbeck, Lankford & Conover, 2001; Iriarte-Diaz, 2002; Daley & Biewener, 2003; Calsbeek & Irschick, 2007), as well as in invertebrates (Fish & Nicastro, 2003; Berwaerts, Aerts & Van Dyk, 2006). We did not examine morphology but, instead, included a meristic trait to explain the swimming performance of the larvae. An interesting observation was the positive correlation between the swimming ability of the larvae and their preanal myomere count. The number of myomeres corresponds to the number of vertebrae, and their function is to produce lateral bending of the axial skeleton during swimming (Pabst, 2000) when the number of body segments is correlated with body curvature (Coughlin, 2002). Vertebral number is partly genetically and partly environmentally determined; the number decreases with increasing incubation temperature (Lindsey, 1988). Furthermore, Swain (1992) reported a dependence of burst swimming speed on vertebral phenotype in larval stickleback (Gasterosteus aculeatus), but the locomotory performance was more directly related to the ratio of abdominal to caudal vertebrae than to the total number of vertebrae. Because the swimming ability of the larvae is closely connected to their predator evasion success (Fuiman & Magurran, 1994), myomere number may be a significant meristic character affecting larval survival. Although the absolute swimming ability of larval fish is generally poor, confusion created by sudden movement during predator attack may be sufficient to increase the probability of survival (Webb, 1981). Indeed, Swain & Lindsey (1984) reported that stickleback larvae with different vertebral numbers were selectively predated under laboratory conditions. Furthermore, there are observations indicating that body segment count distributions shift during larval development in the wild (Swain, 1992; Helminen & Sarvala, 1995). Although, in stickleback, natural selection could be attributed to vertebral ratio rather than their number (Swain, 1992), in vendace (Coregonus albula, a close relative of whitefish), larvae with highest myomere counts were strongly selected during the first 5 weeks after hatching (Helminen & Sarvala, 1995).
It is generally recognized that the early life stages play an important role in population dynamics of fish and other aquatic organisms because, as a result of the high fecundity of many species, relatively small variations in embryonic or larval mortality rates can produce large differences in the sizes of different year classes (May, 1974; Braum, 1978; Watanabe, Yamashita & Oozeki, 1996). It is common that, for example in fish, mortality from egg to adult stage is 99.9% or more (Braum, 1978), and starvation and predation are generally the most important causes of mortality during the larval period (Miller et al., 1988). In the present study, first-feeding success and swimming ability of the larvae were suggested to reflect starvation and predation resistance, respectively. Because of the extremely high natural mortality rate of larvae, their survival is often considered to be random, although there is growing empirical evidence for nonrandom selection on phenotype, so that the survivors are exceptional individuals with respect to some adaptive traits (Fuiman & Cowan, 2003). The results obtained in the present study (i.e. clear family-specific differences observed in both measures of performance) strongly suggest the presence of parental effects in the survival rate of the larvae in the wild.
ACKNOWLEDGEMENTS
We thank Lucile Savigny, Päivi Väisänen, and Kaisa Figueiredo for their assistance during the experiments. Alain Jacob and Harri Kirjavainen, as well as Juha Karjalainen, are acknowledged for their advice regarding plaster casts and the swimming tube system, respectively. Raine Kortet provided valuable comments on an earlier version of the manuscript. The study was financially supported by the Maj and Tor Nessling Foundation (grants 2007075 and 2008109) and the Academy of Finland (grant 121694).




