Patterns of self compatibility, inbreeding depression, outcrossing, and sex allocation in a marine bryozoan suggest the predominating influence of sperm competition
Abstract
Sex allocation by simultaneous hermaphrodites is theoretically influenced by selfing rate, which is in turn influenced by the benefits of enhanced genomic transmission and reproductive assurance relative to the cost of inbreeding depression. The experimental investigation of these influences in seed plants has a rich pedigree, yet although such an approach is equally relevant to colonial invertebrates, which globally dominate subtidal communities on firm substrata, such studies have been scarce. We reared self-compatible genets of the marine bryozoan Celleporella hyalina s.l. in the presence and absence of allosperm, and used molecular genetic markers for paternity analysis of progeny to test theoretical predictions that: (1) genets from focal populations with high selfing rates show less inbreeding depression than from focal populations with low selfing rates; (2) genets whose selfed progeny show inbreeding depression prefer outcross sperm (allosperm); and (3) genets bias sex allocation toward female function when reared in reproductive isolation. Offspring survivorship and paternity analysis were used to estimate levels of inbreeding depression and preference for outcrossing or selfing. Sex allocation was assessed by counting male and female zooids. As predicted, inbreeding depression was severe in selfed progeny of genets derived from the populations with low self-compatibility rates, but, with one exception, was not detected in selfed progeny of genets derived from the populations with higher self-compatibility rates. Also, as predicted, genets whose selfed progeny showed inbreeding depression preferred outcrossing, and a genet whose selfed progeny did not show inbreeding depression preferred selfing. Contrary to prediction, sex allocation in the majority of genets was not influenced by reproductive isolation. Lack of economy of male function may reflect the over-riding influence of allosperm-competition in typically dense breeding populations offering good opportunity for outcrossing. We suggest that hermaphroditism may be a plesiomorphic character of the crown group Bryozoa, prevented by phylogenetic constraint from being replaced by gonochorism and therefore not necessarily adaptive in all extant clades. © 2009 The Linnean Society of London, Biological Journal of the Linnean Society, 2009, 98, 519–531.
INTRODUCTION
Colonial invertebrates such as sponges and corals dominate sublittoral communities on hard substrata throughout the world's oceans (Jackson, 1977). Their sessile habit, modularity, and external dissemination of male gametes are features shared with higher plants, generating selection pressures common to the reproductive biology of both life forms (Harper, 1977; Hughes, 1989b, 2005). A notable consequence of such selection is the frequent occurrence of hermaphroditism (Ghiselin, 1969; Jarne & Charlesworth, 1993; Knowlton & Jackson, 1993). Simultaneous hermaphroditism, as opposed to sequential hermaphroditism, or sex reversal, provides the opportunity for self-fertilization (Darwin, 1876; Stebbins, 1950; Jain, 1976), which has been shown to occur in most seed plants (Jarne & Charlesworth, 1993). The relatively sparse information on modular colonial invertebrates suggests that at least 30% are capable of selfing and further studies are likely to inflate this estimate (Knowlton & Jackson, 1993). Self-fertilization offers a potential selective advantage through increased genomic transmission (Fisher, 1941; Jain, 1976), but such an advantage may be proportionately offset by inbreeding depression (Lloyd, 1979; Lande & Schemske, 1985; Charlesworth & Charlesworth, 1987). It is predicted that such disruptive selection may promote the evolution of a bimodal distribution of outcrossing frequency, t, with populations showing either extremely low or high levels (Lande & Schemske, 1985). An earlier survey of seed plants supported the above prediction by reporting peak frequencies at low (t ≤ 0.2) and high (t > 0.8) outcrossing rates, but also reported intermediate rates in 31% of the populations studied (Schemske & Lande, 1985). Subsequent reanalysis of cumulative data has raised the known frequency of intermediate outcrossing rates to 42% (Goodwillie, Kalisz & Eckert, 2005). Similarly, for hermaphroditic animals, Jarne & Auld (2006) reported intermediate outcrossing rates (0.2 < t ≤ 0.8) in 47% of populations studied. Reconciliation between observation and theory might be sought in terms of evolutionary disequilibrium, or of contingencies such as reproductive assurance, whereby zygotes are still produced when outcross gametes are unavailable (Darwin, 1876; Baker, 1955) and gamete discounting, as discussed further below (Goodwillie et al., 2005; Jarne & Auld, 2006).
Self-fertilization offers the possibility of adjusting sex allocation to economize on male function, with concomitant enhancement of fitness (Maynard Smith, 1978; Charnov 1979). Theoretical models of sex allocation in seed plants vary according to the type of mating system envisaged. Hermaphroditic flowers may exhibit ‘autogamy’ where ovules are fertilized by a flower's own pollen, although mechanisms exist in some species to prevent this, and/or ‘geitonogamy’ where ovules are solely fertilized by pollen from other unisexual flowers on the same plant. Selfing often bears the cost of inbreeding depression, which may be further exacerbated through lost outcrossing opportunity via the reduced export of pollen (pollen discounting) and/or reduced availability of virgin ovules (seed discounting). Pollen discounting will depend on the pollination mechanism, whereas seed discounting is likely to be ubiquitous. Zoophilous pollination involves significant discounting because the supply of both pollen and animal vectors is limited (Lloyd, 1992), whereas anemophilous pollination probably does not because only a negligible fraction of released pollen will be intercepted by flowers on the parental plant and the wind vector is not depleted (de Jong, Klinkhamer & Rademaker, 1999). Models of sex allocation in anemophilous plants, which conform to an evolutionarily stable strategy of selfing rate and inbreeding depression, predict higher allocation to female function relative to the male at higher selfing rate, even despite severe inbreeding depression (Charlesworth & Charlesworth, 1981; de Jong et al., 1999). These models should also apply to colonial invertebrates because fertilization by water-borne sperm is analogous to wind pollination. Pioneering studies have addressed outbreeding/inbreeding depression (Grosberg, 1987; Hoare & Hughes, 2001), characterization of male reproductive success, and assessment of outcrossing rate and levels of inbreeding in relation to spatial distribution and sperm dispersal (Yund & McCartney, 1994; Coffroth & Lasker, 1998; Ayre & Miller, 2006; Sherman, 2008). However, theoretical predictions of sex allocation in relation to the selfing rate remain to be tested in colonial invertebrates.
We measured inbreeding depression, outcrossing rate, and sex allocation among laboratory-reared genets of the marine bryozoan Celleporella hyalina (L.) s.l. to test the following predictions: (1) genets from focal populations with high selfing rates show less inbreeding depression than from focal populations with low selfing rates (deleterious mutations are purged by repeated selfing); (2) genets showing inbreeding depression prefer allosperm (outcrossing takes precedence over selfing, reducing inbreeding depression); and (3) genets bias sex allocation toward female function when reared in reproductive isolation (economy of male function enhances fitness through female function).
Celleporella hyalina encrusts macroalgae and other firm substrata in temperate/polar seas throughout the northern hemisphere and comprises a series of phylogeographically distinct clades (Gómez et al., 2007a). Colonies typically form clusters within fronds. Nevertheless, larvae may settle alone through vagaries of dispersal and, despite efficient uptake and storage of dilute allopserm (Pemberton et al., 2003), the opportunity for outcrossing may be constrained (McCartney, 1997). Celleporella hyalina is amenable to clonal propagation and readily completes its life cycle in culture, facilitating controlled mating trials (Gómez et al., 2007a). Brooded embryos can be monitored in vivo, allowing inbreeding depression to be assessed from an early stage (Hoare & Hughes, 2001). Although male allocation commences earlier than female in colonial ontogeny, colonies are simultaneously hermaphrodite once fully mature (Cancino & Hughes, 1988). Resource allocation can be measured in relative terms by counting the morphologically differentiated male, and female and trophic zooids (Cancino & Hughes, 1988). Mating occurs by geitonogamy. Water-borne sperm are entrained in the feeding currents of trophic zooids and, via unknown mechanisms, are stored and translocated to female zooids (Pemberton et al., 2003), which nourish single embryos within a brood chamber (ovicell) by placental brooding over a gestation period of 3–4 weeks. A free-swimming larva emerges from the brood chamber and settles within 4 h (Cancino, Hughes & Ramirez, 1991). The metamorphosed larva starts budding within 24 h and, by 1–2 weeks, the young colony, comprised of just a few trophic zooids, is capable of storing allosperm (Hughes, Manríquez & Bishop, 2002a), even though the first female zooids will not appear for some further 8 weeks. Different geographical populations of C. hyalina vary in the ability of colonies to produce embryos when grown in reproductive isolation (Hughes et al., 2002b). Enforced inbreeding among sibling and half-sibling progeny of self-incompatible genets typically produces severe inbreeding depression in the F2 generation (Hoare & Hughes, 2001). In a minority of populations studied, however, all colonies appear to be reproductively self compatible and preliminary observation of selfed progeny has revealed no sign of inbreeding depression (Hughes et al., 2002b).
The relative contribution of outcrossing and selfing to the progeny of mixed matings is difficult to measure in the field, and data are correspondingly scarce (Charlesworth & Charlesworth, 1981). Outcrossing and complementary selfing rates are potentially subject to variation within and among individuals, reflecting both extrinsic factors, such as the availability of outcross male gametes, and intrinsic factors, including gametic self compatibility. Population frequency of gametic self compatibility affords a partial index of selfing rate and is readily assessed from the performance of samples maintained in reproductive isolation. Using this approach, Hughes et al. (2002b) identified allopatric populations with high, intermediate, and low frequencies of self compatibility. They established two self-compatible genets from a population with high self-compatibility rate (to which we added two more genets from another population in the same geographical locality), two from a population with intermediate self-compatibility rate, and one from each of two populations with low self-compatibility rate. Constraints of time and resources prohibited the sourcing and inclusion of genets from additional populations and therefore the influence of population as a main effect in the experimental design could not be tested. Conversely, vegetative propagation enabled good replication within genets, facilitating a two-way experimental design with genet and mating type (selfing/outcrossing) as main effects. Therefore, we were able to achieve high resolution of mating characteristics and sex allocation for a set of up to eight genets derived from focal populations with markedly different self-compatibility rates, thus enabling the above hypotheses to be tested.
The results are interpreted in the light of similarities and differences between the mating systems of seed plants and sessile colonial invertebrates. We conclude by considering the evolutionary persistence of universal hermaphroditism in Bryozoa.
MATERIAL AND METHODS
Source of genets
Genets derived from five geographically isolated populations showing different frequencies of gametic self compatibility (Table 1; Hughes et al., 2002b; Gómez et al., 2007a, b; R. N. Hughes & P. J. Wright, unpubl. data) were selected from established cultures kept in reproductive isolation from the moment of larval metamorphosis. Genets were named according to population of origin: Amlwch, North Wales (WA); Lough Hyne, South-West Ireland (IR); Halifax, Nova Scotia (NS); Woods Hole, Massachusetts (WH); and Sakkonet, Rhode Island (RI). NS, WH, and RI each were represented by two self-compatible genets that were able to serve for both selfing and reciprocal outcrossing. WA and IR each were represented by one self-compatible genet, requiring a self-incompatible genet to be included as the outcross partner. The populations belong to phylogeographically distinct clades (Gómez et al., 2007a). WA and IR represent a NE Atlantic clade distributed from Iceland to North West Spain; NS represents a Fennoscandian clade and was probably introduced to the Halifax marina via boat traffic; WH1, WH2, RI1, and RI2 represent the ‘Woods Hole’ clade of Gómez et al. (2007a), occurring between Martha's Vineyard and the northern tip of Long Island.
| Source population | Population self-compatibility rate (proportion self-compatible) | Experimental genet | Genet self-compatibility | Paternity marker | Mating trial (paired genets) | Expected mating type |
|---|---|---|---|---|---|---|
| Amlwch, North Wales 53°25′N, 04°20′W | Low(0.05 ± 0.03, N = 56) | WA1WA2 | Compatible Incompatible | MicrosatelliteCHY1 | WA1 × WA1WA2 × WA2WA1 × WA2 | SelfNo progenyWA1 self & outcross*WA2 outcross* |
| Lough Hyne, South-West Ireland51°32′N, 09°15′W | IR1IR2 | Compatible Incompatible | MicrosatelliteCHY1 | IR1 × IR1IR2 × IR2IR1 × IR2 | SelfNo progenyIR1 self & outcross*IR2 outcross* | |
| Halifax, Nova Scotia44°40′N, 63°35′W | Intermediate(0.50 ± 0.29, N = 4) | NS1NS2 | CompatibleCompatible | None | NS1 × NS1NS2 × NS2NS1 × NS2 | SelfSelfSelf & outcross |
| Woods Hole, Massachusetts41°32′N, 70°40′W | High(1.00, N = 8) | WH1WH2 | CompatibleCompatible | AFLPs | WH1 × WH1WH2 × WH2WH1 × WH2 | SelfSelfWH1 self & outcross*WH2 self & outcross* |
| Sakkonet, Rhode Island41°27′N, 71°11′W | High(1.00, N = 6) | RI1RI2 | CompatibleCompatible | None | RI1 × RI1RI2 × RI2RI1 × RI2 | SelfSelfRI1 self & outcrossRI2 self & outcross |
- * Paternity analysis.
- N, number of genets tested for self compatibility. Standard errors are given where appropriate. Population self-compatibility rates are derived from Hughes et al. (2002b) and RNH & PJW, unpublished data. The single estimate representing the Welsh and Irish populations is derived from combined geographical samples of the North-East Atlantic clade (Gómez et al., 2007a). N, number of colonies tested for self compatibility. Standard errors are derived assuming a binomial distribution. Each pair of genets was represented by 20 replicates, except WH1 × WH1, WH1 × WH2 and IR1 × IR2 with 19 replicates.
- AFLP, amplified fragment length polymorphism; WA, Amlwch, North Wales; IR, Lough Hyne, South-West Ireland; NS, Halifax, Nova Scotia; WH, Woods Hole, Massachusetts; RI, Sakkonet, Rhode Island.
Propagation of genets and establishment of mating trials
Genets were propagated from cuttings to produce the required number of ramets. Because of the capacity of immature colonies to store allosperm, culture jars were fitted with aeration and feeding ports designed to prevent cross-colony contamination by water droplets or aerosols (Manríquez, Hughes & Bishop, 2001). Immediately prior to experimentation, ramets were reduced to a standard size of ten trophic zooids. Next, mating trials (Table 1) were established. A ramet from each of the two genets to be mated was placed in a jar containing 225 mL of 2 mm-filtered, ultraviolet-irradiated seawater. A daily food ration of 20 mL of the microflagellate Rhinomonas reticulata was injected into the jar via the feeding port and the water changed twice weekly. Each mating trial was simultaneously replicated using 20 pairs of ramets whose culture jars were positioned randomly with respect to genet and treatment. On the basis of previous experience obtained using our system, high replication within genets was deemed necessary to compensate for ‘jar effects’ (i.e. residual variation in performance among ramets within treatments).
Inbreeding depression
To measure inbreeding depression (i.e. the first prediction), reproductive success was grouped into three stages: (1) conception rate and survivorship of younger embryos; (2) survivorship of older embryos and free-swimming larvae; (3) and a post-larval survivorship. Stage 1 was measured by counting the number of brooded embryos and the total number of ovicells for both ramets in each jar. Analysis of covariance (ancova) was used to adjust embryo count to the covariate ovicell count with genet and mating type as crossed factors (self-incompatible outcross partners WA2 and IR2 were excluded from this and all subsequent analyses). Stage 2 was measured by transferring each ramet to a separate culture jar and counting the number of larvae settling over the next 3 weeks. Because brooding lasts for at least 3 weeks (Cancino & Hughes, 1988), any settled larvae must have originated from the previously counted embryos. ancova was used to adjust cumulative number of settled larvae to the covariate embryo count, with genet and mating type as crossed factors. Stage 3 was measured by transferring settled larvae to new jars and following subsequent colonial survival until the first female zooids appeared, 8–10 weeks after settlement. Data were subjected to two-way analysis of variance (anova) with genet and mating type as crossed factors. Inbreeding depression was evaluated by comparing reproductive success between mating types for each of the above stages.
Outcrossing rate
To measure outcrossing rate (i.e. the second prediction), offspring derived from larvae released by each maternal colony over 1–5 days were grown to a colonial diameter of 4–5 mm over a period of 10–12 weeks and analysed for paternity using a range of microsatellite and amplified fragment length polymorphism (AFLP) molecular genetic markers. Offspring were removed from the acetate sheet upon which they were grown and the DNA extracted according to the manufacturer's instructions using a DNeasy kit (Qiagen). Colonies from the source populations were screened with a selection of 21 microsatellite loci (Hoare, Hughes & Gliddon, 1998; Freeland et al. 1999, 2000; Craig et al. 2001) to identify potential genetic markers. Of these, only one locus (CH1; Hoare et al., 1998) was sufficiently polymorphic for assigning parenthood in the Amlwch samples. Consequently, a further 35 microsatellite loci were isolated using a subtractive hybridization protocol (Tysklind et al., 2009), of which one (IRL6-25, CAn) was suitable for the Irish samples. Paternity was determined in the Amlwch and Irish crossings by amplifying the CH1 and IRL6-25 loci, respectively, in the parents and offspring using the polymerase chain reaction (PCR). The primers described in Hoare et al. (1998) were used to amplify CH1, whereas CAGAGCAGTTCGACCAATCA (forward, 5′ to 3′) and AGCGACGAAAGACTTTGGAA (reverse, 5′ to 3′) were used for IRL6-25. PCR reactions (10 µL) contained 2 µL of 10× diluted template DNA, 1× NH4 reaction buffer (Bioline), 1.5 mm MgCl2, 0.2 mm dNTPs, 0.5 µm of each primer (forward primer end-labelled with Cy5 fluorescent dye), and 0.025 U µL–1 Taq polymerase (Bioline). Thermal cycling utilized one cycle of 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 62 or 55 °C for 30 s (CH1 or IRL6-25), and 72 °C for 30 s; with a final extension of 72 °C for 10 min. The resulting PCR products were sized on a Beckman Coulter CEQ8000 automated sequencer using a 400-bp size ladder, according to the manufacturer's instructions.
Because of the rarity of informative microsatellite markers, additional AFLP markers were screened for suitability with the remaining populations. AFLP analysis followed that of Vos et al. (1995) with a few minor modifications. Genomic DNA was digested at 37 °C for 3 h using a ‘frequent’MseI (New England Biolabs) and ‘rare’EcoRI (New England Biolabs) restriction enzyme. MseI and EcoRI adapters were ligated to the cut ends of the restricted fragments by incubating with T4 Ligase (New England Biolabs) at 16 °C for 16 h. The resulting fragments were PCR-amplified using pairs of single nucleotide ‘pre-selective’ primers (EcoRI-A and MseI-C) and 25-µL reactions containing 2.5 µL of 10× diluted template DNA, 1× NH4 reaction buffer (Bioline), 1.5 mm MgCl2, 0.2 mm dNTPs, 0.4 µm of each primer, and 0.02 U µL–1 Taq polymerase (Bioline). Thermal cycling conditions included one cycle of 94 °C for 2 min; 20 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 2 min. The PCR products were subsequently re-amplified using EcoRI-A and MseI-C primers that included an additional one or two selective nucleotides on the 3′ end. Reactions were prepared as before with minor modifications (5 µL of 10× diluted PCR product for template, 0.25 µm of each primer of which the EcoRI primers were end-labelled with Cy5 or Cy5.5 dye, and 0.02 U µL–1 Taq polymerase in a 20 µL volume). Thermal cycling conditions included one cycle of 94 °C for 2 min; 10 cycles of 94 °C for 30 s, 66–57 °C for 30 s (dropping by 1 °C in each cycle), and 72 °C for 2 min; 20 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 2 min; with a final extension of 72 °C for 30 min. The PCR products were sized on a Beckman Coulter CEQ8000 sequencer using a 600-bp ladder. Individual AFLP bands were scored as present or absent in each of the parental and offspring samples, with multiple extractions, restrictions, and PCR amplifications being performed for each selective primer pair to test for reproducibility. The use of three primer pairs (MseI-CG + EcoRI-ACA; MseI-CAA + EcoRI-ACA; MseI-CAG + EcoRI-ATA) yielded two diagnostic bands (i.e. only present in one or other colony) for the WH1 colony and three for the WH2 colony. With the exception of ten offspring, genotypes either matched precisely that of the maternal genotype (i.e. selfing) or possessed all diagnostic bands from both colonies (i.e. outbreeding), implying that both parental colonies were homozygous for the presence/absence of the band at all five loci. The ten offspring that showed incompatible genotypes also showed the loss of one or more maternal band, and/or failure to PCR at other loci, and thus were excluded from the analysis. Thus, we were able to determine paternity with absolute accuracy in the Massachusetts crossings without requiring a maximum likelihood or Bayesian based approach, as is often required in AFLP-based paternity analyses (Meudt & Clarke 2007).
Development of genetic markers for the Nova Scotia genets proved unsuccessful, and delays encountered in cloning the Rhode Island genets precluded paternity analysis in the time available. Paternity analysis therefore was possible only for progeny arrays of the self-compatible genets WA1, IR1, WH1, and WH2. Progeny arrays of the outcross partners WA2 and IR2 also were included to verify self-incompatibility. A target of eight offspring per ramet was adopted as the maximum affordable with limited time and resources, given a potential of 8 offspring × 20 replicates × 8 genets = 960 offspring to be genotyped (Table 2).
| Maternal genet | Outcross paternal genet | Replicate pairs of ramets | Total offspring analysed | Mean per maternal ramet |
|---|---|---|---|---|
| WA1 | WA2 | 20 | 106 | 5.3 |
| WA2* | WA1 | 20 | 159 | 8.0 |
| IR1 | IR2 | 19 | 110 | 5.8 |
| IR2* | IR1 | 20 | 113 | 5.7 |
| WH1 | WH2 | 19 | 112 | 5.9 |
| WH2 | WH1 | 20 | 155 | 7.8 |
- * Self-incompatible genets (not used to test preference for outcross sperm).
- WA, Amlwch, North Wales; IR, Lough Hyne, South-West Ireland; WH, Woods Hole, Massachusetts.
Sex allocation
To measure sex allocation (i.e. the third prediction), each ramet was grown to a size of approximately 1000–2000 trophic zooids, representing the upper limit of natural growth (Cancino, 1986). During this growth period, the ramet was recorded weekly by video through a binocular microscope and the captured images processed (UTHSCSA Image Tool; http://ddsdx.uthscsa.edu/dig/itdesc.html) to count the number of trophic zooids viewed from beneath. On reaching the target size, the ramet was recorded from above to count the number of male zooids and ovicells budded on top of the basal layer of trophic zooids. Any males in the basal layer were scored during the trophic zooid count. Females are not normally produced in the basal layer and none were observed. The abundance of male zooids in each partner ramet was used to represent the relative availability of self and outcross sperm. An index of sex allocation was obtained using ancova to adjust ovicell count to the covariate male zooid count.
All the two-way models produced statistically significant interaction terms that precluded comparison of levels within main effects (see Appendix). Consequently, one-way ancova models with mating type as main effect were run for each genet and anova models replaced by t-tests. The Simes–Hochberg method for sequential Bonferroni correction for multiple comparisons (Simes, 1986; Hochberg, 1988) was applied. SPSS, version 12.0, was used for all statistical analyses.
RESULTS
Mating trials were each represented by the full complement of 20 mating pairs except in three cases (WH1 × WH1; WH1 × WH2; IR1 × IR2), where only 19 pairs were available as a result of losses in culture (Table 1). The mean number of trophic zooids, which determines the overall size of the ramet, varied among treatment cells (Fig. 1). Inclusion of autozooid count as a covariate, however, did not improve goodness-of-fit of preliminary statistical models and so was discontinued in subsequent analyses.
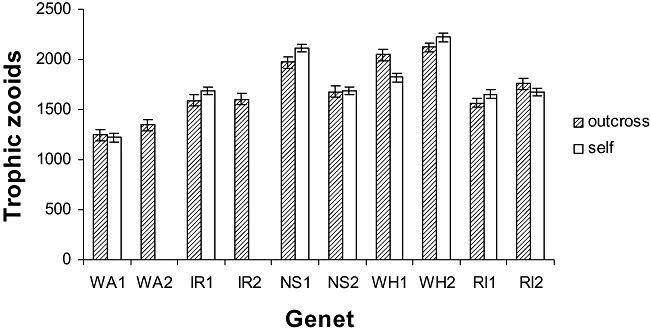
Colony size measured as number of trophic zooids. Data are the mean ± SE and represent self and outcross mating trials for each genet. The self-incompatible genets WA2 and IR2, used, respectively, as outcrossing partners for WA1 and IR1, are included for comparison. WA, Amlwch, North Wales; IR, Lough Hyne, South-West Ireland.
Inbreeding depression
Applying sequential Bonferroni correction, reproductive success at stage 1 (conception rate and survivorship of younger embryos) (Fig. 2A) was significantly greater in outcross than selfed progeny of NS1, but significantly lower than in the selfed progeny of NS2, and not significantly different in the other genets, therefore revealing no consistent pattern with mating type (one-way ancova: pairwise comparison of mating type: WA1, P = 0.378; IR1, P = 0.527; NS1, P = 0.012; NS2, P = 0.004; WH1, P = 0.157; WH2, P = 0.182; RI1, P = 0.063; RI2, P = 0.057).
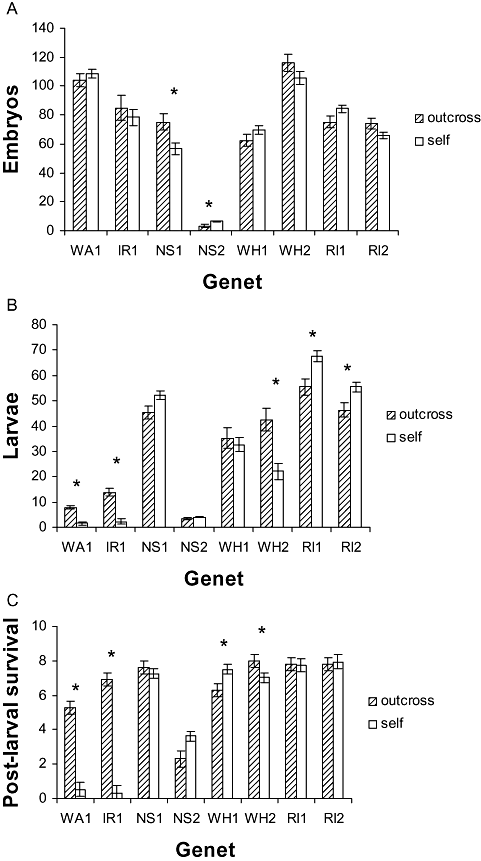
Inbreeding depression. A, stage 1 reproductive success. Mean embryo counts were adjusted by one-way analysis of covariance to the following ovicell counts: WA1 = 192, IR1 = 465, NS1 = 364, NS2 = 103, WH1 = 267, WH2 = 293, RI1 = 223, RI2 = 210. B, stage 2 reproductive success. Mean counts of settled larvae were adjusted to the following prior embryo counts: WA1 = 108, IR1 = 82, NS1 = 63, NS2 = 6, WH1 = 67, WH2 = 109, RI1 = 82, RI2 = 70. C, stage 3 reproductive success. Post-larval survivorship measured as number per batch of 8 settled larvae surviving to colonial sexual maturity. Data are means with standard errors. Asterisks indicate statistical significance of paired comparisons after sequential Bonferroni correction. WA, Amlwch, North Wales; IR, Lough Hyne, South-West Ireland; NS, Halifax, Nova Scotia; WH, Woods Hole, Massachusetts; RI, Sakkonet, Rhode Island.
Reproductive success at stage 2 (survivorship of older embryos and free-swimming larvae) (Fig. 2B) was significantly lower in self than outcrossed progeny of genets WA1, IR1, and WH2, but significantly greater in selfed progeny of genets RI1 and RI2 (one-way ancova: pairwise comparison of mating type: WA1, P < 0.001; IR1, P < 0.001; NS1, P = 0.036; NS2, P = 0.324; WH1, P = 0.603; WH2, P < 0.001; RI1, P = 0.003; RI2, P = 0.008).
Reproductive success at stage 3 (post-larval survivorship) (Fig. 2C) was significantly lower in self than outcrossed progeny of genets WA1, IR1, and WH2, but significantly greater in selfed progeny of WH1 (pairwise comparison of mating types: t-test (equal variances not assumed): WA1, t48 = 7.826, P < 0.001, IR1, t48 = 13.524, P < 0.001; NS1, t56 = 0.903, P = 0.371; NS2, t51 = 1.852, P = 0.073; WH1, t54 = 2.281, P = 0.031; WH2, t53 = 2.951, P = 0.006; RI1, t56 = 0.097, P = 0.923; RI2, t56 = 0.579, P = 0.568).
Outcrossing rate
An average of 6.4 offspring per ramet was recovered and paternity was successfully determined in all 751 of the available offspring (Table 2). The putatively self-incompatible genets WA2 and IR2 used as outcross partners for WA1 and IR1 were confirmed to produce only outcrossed progeny, apart from one apparently selfed offspring by a ramet of IR2. Outcross rate of self-compatible genets was not significantly correlated with the ratio of self and nonself male zooids (Pearson Correlation: WA1, r19 = −0.103, P = 0.667; IR1, r19 = −0.046, P = 0.851; WH1, r19 = −0.100, P = 0.413; WH2, r19 = 0.204, P = 0.077).
Outcross and self offspring (Fig. 3) were represented unequally in progeny arrays (mean number per ramet with standard errors: WA1 outcross 5.05 ± 0.57, self 0.25 ± 0.12, t-test unequal variances: t20.1 = 8.00, P < 0.001; IR1 outcross 5.58 ± 0.41, self 0.21 ± 0.12, t-test unequal variances: t21.3 = 12.63, P < 0.001; WH1 outcross 0.89 ± 0.26, self 5.00 ± 0.66, t-test unequal variances: t23.6 = 5.79, P < 0.001, WH2 outcross 5.70 ± 0.47, self 2.05 ± 0.49, t-test unequal variances: t37.9 = 5.35, P < 0.001). All paired comparisons were significant after sequential Bonferroni correction; hence, all genets except WH1 preferred outcrossing. The apparent preference for selfing shown by WH1 could not be explained by poor performance in outcross trials because this genet produced more offspring in outcross than in self-mating trials (t53 = 4.665, P < 0.001) and marginally more offspring than WH2 in outcross trials (t75 = 1.889, P = 0.063).
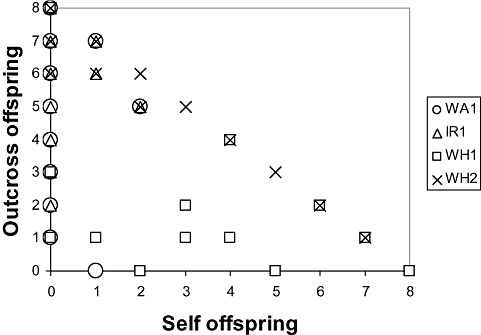
Outcrossing and selfing rates determined by paternity analysis of progeny arrays (Table 2). Each datum represents the progeny array of one ramet (WA1, N = 20; IR1, N = 19; WH1, N = 19; WH2, N = 20). An array can have a maximum of eight progeny. WA, Amlwch, North Wales; IR, Lough Hyne, South-West Ireland; WH, Woods Hole, Massachusetts.
Sex allocation
After sequential Bonferroni correction, the sex ratio measured as adjusted ovicell count (Fig. 4) was significantly less in self than in outcross matings for NS1, but not significantly different between mating types for other genets (ancova: pairwise comparison of mating type: WA1, P = 0.612; IR1, P = 0.675; NS1, P = 0.001; NS2, P = 0.413; WH1, P = 0.017; WH2, P = 0.283; RI1, P = 0.181; RI2, P = 0.285).
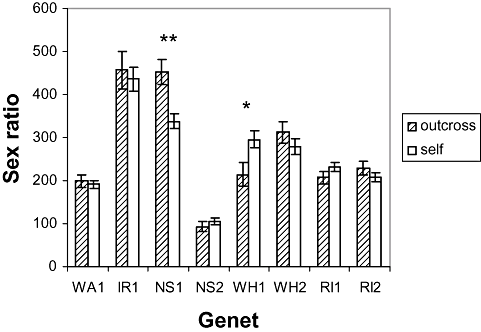
Sex ratio: mean ovicell counts were adjusted by one-way analysis of covariance to the following male zooid counts: WA1 = 341, IR1 = 417, NS1 = 72, NS2 = 36, WH1 = 341, WH2 = 490, RI1 = 135, RI2 = 142. Data are the mean ± SE. Asterisks indicate statistical significance of paired comparisons after sequential Bonferroni correction. WA, Amlwch, North Wales; IR, Lough Hyne, South-West Ireland; NS, Halifax, Nova Scotia; WH, Woods Hole, Massachusetts; RI, Sakkonet, Rhode Island.
DISCUSSION
Inbreeding depression, preferential outcrossing, and reproductive assurance
Inbreeding depression was clearly evident at reproductive stages 2 and 3 for genets derived from the Welsh and Irish populations with low self-compatibility rates. These results for selfed progeny are corroborated by the strong inbreeding depression recorded among progeny of full sib and half sib matings of a wider range of genotypes from Welsh populations (Hoare & Hughes, 2001). The results obtained for genets derived from the populations with intermediate or high rates of self compatibility are mixed, being commensurable with outbreeding depression at reproductive stage 2 for genets RI1 and RI2, and at stage 3 for WH1, and inbreeding depression in stages 2 and 3 for WH2. In general, however, the results support prediction (1) that genets derived from the focal populations with high selfing rates show less inbreeding depression than from the populations with low selfing rates.
There was no evidence that outcrossing rate was limited by the availability of allosperm or, reciprocally, that selfing rate was limited by the availability of autosperm, assuming that sperm output was adequately represented by the number of male zooids.
Preference for outcrossing shown by the genets whose selfed progeny exhibited inbreeding depression and preference for selfing shown by the genet whose selfed progeny was apparently free of inbreeding depression support prediction (2). Inbreeding depression may be expected to promote the preferential use of outcross gametes, with selfing reserved for reproductive assurance. Conversely, outbreeding depression, through the disruption of coadapted homozygous gene complexes evolved under persistent selfing, may be expected to promote preference for self gametes (Parker, Simmons & Kirk, 1990). Accordingly, genets from the Welsh and Irish populations with low self-compatibility rates showed strong preference for outcrossing and one genet (WH1) derived from a population with high self-compatibility rate and no demonstrable inbreeding depression showed preferential inbreeding. By contrast to expectations, however, the other genet (WH2) derived from the same population showed preferential outcrossing and significant inbreeding depression in terms of post-larval survivorship. Because all source populations occurred at high density, apparently with good opportunity for outcrossing, the observed variation of individual propensity for selfing suggests evolutionary disequilibrium rather than reproductive assurance (Goodwillie et al., 2005; Jarne & Auld, 2006). The opposite conclusion was reached by McCartney (1997) for a Maine population of C. hyalina. In that study, allozyme markers indicated an inverse relationship between selfing rate per focal colony and the number of potential paternal colonies, consistent with reproductive assurance in a regime of preferred outcrossing when the total supply of allosperm remains insufficient to fertilize the total supply of eggs. The experiments conducted in the present study were not designed to test the reproductive assurance hypothesis per se and so we did not intentionally vary the proportion of allosperm available to maternal colonies. By contrast, we tried to equalize the proportions of autosperm and allosperm by using partner colonies of comparable size so that outcrossing rate should not be limited by the availability of allosperm. The residual variation in number of male zooids between potential mates was not reflected in the proportions of selfed and outcrossed progeny; however, although this result satisfied our intention, it could not inform debate on reproductive assurance. More critical evidence indicating that the reproductive assurance hypothesis does not generally apply to C. hyalina is provided by our demonstration (Hughes et al., 2002b; present study) that the self-compatibility rate is low in clades from both sides of the North Atlantic and North East Pacific.
Sex allocation
The data obtained in the present study show conclusively that seven out of eight genets did not increase male allocation when denied opportunity for outcross mating. Such a lack of response irrespective of self-compatibility rates in source populations belies general applicability of prediction (3) to C. hyalina sensu lato. Theoretical prediction of a female bias in sex allocation at high selfing rates (Charlesworth & Charlesworth, 1981; Charnov, 1982; de Jong et al., 1999) assumes that any reduction in male function allows a corresponding increase in female function (Darwin, 1877; Heath, 1977; Maynard Smith, 1978). Accordingly, Gilia achilleifolia economizes on male function and produces larger fruit when selfing, consistent with redirection of resources to female function (Schoen, 1982). In the case of C. hyalina, however, experimental evidence of a sex allocation trade off is tenuous (Hunter & Hughes, 1995), although geometrical considerations suggest that such a trade off must exist (McCartney, 1997). Sexual zooids lack trophic apparatus and depend on translocation of metabolites from trophic zooids, with this being particularly critical for the prolonged, energetically expensive, process of placental brooding (Manríquez, 1999). Trophic zooids form the basal layer of the colony, which grows in area by distal budding. Sexual zooids, beginning with males at approximately 4–7 weeks after larval settlement and then accompanied by females from approximately 6–10 weeks, are budded from the upper surface of the trophic zooids, adding a centrifugally expanding second layer to the colony. Male zooids are also produced in the basal layer, but become significant in number only as a general response to stress (Hughes et al., 2003) and were relatively sparse in our experiments, indicating good growth conditions. In the North-East Atlantic and Fennoscandian clades, trophic zooids are produced mainly at the periphery of the basal layer and, because colonies retain an approximately circular shape, colonial trophic capacity per sexual zooid is progressively reduced by a declining perimeter/area ratio. In the Woods Hole clade, significant numbers of trophic zooids are also produced in the upper layer among the sexual zooids, presumably compensating the perimeter-area effect to some extent.
Any sex allocation trade off perhaps is rendered elusive by three-way partitioning of resources among trophic, male and female functions according to hierarchical rules (Tomimatsu & Ohara, 2006) not yet understood for C. hyalina. The problem also may be exacerbated by adopting a relative measure of sex allocation that is based only on numbers of zooids and hence is insensitive to differential costs in materials, energy, and time between sperm production and placental brooding. The observed lack of correspondence between sex allocation by C. hyalina and either self-compatibility rate in source populations or individual opportunity for outcrossing contrasts with numerous botanical examples of economy in male function under routine selfing (Cruden, 1977). Both zoophilous (Schoen, 1982) and anemophilous plants (Charnov, 1987) behave similarly in this regard and so, other things being equal, there is little reason to suspect that colonial invertebrates mating via water-borne sperm should not follow suit.
Theoretical prediction of male economy under selfing, however, also assumes that fitness gain through the male function decelerates with increasing male allocation (Charnov, 1982, 1987; Lloyd & Bawa, 1984). Decelerating male gain curves have been demonstrated in a number of seed plants, presumably in small reproductive populations where competition among self pollen, a type of ‘local mate competition’, prevails over seed competition, or ‘local resource competition’ (Charnov, 1982; Lloyd & Bawa, 1984). Among animals, facultative economy in male function at high population selfing rates has been demonstrated in the freshwater bivalve Utterbackia imbecillis, although, in this case, the shape of the male gain curve was not studied (Johnston, Das & Hoeh, 1998). In large breeding populations, by contrast, relaxation of local mate competition and intensification of competition among allosperm should linearize the male gain curve, opposing economy in male function (Charnov, 1982). This may apply with particular force to colonial invertebrates, which tend to have efficient mechanisms for capturing and/or storing water-borne sperm from far afield (McCartney, 1997; Bishop, 1998; Pemberton et al., 2003). Indeed, allosperm competition in such animals is known to be important over a wide range of population densities (Levitan & Petersen, 1995; Yund, 1998). Linearity of the male gain curve has been demonstrated experimentally in the field for a Maine population of C. hyalina, in which colonies bearing more male zooids fertilized a greater proportion of eggs borne by focal receptor colonies (Yund & McCartney, 1994). Lack of economy of male function under selfing therefore may reflect the weak effect of local mate competition compared to allosperm competition in a selective environment characterized by good opportunity for outcross mating, as for example in C. hyalina, where colonies of different parentage typically are randomly mingled in dense aggregations (Hoare & Hughes, 2001).
Hermaphroditism in Bryozoa
All species of Bryozoa are simultaneous hermaphrodites, with colonies bearing either bisexual zooids or distinct male and female zooids depending on the taxon (Ryland, 1970). No other Phylum of colonial invertebrates (Bergquist, 1978; Fautin, 2002) or any Division of seed plants (Stebbins, 1950) displays such universal hermaphroditism. Unfortunately, the bryozoan fossil record yields little information on hermaphroditism because, unlike the Hippothoidae to which C. hyalina belongs, most taxa lack recognizable male zooids even though they have skeletally distinct female zooids with brood chambers. Nevertheless, inheritance from a common ancestor is a more parsimonious hypothesis for the ubiquity of hermaphroditism than the alternative hypothesis of independent evolution among extant clades.
Acceptance that hermaphroditism is plesiomorphic to the crown group Bryozoa, however, raises the problem of its persistence at least since the Ordovician when the phylum first appeared in the fossil record (Taylor & Ernst, 2004). On the one hand, vagaries of larval dispersal, habitat fragmentation, and climatic oscillations might create episodes of low population density that promote hermaphroditism through reproductive assurance (Ryland, 1976) or local mate competition (McCartney, 1997). On the other hand, alterations to the ratio of benefits in genomic transmission and reproductive assurance to costs of inbreeding depression, caused for example by environmental change and pathogens (Gow et al., 2005), may render hermaphroditism vulnerable to replacement by gonochorism (Charlesworth & Charlesworth, 1981). Moreover, intraspecific variation in sex allocation may encompass effective gonochorism. For example, occasional colonies of C. hyalina bear numerous male but very few female zooids and vice versa (Hunter & Hughes, 1995; McCartney, 1997). Much of this variation in sex allocation is genetic (Hughes, 1989a, b; Hunter & Hughes, 1995) and therefore should be visible to natural selection.
The above considerations suggest that phylogenetic constraint (Gould & Lewontin, 1979) may prevent gonochorism from becoming established in any bryozoan population. A corollary of this explanation is that hermaphroditism is not necessarily adaptive in all extant clades of Bryozoa. Phylogenetic constraint is often invoked when adaptive explanation of an observed trend remains elusive, as for example with egg size in trematodes (Poulin, 1997), semelparity in insects (Tallamy & Brown, 1999), parental care in ducks (Johnson et al., 1999), and the mode of embryological development in limpets (Collin, 2004). Regarding hermaphroditism in Bryozoa, however, the apparent absence of variation precludes statistical inference (Pagel, 1999), and evolutionary understanding of the phenomenon will require confirmation of character state by more comprehensive in vivo studies conducted across the phylum, together with a wider comparison among the lophotrochozoa and ancestral lineages.
ACKNOWLEDGEMENTS
This research was funded by The Natural Environment Research Council (NER/A/S/2003/00599). Comments by two anonymous referees significantly helped to improve the manuscript.
Appendix
Two -way models: inbreeding depression
Reproductive success stage 1. ancova: Response variable embryo count; main effects genet, mating type; covariate ovicell count: genet F7,434 = 3.751, P < 0.001; mating type F1,434 = 0.235, P = 0.628; ovicells F1,434 = 127.410, P = < 0.001; genet × mating type F7,434 = 0.733, P = 0.644; genet × mating type × ovicells F15,434 = 26.952, P < 0.001.
Reproductive success stage 2. ancova: Response variable larvae; main effects genet, mating type; covariate embryo count: genet F7,433 = 5.903, P = 0.017; mating type F1,433 = 5.139, P = 0.024; embryos F1,433 = 4629.163, P = < 0.001; genet × mating type F7,433 = 0.649, P = 0.715; genet × mating type × embryos F15,433 = 36.923, P < 0.001.
Reproductive success stage 3. anova: Response variable post-larval survivorship; main effects genet, mating type: genet F7,359 = 68.869, P < 0.001; mating type F1,359 = 44.471, P < 0.001; genet × mating type F7,359 = 26.870, P < 0.001.




