A test for negative frequency-dependent mating success as a function of male colour pattern in the bluefin killifish
Abstract
Rare male mating advantage (a form of negative frequency dependence) is frequently proposed as a mechanism for the maintenance of genetic variation within populations. This hypothesis is attractive for systems with pronounced male colour polymorphism because it can maintain particularly high levels of variation. We tested for negative frequency-dependent mating success between yellow and red male colour patterns in bluefin killifish, Lucania goodei. Lucania goodei populations harbour substantial colour pattern polymorphism, and a large proportion of this variation has a genetic basis. We established outdoor mesocosms with red and yellow males in three different ratios: yellow rare (one yellow ♂ : five red ♂), even (three yellow ♂ : three red ♂), and red rare (five yellow ♂ : one red ♂). We obtained eggs and used microsatellites to determine paternity. By contrast to expectations, we found no support for a rare male mating advantage. Red males had slightly higher spawning success than yellow males, particularly in replicates with large clutches and when red males were rare. However, yellow males did not have higher mating success when rare. We discuss alternative mechanisms for the maintenance of the polymorphism as well as the potential reasons for the lack of a rare male mating advantage. © 2009 The Linnean Society of London, Biological Journal of the Linnean Society, 2009, 98, 489–500.
INTRODUCTION
Genetically based colour polymorphisms that occur within populations require explanation. In the absence of balancing selection, both natural (and/or sexual) selection and genetic drift should favour the erosion of variation resulting in a single colour pattern within a given population (Lewontin, 1974; Charlesworth, 1987). This is particularly so when colour patterns are affected by a small number of alleles, as is the case in many systems (Nachman, Hoekstra & D'Agostino, 2003; Fuller & Travis, 2004; Hoekstra et al., 2006). Yet, many species harbour substantial levels of genetic polymorphism in male colour pattern within populations (Endler, 1983; Houde, 1997; Horth, 2004; Gray & McKinnon, 2007). A variety of mechanisms can allow for the maintenance of multiple genetic colour morphs at appreciable levels within populations (Barton & Turelli, 1989), including negative frequency dependence (Maynard Smith, 1982; Parker, 1984; Gray & McKinnon, 2007), spatially variable selection (i.e. fine-scaled microhabitat variation in fitness; Hedrick, 1986; Chunco, McKinnon & Servedio, 2007), and overdominance (i.e. heterozygote advantage; Gillespie, 1984; Hartl, 1988).
Negative frequency dependence has garnered a large amount of attention because, in theory, it can account for the maintenance of particularly large amounts of genetic variation within populations (Wright, 1939, 1948). Negative frequency dependence occurs when the fitness of a genotype is inversely proportional to its frequency in the population. Under this scenario, a given colour morph has higher fitness than other colour morphs when rare, but has lower fitness than other colour morphs when common. Frequency-dependent fitnesses can arise through several mechanisms: vulnerability to predators and pathogens (Olendorf et al., 2006); for a recent review, see Punzalan, Rodd & Hughes, 2005), costly competition between similar phenotypes (Seehausen & Schluter, 2004), sexual selection associated with distinct behavioural strategies (Sinervo & Lively, 1996; Widemo, 1998; Horth, 2003; Sinervo & Calsbeek, 2006; Bleay, Comendant & Sinervo, 2007), or mate preferences of females for rare males (Farr, 1977; Partridge, 1983). For the purpose of the present study, we distinguish between negative frequency dependence that arises from the dynamics of the mating system and creates a ‘rare male’ mating advantage (Partridge, 1983) versus negative frequency dependence that arises from external, ecological factors, such as predation, parasitism, or other ecological interactions (Hori, 1993; Punzalan et al., 2005).
Rare male mating advantage occurs when males with rare colour patterns have increased mating success (Partridge, 1983) and can arise through two main mechanisms. First, a rare male mating advantage can be generated if females prefer to mate with unfamiliar colour morphs (Hughes et al., 1999) or rare colour morphs (Royle, Lindstrom & Metcalfe, 2008; Zajitschek & Brooks, 2008). Second, a rare male mating advantage can also be generated through male–male competition if males engage in more costly fights with individuals of the same colour morph, as has been proposed in cichlids (Seehausen & Schluter, 2004; Dijkstra et al., 2007; but see Dijkstra et al., 2008). Rare male mating advantage is frequently invoked as an explanation for the maintenance of high levels of colour pattern polymorphism, and has received empirical support in some vertebrate systems (guppies: Farr, 1977; Hughes et al., 1999; Zajitschek & Brooks, 2008; green swordtails: Royle et al., 2008; lady birds: O'Donald & Muggleton, 1979). Similarly, rare male mating advantage has been well-documented in many species of Drosophila, but has received less support in other insect taxa (Knoppien, 1985).
In the present study, we tested for a rare male mating advantage generated via negative frequency dependence between yellow and red male colour morphs in the bluefin killifish, Lucania goodei. Lucania goodei populations harbour large amounts of variation in male colour pattern, with a minimum of two colour morphs and as many as twelve colour morphs occurring within populations (Fuller, 2002). We focused on the male anal fin colour pattern. The male anal fin can be solid red, solid yellow, solid blue, solid orange, a combination of blue and red, or a combination of blue and yellow. The blue and red (or blue and yellow) colour elements exist in a variety of configurations on the anal fin, which leads to the large number of colour patterns. Females do not express the male colour pattern in nature, but treatment with methyltestosterone will induce females to express the male colour pattern (Fuller & Travis, 2004). Females do have mating preferences and spawn more quickly with preferred males, although there is no evidence for an overall preference as a function of body size or colour pattern (McGhee, Fuller & Travis, 2007). Lucania goodei is an external fertilizer. Sperm competition is largely absent from this system most likely because females release small numbers of eggs in any given spawning event (Foster, 1967).
For the present study, we chose to focus on the maintenance of red versus yellow colour morphs. Yellow and red morphs are the two most abundant colour patterns in clear water populations and are present in 29 of our 30 study populations (Fuller, 2002). Among our 29 study populations where both morphs are present, the relative abundance of males with red coloration is in the range 0.14–0.85. The relative abundance of males with yellow coloration ranges coloration is in the ranges 0.05–0.71. The variation in yellow versus red has a genetic component that is largely controlled a polymorphism at a single, autosomal locus (Fuller & Travis, 2004). The fact that both morphs are present in almost all of our populations and is clearly influenced by a locus of large effect indicates that these morphs are likely being maintained via balancing selection. We chose not to focus on the orange or blue morphs. We know little about the genetic basis of the orange colour pattern. The variation in blue colour coloration is much more complex than the variation in yellow versus red. The variation in blue coloration has large environmental (E), genetic (G), and G × E components (Fuller & Travis, 2004). We chose to focus on the red versus yellow morphs because the maintenance of these colour patterns requires balancing selection to maintain genetic variation within populations.
The red and yellow colour patterns do not appear to be linked to different mating strategies. There is no evidence that yellow and red males differ in body size (Fuller, 2002). In nature, red and yellow males do not differ in their courtship of females, nor in aggressive interactions with conspecifics or heterospecifics (Fuller, 2001). In a study of male competition, McGhee et al. (2007) found no evidence for differences in competitive ability or different mating strategies between yellow and red morphs. The factors that allow these two morphs to persist in almost all populations, and particularly in clear water habitats, are unknown. Negative frequency dependence predicts that the fitness of each morph is highest when it is rare.
MATERIAL AND METHODS
We tested whether the mating success of yellow and red colour morphs exhibits negative frequency dependence. Accordingly, we created breeding populations and determined the spawning success of males under three different treatments: (1) red males rare (i.e. red males less abundant than in natural populations); (2) red and yellow males equal in frequency; and (3) yellow males rare (i.e. yellow males less abundant than in natural populations; see the results for data on the frequency of red and yellow colour morphs in the Wakulla River). We placed animals in outdoor stock tanks, allowed them to mate over several weeks, obtained the offspring, and determined their paternity to test whether each colour morph had increased mating success when rare.
To determine the natural frequencies of the morphs in the population, we collected fish with seines and dipnets from the Upper Bridge Site at the Wakulla River (Wakulla Co., FL) in April, 2003. We returned the fish to Florida State University where we recorded sex, male colour pattern, and standard length. We used these same fish to establish eight replicates of each of the following three treatments: red rare (one red male : five yellow males : six females), even (three red males : three yellow males : six females), and yellow rare (five red males : one yellow male : six females). We chose these ratios because they created experimental populations where each colour morph was rarer than in nature (see results for natural frequencies). Theory predicts that the two morphs should have equal fitness at the equilibrium point, assuming that negative frequency dependence is occurring (Endler, 1986). In L. goodei, it is unknown whether there is an equilibrium where the two morphs are equally abundant (50 : 50 ratio) or whether the ratios found in nature represent equilibrium frequencies. Hence, it is prudent to use treatments where each morph is rarer than found in nature because negative frequent-dependent mating success predicts that each morph should have increased mating success when its relative abundance is lower than the equilibrium frequencies.
Replicates were placed in round stock tanks (maximum of 850 L) filled with well water. All trials were established on 1 May 2003. We chose not to fin clip the animals at the onset of the experiment for fear of unduly altering the appearance of male fins, which are presumably the target of sexual selection. The stock tanks were located on an old agricultural field at the Florida State Mission Road Greenhouse Facility in Tallahassee, FL. Tanks were set in a block of eight rows with three tanks per row. We randomized the location of the treatments in each row. Each tank was covered with mesh screens to prevent aquatic beetles and dragon fly larva from colonizing the stock tanks and preying upon the fish. Fish were fed once each week with brine shrimp and also fed on small aquatic invertebrates and algae that naturally occurred in the stock tanks.
The fish readily spawned their eggs in the tanks on yarn mops that served as spawning substrate. Each tank was stocked with 24 yarn mops. Twelve mops were attached to small pieces of PVC, which made them negatively buoyant and sink to the bottom of the tank (hereafter referred to as bottom spawning substrate). The remaining twelve were attached to Styrofoam balls, which made them float to the surface (hereafter referred to as floating spawning substrate). We removed eggs from the spawning substrate for all replicates and transferred them to the laboratory on 12–19 June 2003. Eggs were placed in small tubs of water with a dilute amount of methylene blue dye to prevent fungus infection. Eggs were allowed to hatch and resulting fry were euthanized in an overdose of MS-222. The fry were then placed in 1.5-mL Eppendorf tubes and stored at −20 °C. The breeding adults and any remaining juvenile fish were removed from all the stock tanks in 11–13 August 2003, measured (standard length and male colour pattern), euthanized, and stored in 1.5-mL Eppendorf tubes at −20 °C. All of the samples were later transferred on dry ice to the University of Illinois. Samples were later stored in 95% ethanol.
Genotyping with microsatellites
We used microsatellites to determine the paternity and maternity of the offspring. We extracted genomic DNA using proteinase K extraction. Individuals were genotyped at three polymorphic loci: AC17 (Burg, Wilcox & Martin, 2002), Lg1 (Creer & Trexler, 2006), and CA (Fuller and Olendorf unpublished data). Forward primers were labelled with 6Fam (AC17), Pet (Lg1), or VIC (CA). The loci were amplified in 20-µL reactions at 5.0 mm MgCl2 (2.5 mm MgCl2 for AC17). Forty cycles of polymerase chain reaction (PCR) were performed (94 °C for 30 s, 52 °C for 30 s, and 72 °C for 90 s). PCR products were cleaned using silica membrane plates and then analysed with an ABI Prism 3730xl Analyzer at the W. M. Keck Center for Comparative and Functional Genomics on the University of Illinois campus. The PCR products were scored using Applied Biosystems GeneMapper and verified manually.
Summary statistics for these primers are listed in the Supporting Information (Table S1). AC17 did not differ from Hardy–Weinberg expectations. For the population as a whole, CA did differ from Hardy–Weinberg expectations, but this was not the case when sexes were analysed separately. In an earlier study, Lg1 did not differ significantly from Hardy–Weinberg expectations (Creer & Trexler, 2006), although they did in the present study because of an excess of homozygotes. However, the frequency of null alleles was estimated at 0.10. Dakin & Avise (2004) have shown that null alleles at low frequencies have minimal effects on the average exclusion probability, but may increase the probability of false exclusion. For this reason, we chose to examine replicates where we could determine the paternity for 80% or more of the offspring (see below).
Paternity assignment
We determined paternity only for offspring for which we could determine their genotypes with all three primers. Across all 24 replicates, we collected a total of 1455 offspring. Of these, we successfully determined the genotype at all three loci for 1186 offspring (81% of all offspring; for details for each replicate, see Supporting information, Table S2). We used CERVUS software to determine the identity of both the father and the mother. CERVUS uses a likelihood approach and determines the most likely parents based on the number of matches and mismatches between offspring and potential parents at the same time as taking into account the frequency of each of the alleles in the population, as well as whether or not the parent is a homozygote. We used the genotype frequencies from the collective group of parents to determine the allele frequencies. Simulations were run assuming that we had collected 83% of the mothers and fathers (five out of six) and that genotypes had been determined with a 1% error rate.
We considered paternity to have high certainty when CERVUS could assign the father with either 95% or 80% confidence. Cervus determines the confidence for two separate analyses. It first determines the confidence for an analysis of paternity alone. It also determines the confidence for an analysis of both the maternity and paternity. Occasionally, the confidence for the father identity is high, but the confidence for the pair (mother and father) is low. This can happen when there are two different females that could potentially be the mother or if the mother died before we collected the offspring (for data on mortality, see Results). In this case, we relied on the confidence as determined by the analysis of paternity performed. Alternatively, the confidence for the pair can be high, but the confidence for the father alone can be low. This happens when the mother can be identified with high confidence, but the analysis on the father alone has low confidence as a result of the father carrying common alleles. Often, a determination of the mother's genotype allows the father to be identified with higher confidence. In this case, we relied on the confidence for the pair.
We considered the paternity for all offspring typed at three loci and having zero or one mismatches with their putative fathers. In some cases, there were multiple males that were equally likely putative fathers. For these cases, we determined the phenotype of the sire. If the two males had the same colour pattern (i.e. yellow), then the offspring was still informative because we knew the sire's colour pattern (i.e. yellow). If the two putative fathers differed in colour pattern, then the offspring's paternity was unresolved. Offspring that were typed at all three loci, but had two mismatches with its putative father were also considered unresolved. For this analysis, we only considered replicates in which the father's phenotype could be resolved for 80% of the offspring. Identical results were obtained when we only considered the paternity for offspring where the father could be identified with high certainty for 80% of the offspring.
It is unclear why some replicates had lower levels of paternity assignment. Some replicates had slight deviations in the number of dams and sires in the stock tank (see Results and Supporting information, Table S2). Other possibilities include genotyping errors, multiple sires with similar genotypes (see above), or the presence of null alleles. Regardless, the results presented are conservative because we only present data for replicates for which we could determine paternity for 80% or more of the offspring.
Statistical analysis
The present study aimed to determine whether each colour morph had its highest mating success when rare. Accordingly, we relied on chi-square analysis. For each replicate, we determined whether the number of offspring sired by red and yellow males varied from the expected values based on the frequency of red and yellow sires. The expected number of red babies was equal to the total number of babies assigned to fathers multiplied by the proportion of red sires present in the stock tank at the end of the experiment (and vice versa for yellow). Note that there were some slight deviations in the frequency of yellow and red sires from the starting frequencies (see ResultsSupporting information, Table S2). The chi-square value is then simply the sum of [(observed − expected)2]/expected for the red and yellow colour morphs. The advantage of using a chi-square analysis is two-fold. First, it allows us to account for variation in the total number of offspring. The total number of offspring typed at three loci varied across replicates in the range 8–164 (Table 1). The consequence of this is that replicates with high sample size have more power to detect significant deviations from null expectations. The hypothesis of negative frequency-dependent mating success predicts large deviations away from null expectations in the red rare treatment (i.e. where red males should have higher mating success) and in the yellow rare treatment (i.e. where yellow males should have higher mating success). The second advantage of the chi-square analysis is that it takes into consideration the fact that red and yellow spawning success are not independent variables.
| Treatment | Number of red sires | Number of yellow sires | Number of red offspring | Number of yellow offspring | Number of offspring typed at three loci | Expected number of red offspring | Expected number of yellow offspring | χ2 |
|---|---|---|---|---|---|---|---|---|
| Red rare | 1 | 5 | 1 | 6 | 7 | 1.17 | 5.83 | 0.03 |
| Red rare | 1 | 5 | 0 | 8 | 8 | 1.33 | 6.67 | 1.60 |
| Red rare | 1 | 5 | 44 | 102 | 155 | 24.33 | 121.67 | 19.07 |
| Red rare | 1 | 5 | 11 | 10 | 22 | 3.50 | 17.50 | 19.29 |
| Red rare | 1 | 5 | 50 | 63 | 127 | 18.83 | 94.17 | 61.89 |
| Even | 2 | 4 | 52 | 102 | 164 | 51.33 | 102.67 | 0.01 |
| Even | 3 | 3 | 25 | 24 | 50 | 24.50 | 24.50 | 0.02 |
| Even | 3 | 3 | 10 | 8 | 18 | 9.00 | 9.00 | 0.22 |
| Even | 3 | 5 | 14 | 18 | 37 | 12.00 | 20.00 | 0.53 |
| Even | 2 | 3 | 8 | 23 | 31 | 12.40 | 18.60 | 2.60 |
| Even | 3 | 3 | 23 | 6 | 35 | 14.50 | 14.50 | 9.97 |
| Even | 3 | 3 | 86 | 24 | 124 | 55.00 | 55.00 | 34.95 |
| Yellow rare | 5 | 1 | 14 | 3 | 18 | 14.17 | 2.83 | 0.01 |
| Yellow rare | 5 | 1 | 22 | 6 | 29 | 23.33 | 4.67 | 0.46 |
| Yellow rare | 5 | 1 | 11 | 1 | 12 | 10.00 | 2.00 | 0.60 |
| Yellow rare | 5 | 1 | 11 | 0 | 11 | 9.17 | 1.83 | 2.20 |
| Yellow rare | 4 | 2 | 12 | 2 | 15 | 9.33 | 4.67 | 2.29 |
| Yellow rare | 5 | 1 | 74 | 3 | 78 | 64.17 | 12.83 | 9.04 |
- Values in bold denote replicates that deviated from random expectations.
With these data, we then asked whether some treatments were more likely to vary from null expectations than others. To address this question, we used a general linear model that considered the chi-square values for each replicate as a function of treatment. We tested whether there were overall differences in the mating success of red versus yellow males by calculating the signed deviation from expected values (i.e. observed number of red babies − expected number of red babies) and determining whether these differed significantly from zero. We also examined the relationship between the proportion of babies sired by red males and the final frequency of red and yellow males, as well as the relationship between average spawning success of each colour morph and sire colour frequency. Average red male mating success was equal to the proportion of babies sired by red males divided by the number of red males in the stock tank at the end of the experiment, and vice versa for yellow. Preliminary analyses indicated that there were no effects of location (i.e. whether eggs were on the floating or sinking spawning substrates) on red versus yellow spawning success, so we did not consider this variable in the analysis. All analyses were performed using SAS PROC GLM, version 9.1 (SAS Institute).
RESULTS
Natural frequencies of red and yellow morphs
The natural frequencies of red and yellow males were intermediate between the red rare and yellow rare treatments. In the natural population, 39% of males had red anal fins (85 of 218), 58% had yellow anal fins (126 of 218), and 3% had orange fins (seven of 218). Our ‘red rare’ (one red ♂ : five yellow ♂) and ‘yellow rare’ (five red ♂ : one yellow ♂) treatments resulted in breeding populations where each colour morph was 16.7% of the males and, thus, was rarer than in the natural population. The sex ratio was approximately even with 52% female (242 of 432) and 48% male (218 of 432).
Survival of dams and sires and permanence of colour morphs
Female survival was high over the 3-month period (97.97%; see also Supporting information, Table S2), and there was no evidence that survival differed as a function of treatment. Male survival was also very high (∼100%; see below) and did not differ among treatments (see Supporting information, Table S2).
Male colour patterns and the frequency of the male colour morphs were largely stable throughout the experiment (Pearson correlation of starting and ending frequency of red males: R = 0.964, P < 0.001, N = 24). However, there were slight deviations from our starting frequencies in seven of the 24 replicates. In one ‘even’ replicate, a red male died. In two replicates, we had more than six males at the end of the experiment. This most likely occurred because we had misidentified small adults as females that were actually males. In one ‘even’ replicate, there were two additional yellow males (and two missing females). In one ‘yellow rare’ replicate, there was an additional red male (and one missing female).
Four out of 144 males had apparently switched colour pattern at the end of the experiment, which caused slight deviations in the frequencies of the colour morphs. In one ‘even’ replicate, there were four yellow males and two red males present at the end of the experiment. In two ‘yellow rare’ replicates, there were four red males and two yellow males at the end of the experiment. In one ‘red rare’ experiment, there were four yellow males and two red males at the end of the experiment. These changes in colour pattern frequency highlight the fact that, although there is a large genetic component to these colour patterns, there is also environmental variation present. In previous studies, we have similarly found that male colour patterns are largely stable, but that a few males apparently change colour (R. C. Fuller, unpubl. data). Three of the seven replicates (where males died or colour pattern frequencies slightly changed) were excluded from the analysis because of low levels of paternity assignment. The remaining four had little impact on the test for negative frequency dependence (i.e. excluding them does not alter the results of the study).
Spawning success of red and yellow males
The proportion of offspring sired by red males increased with the frequency of red males in the treatment (F1,16 = 47.81, P < 0.001, slope = 0.977 ± 0.158; Fig. 1A). However, in six of the 18 replicates, the observed number of red versus yellow offspring differed significantly from null expectations (Table 1; dark diamonds in Fig. 1). Three of these replicates occurred in the red rare treatment, two were in the even treatments, and one was in the yellow rare treatment. In all six cases, red males sired greater than expected numbers of offspring. In no replicate did yellow males have greater than expected mating success. The overall signed deviation from expected values (i.e. number of observed red babies − number of expected red babies) indicated that red males had significantly higher than expected mating success (Signed t-test: t = 2.44, d.f. = 17, P = 0.0258).
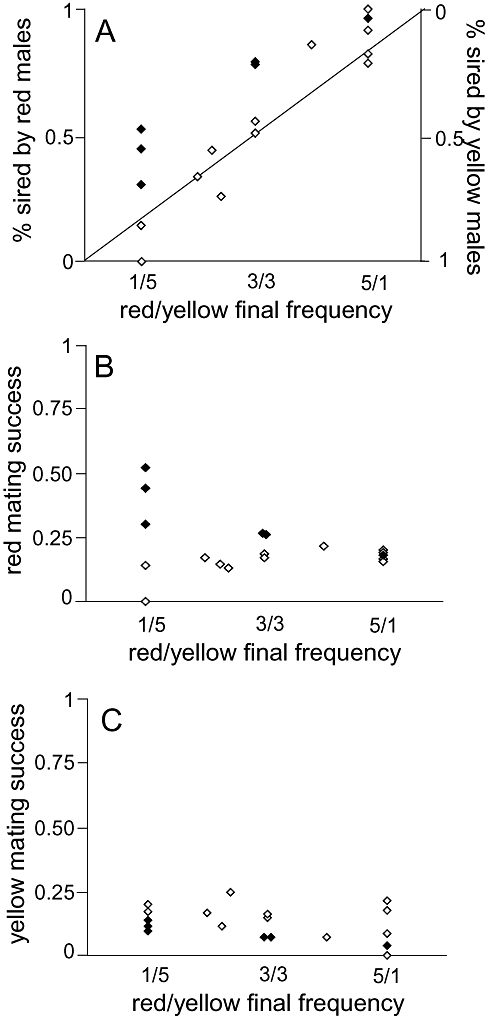
The proportion of offspring sired by red males (A), average red male mating success (proportion sired by red males/total number of red males) (B), and average yellow male mating success (proportion sired by yellow males/total number of yellow males (C). Dark diamonds indicate replicates where the paternity of red versus yellow sires differed significantly from random expectations. Open diamonds did not differ from random expectations. Null expectations are shown by the solid line in (A).
Analysis of variance of the individual chi-square values suggested that treatments did not vary systematically in their deviations between expected and observed numbers of offspring sired by red versus yellow males (F2,15 = 1.98, P = 0.1724). However, there were two replicates with fewer than ten offspring (i.e. the two red rare replicates that did not differ from random). Excluding these two data points resulted in a pattern where the chi-square values differed among treatments with the greatest deviations from null expectations occurring in the red rare treatment (F2,13 = 5.89, P = 0.0151; Fig. 1B). Furthermore, red males had greater spawning success when rare (correlation between red mating success and final red sire frequency R = −0.601, P = 0.0138, N = 16). However, there was no indication that yellow males had increased mating success when rare (F2,13 = 0.66, correlation between yellow mating success and final yellow sire frequency R = 0.222, N = 16; Fig. 1C).
DISCUSSION
In theory, a rare male mating advantage can allow for the maintenance of multiple genetic colour patterns either through female mating preferences for rare colour patterns or through the presence of costly competition between like-coloured morphs. In the present study, we found no support for the idea that negative frequency-dependent mating success maintains the polymorphism between yellow and red colour patterns. Hence, colour morphs do not generally have increased mating success when rare. This result is in keeping with the results obtained by McGhee et al. (2007), who found no support for the idea that familiarity with a particular colour pattern affects female mating preferences. Similarly, there is no available evidence that competition between like-coloured males results in higher levels of overall aggression than competition between differently coloured males (K. E. McGhee pers. comm.).
We did find that red males had slightly higher mating success than yellow males. A subset of the data supported the notion that there were greater deviations from the null expectations and that red male mating success was higher when red males were rare. However, there was no evidence that yellow males had higher mating success when rare. Given the abundance of the yellow male in its natal population (∼60%), the yellow rare treatment (yellow males 16.7% of the population) should have been particularly effective in creating a situation where yellow males would be expected to have high fitness provided that negative frequency-dependent dynamics were occurring. Yellow male mating success did not significantly exceed null expectations in any of the replicates where yellow males were rare. Hence, there is no support for the hypothesis that a rare male mating advantage maintains the yellow/red colour polymorphism in L. goodei.
Why should red males have higher mating success when rare? One possibility is that there is a general preference for red males. When red males are rare, they receive an excess amount of the reproductive output of females. When red males are common, there are more red males for females to mate with, and the mating advantage of red males relative to yellow males decreases. This pattern is described in the Fisher runaway models for the evolution of costly male traits and female mating preferences (Lande, 1981; Kirkpatrick, 1982). Although red males may have higher fitness when they are rare, there is little evidence that this is due to rarity per se. Yellow males never had higher than expected mating success when rare. Hence, the problem remains in explaining why the yellow morph, and the colour polymorphism, is maintained in natural populations.
Asymmetric effects of colour morph frequencies have been demonstrated in other species. In the cichlid group, Pumdamilia, there are red and blue colour morphs that co-occur in many populations. Dijkstra et al. (2008) tested whether males of each colour morph are more likely to display aggression towards individuals of the same colour. They found that groups with all red individuals had higher levels of aggression than mixed groups with both red and blue individuals. However, groups with all blue individuals did not differ in overall aggression levels from mixed groups with both morphs. Hence, additional factors must be present to account for the maintenance of both morphs.
Negative frequency dependence may still be important in maintaining a subset of the other colour patterns seen in L. goodei populations. Lucania goodei populations are highly polymorphic for male colour pattern, and this includes colour patterns other than red and yellow. Males can have solid blue anal fins, solid orange anal fins, or anal fins that have combinations of blue and yellow or blue and red. There are also many ways in which these colour elements can be combined and create various patterns. Conceivably, negative frequency dependence could have emerged if we had used a different set of colour patterns, such as red and blue males, as opposed to the red and yellow males used in the present study. Nevertheless, negative frequency dependence does not appear to be maintaining the variation in red versus yellow males. We chose to focus on the red and yellow morphs because both morphs are maintained in almost all of our study populations (Fuller, 2002) and because there is a simple genetic basis to these colour patterns (Fuller & Travis, 2004), which suggests that some sort of balancing selection is maintaining the genetic polymorphism within populations. Again, the fact that yellow males did not have increased mating success when rare suggests that rarity per se does not lead to increased male mating success at least for the yellow colour morph.
The experimental approach that we employed in the present study stands in contrast to the approach taken in other studies of sexual selection and negative frequency dependence (but see Horth & Travis, 2002; Bleay et al., 2007). There are many behavioural studies of female mating preference for rare males where female mating preferences are measured under various social situations (Hughes et al., 1999; Zajitschek & Brooks, 2008; for a review, see Singh & Sisodia, 2000). Similarly, there are mounting behavioural studies of aggression between like-coloured versus differently coloured male morphs to determine whether competition among males can generate negative frequency dependence (Dijkstra et al., 2007, 2008). Although these studies are highly informative, they are less informative with respect to whether the measured behaviour actually results in negative frequency-dependent mating success. This is not a fatal flaw by any means. Such behavioural experiments are designed to test the purported mechanism that conceivably generates negative frequency-dependent fitness.
Our approach in the present study was to test for negative frequency-dependent mating success between two colour morphs in L. goodei without regard to whether the pattern was generated by female or male behaviour. We designed the experiment so that animals were in similar densities to those seen in natural conditions with similar levels of spawning substrate and assumed that our treatments allowed the mating dynamics of the system to be fully expressed. Admittedly, it could be argued that female mating preference for rare males might still be present in the system, but that it is pre-empted by competition among males (or vice versa that female mating preferences circumvent negative frequency-dependent dynamics generated through male competition). However, our previous behavioural studies have indicated that prior housing with a particular male has no effect on female mating preference and has provided no evidence for distinct competitive strategies nor for the idea that males engage in more costly fights with like-coloured males (Fuller, 2001; McGhee et al., 2007). These behavioural observations, when taken in conjunction with the present study that directly tested for negative frequency-dependent mating success, argues strongly against negative frequency dependence maintaining the yellow and red colour polymorphism in natural populations.
Alternative mechanisms for the maintenance of colour polymorphism in L. goodei
A variety of other mechanisms may possibly allow for the maintenance of genetic variation in L. goodei. Numerous studies have demonstrated negative frequency dependence among colour morphs as a result of predation (Endler, 1988; Bond & Kamil, 1998, 2002). The hypothesis is that predators form search images for (and prey upon) the most common colour patterns which generates negative frequency dependence (Punzalan et al., 2005). There are a variety of visually oriented predators in L. goodei populations including large mouth bass (Micropterus salmoides), warmouth (Lepomis gulosus), and wading birds that potentially prey upon L. goodei (Fuller & Noa, 2008).
In theory, fine-scale variation in lighting environments may result in a situation where each colour morph has its highest fitness in a particular microhabitat where it is most conspicuous (Chunco et al., 2007; Gray et al., 2008). The fact that the relative abundance of blue, yellow, and red colour morphs varies across populations as a function of lighting habitat suggests that lighting environments are important in L. goodei (Fuller, 2002; Fuller & Travis, 2004). Among populations, blue morphs are much more common in tea-stained habitats. Lighting conditions are also variable within populations because shorter wavelengths are increasingly filtered out with increasing depth, and this is particularly acute in tea-stained habitats (R. C. Fuller, unpubl. data). We found no evidence that red versus yellow male mating success varied as a function of depth, but this alternative deserves further examination.
Alternatively, the variation in yellow versus red coloration may theoretically be maintained through overdominance. Fuller & Travis (2004) showed that there is an allele of large effect that contributes to the variation in yellow versus red. The yellow allele (Y) is dominant over the red allele (y). Thus, yellow males can be either homozygous dominant (YY) or heterozygous (Yy), whereas red males are homozygous recessive (yy). The overdominance hypothesis predicts that heterozygous yellow males (Yy) have higher fitness than the other two genotypes. The fact that red males (yy) had higher mating success than yellow males (YY and Yy) suggests that overdominance is not occurring as a function of mating success. However, it is possible that heterozygous yellow males or females carrying the heterozygous genotype derive some unknown benefit (e.g. juvenile survival) that contributes to the maintenance of the colour morphs within populations.
Other mechanisms for the maintenance of genetic variation seem less likely, but could, in theory, account for the maintenance of these colour morphs. Antagonistic pleiotropy proposes that there are trade-offs in life-history components that create approximately equal fitness between the two colour patterns but theoretical analyses cast doubt on the likelihood of this mechanism (Curtsinger, Service & Prout, 1994; Hedrick, 1999). Another possibility is that there is sexually antagonistic selection where there are different fitness consequences of the alleles in males versus females (Chippindale, Gibson & Rice, 2001; Rice & Chippindale, 2001; Delph et al., 2004). This hypothesis assumes that there are pleiotropic effects of the alleles for the red and yellow colour pattern that have other effects on the phenotype. Disassortative mating (i.e. females that carry YY mate with males that carry yy, and vice versa) could also account for the maintenance of genetic variation in this system (for an example in scale eating cichlids, see Takahashi & Hori, 2008).
Egg cannibalism is unlikely to account for the pattern in male mating success shown in the present study. Although egg cannibalism is very high in L. goodei, there is no evidence that fish can differentiate their own eggs from those of others (B. Sandkam, unpubl. data). Fuller & Travis (2001) measured egg cannibalism rates of yellow and red males and found little difference between the two morphs. Cannibalization rates are higher in the sinking mops compared to the floating mops (B. Sandkam & R. C. Fuller, unpubl. data), although there is no evidence that morphs differ in the position in which they spawn in the water column.
Understanding the taxonomic distribution of rare male mating advantage
Clearly, rare male mating advantage has been demonstrated in a variety of animal taxa including guppies (Farr, 1977; Hughes et al., 1999; Zajitschek & Brooks, 2008), green swordtails (Royle et al., 2008), and Drosophila (Knoppien, 1985; Singh & Sisodia, 2000). The evidence in guppies is particularly striking because it has been documented by at least four separate groups and has been shown repeatedly across a span of 30 years (Farr, 1977; Hughes et al., 1999; Eakley & Houde, 2004; Zajitschek & Brooks, 2008). Similarly, in Drosophila, rare male mating advantage has been shown repeatedly by a number of investigators and laboratories (Singh & Sisodia, 2000). However, the present study and others (Childress & McDonald, 1973; Knoppien, 1985; Baer, Dantzker & Ryan, 1995) indicate that rare male mating advantage is not universal. Publication bias may also be present because studies with negative results are more likely to go unreported. The fact that rare male mating advantage is strongly supported in some systems but not others suggests that rare male mating advantage only occurs under certain conditions.
There are a variety of reasons why rare colour patterns may not be favoured. First, if rare males represent migrants, then they may not be locally adapted to a given habitat. Some male genotypes may also be rare simply because they have low fitness. In either case, rare males (and their offspring) would experience decreased survival and viability, and provide few genetic benefits to females (Partridge, 1983). Similarly, if rare males are suboptimal for local conditions, then they may fare poorly in competition with other males. Given these considerations, why should rare male mating advantage occur?
Inbreeding avoidance is frequently suggested to promote female choice for rare males (Partridge, 1983). In L. goodei, inbreeding is detrimental to fitness (McCune et al., 2002). However, in natural populations, inbreeding is most likely low for two reasons. First, L. goodei populations are typically quite large with high levels of dispersal (and presumably gene flow) (Loftus & Kushlan, 1987; Ruetz et al., 2005). Because the probability of mating with a relative decreases with population size, inbreeding should be low. Second, female L. goodei are highly iteroparous. When gravid, females spawn on many consecutive days (Breder & Rosen, 1966). Each day, they typically spawn six to ten eggs. Furthermore, females release one or two eggs per spawn and appear to distribute their eggs across many males. Even if a female were to spawn with a relative, the effect this would have on female fitness is minimal because each individual spawn represents a small proportion of a female's total reproductive output. By contrast, guppies typically have small population sizes and also have internal fertilization where a single male can fertilize a large proportion of a given female's eggs, which may generate stronger selection on inbreeding avoidance.
In conclusion, the present study found no evidence for negative frequency-dependent male mating success with respect to yellow and red colour patterns in L. goodei. Red males did have slightly higher mating success than yellow males. The selective forces allowing yellow and red males to coexist at appreciable frequencies across multiple populations remain unknown.
ACKNOWLEDGEMENTS
We thank J. Birdsley, M. Gunzburger, B. Hale, and K. McGhee for their assistance in the field, the establishment of the stock tanks, and the collection and storage of fry. J. Travis provided valuable comments on the statistical analysis and the manuscript. A. Bell, O. Sanogo, K. Laskowski, E. Berdan, D. Welsh, M. Zhou, L. Marquardt, and E. Giesing also provided valuable comments on the manuscript. We thank R. Olendorf for assisting with the development of the microsatellites. This work was funded by the University of Illinois and a National Science Foundation Grant (IOB 0445127).




