Sperm release and use at fertilization by yellow dung fly females (Scathophaga stercoraria)
Abstract
Females of many species mate multiple times and store transferred sperm in storage organs. The mechanisms underlying sperm release from the stores at fertilization remain poorly understood, although they are central to an understanding of the female influence on post-copulatory male competition. Using double-mated females of the yellow dung fly, we counted the sperm sticking to the surface of deposited eggs of two successive clutches to obtain insight into the physiological processes associated with fertilization. The number of sperm released to fertilize an egg decreased between the first and second clutches, as well as within clutches from early to late eggs. These results indicate that: (1) sperm are lost from the stores over time independent of egg laying and (2) the number of sperm released depends on the amount of sperm stored. The lower number of sperm on eggs of the second clutches was accompanied by a strong increase of the proportion of sperm adhering to the micropyle region, suggesting that sperm use is more efficient and sperm release better controlled when sperm supply is substantially reduced. Finally, our approach indicates that sperm storage capacity of the female is higher than assumed from counts of spermathecal sperm. © 2009 The Linnean Society of London, Biological Journal of the Linnean Society, 2009, 98, 511–518.
INTRODUCTION
Storage of sperm from multiple mates is an integral part of female reproductive strategies in many animal species (Birkhead & Møller, 1993; Neubaum & Wolfner, 1999) and creates the opportunity for post-copulatory sexual selection through sperm competition and/or cryptic female choice (Parker, 1984; Eberhard, 1996; Birkhead & Møller, 1998; Parker, 1998). Extensive research over the last decades has revealed an extraordinary diversity of male traits that have evolved in the context of sperm competition (Birkhead & Møller, 1998; Simmons, 2001). The significance of cryptic female choice remains more controversial (but see also Pizzari & Birkhead, 2000). This is partly a result of the methodological difficulties of studying patterns of sperm use when fertilization occurs internally (Birkhead, 1998). Females of many species possess specialized sperm storage organs and fertilization of the ova frequently occurs long after insemination has taken place (Walker, 1980; Birkhead & Møller, 1993; Neubaum & Wolfner, 1999). When insemination and fertilization are temporally separated, males may directly influence sperm transfer and storage (Gage & Baker, 1991; Martin & Hosken, 2002). However, the fate of inseminated sperm depends on the female's efficiency of sperm uptake, storage, and utilization (Walker, 1980; Neubaum & Wolfner, 1999). The potential of female control of these processes is therefore of major interest for evaluating the evolutionary significance of cryptic female choice (Eberhard, 1996; Birkhead & Møller, 1998).
One of the best-studied species in the context of post-copulatory sexual selection is the yellow dung fly Scathophaga stercoraria. When females arrive at a dung pat to mate and deposit their eggs, they are typically grasped by one of the waiting males and forced to copulate (Parker, 1970a). Most females arrive at the pat already inseminated (Parker, Simmons & Kirk, 1990). The last male mating with a female before oviposition typically fertilizes approximately 80% of the eggs of the successive clutch (Parker, 1970a). The pattern of last male sperm precedence has been attributed to an indirect displacement of stored sperm by the last mating male (Simmons, Parker & Stockley, 1999). However, there is also evidence that females are able to use sperm of different males differentially to fertilize their eggs (Ward, 2000).
The sperm storage organs of the yellow dung fly consist of three, occasionally four, spermathecae, each of which is linked to the bursa copulatrix by a narrow duct (Ward, 2007); for graphical illustration, see Arthur et al. (2008). The site of insemination is the bursa copulatrix (Hosken, Meyer & Ward, 1999) and sperm then have to move into the spermathecae before they are used for fertilization (Simmons et al., 1999). Sperm transfer into the spermathecae appears to depend on multiple processes, including ejaculation by the male, active movement of the sperm, and female muscular activities (Otronen, Reguera & Ward, 1997; Simmons et al., 1999; Hellriegel & Bernasconi, 2000; Hosken & Ward, 2000). Using males with different sperm length, Hellriegel & Bernasconi (2000) found that the distribution of sperm in the spermathecae is not random. Nonrandom sperm storage was confirmed by a recent genetic analysis of spermathecal sperm using a microsatellite based competitive-multiplex-polymerase chain reaction approach (Bussière et al., 2009). These findings strongly indicate that yellow dung fly females are able to affect sperm storage to some extent but, so far, no data are available on sperm release and utilization at the time of fertilization.
The present study aimed to gain insight into the physiological mechanisms underlying sperm release and utilization in a sperm competitive situation. Knowledge of the physiological mechanisms should help to elucidate whether females have an active role in sperm utilization and thus the potential for nonrandom sperm use. Because fertilization takes place in the bursa copulatrix (Hosken et al., 1999), sperm release cannot be observed in situ. To address this problem, we counted the sperm sticking to the surface of freshly-deposited eggs. By counting this sperm, we first investigated whether sperm are released independent of the sperm supply within the female's storage system or whether the number of sperm per egg decreases when sperm reserves are depleted, as shown for some insects (Harbo, 1979; Smith, Browne & Vangerwen, 1988) and birds (Birkhead & Fletcher, 1994; Birkhead, Sheldon & Fletcher, 1994). Second, we investigated whether ovulation and the release of sperm from the stores are coordinated by the female (Bloch Qazi & Wolfner, 2006). Only if these processes are tightly linked can the majority of sperm be expected to be located at the ventral micropylar region of the egg in the yellow dung fly. This prediction is based on a recent morphological study showing that the ventral orientation of the micropyle in the bursa copulatrix impedes direct access of the sperm released from the dorsal openings of the spermathecal ducts (Arthur et al., 2008; Fig. 1).
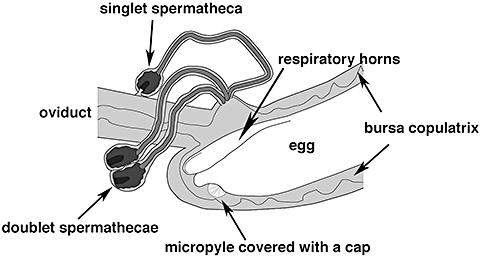
Line drawing of the anterior part of the bursa copulatrix at the time of fertilization with the doublet spermathecae detached from the oviduct. The micropyle of the egg is ventral and is oriented away from the entrances of the spermathecal ducts and covered by an acellular cap. The two respiratory horns of the egg are closed over the dorsal plastron of the egg.
MATERIAL AND METHODS
Origin and breeding of flies
Flies were derived from the second to fifth generations of a laboratory stock established from a population of S. stercoraria near Fehraltorf, Switzerland. Freshly-deposited eggs were transferred into small containers with cow dung and larvae were reared in this container until emergence of the adults. After emergence, flies were kept individually in bottles and fed with sugar, water, and adult Drosophila ad libitum. Flies were housed in a climate chamber under a 13 : 11 h light/dark cycle under 60% relative humidity and at 18–20 °C. Females used in the experiment were aged 19–60 days old and males were aged 16–61 days old. Because male and female age was quite variable, we tested for the effects of age prior to further data exploration. Neither the age of the first male, nor that of the second male, nor female age, significantly influenced the number of sperm released (linear regressions: all P > 0.5).
Experimental design
Virgin females were mated to two different males, with an interval of approximately 24 h between the first and the second copulation. Copulations took place in the absence of dung to prevent oviposition. If a pair did not copulate after approximately 15 min, the male was replaced. Ten to 20 min after the second copulation, the female was placed on a piece of filter paper with a smear of dung and allowed to deposit her eggs. During oviposition, the female was enclosed under a 50-mL glass vial and observed. Females tend to lay eggs close together. We therefore moved the vial with the female across the dung surface when females paused during oviposition to subdivide a clutch into batches of approximately 12 eggs. After moving the vial with the female to the next section of the dung, the number of eggs laid was counted and three eggs per batch were processed for microscopy (see below). After oviposition, the female was returned to standard culture conditions. Seven to 11 days later, females were allowed to lay a second clutch without further re-mating, in accordance with the procedure described above.
Egg preparation and sperm count
Preliminary experiments revealed that sperm stick to the surface of freshly-deposited eggs and that 4,6-diamidino-2-phenylindole (DAPI)-stained unfixed sperm heads are identifiable for at least 5 h when preparations are stored at 5 °C (S. H. Sbilordo, unpubl. data).
Three eggs per batch were removed from the dung and placed on a glass coverslip (40 mm × 50−60 mm) with two spacers (0.13–0.16 mm), held in place with a drop of nail polish. Eggs were mounted in a mounting medium containing DAPI (Vectashield VC-H-1500) and covered with a second coverslip, again held in place with nail polish. All preparations were stored in a fridge until they were examined under a fluorescence microscope (Leica DMR) from both sides. Sperm heads were counted at a magnification of ×400. We further recorded the distribution of sperm on the egg surface by dividing the egg into three regions: sperm aggregated in the micropylar region; sperm scattered over the ventral part of the egg; and sperm located on the dorsal part of the egg. Some sperm aggregations were too densely clumped to distinguish each single sperm. In these cases, we stopped counting at 100 sperm (= maximum). Digital photographs for illustration were taken with a Leica DMR microscope fitted with a SPOT RT camera (Visitron Systems GmbH).
Sample size and statistical analysis
Fourteen females met all conditions for our approach: they had mated twice on two subsequent days, deposited their first clutch the same day that they copulated with the second male, and laid their second clutch within 11 days after the first one. Five females met all other assumptions but failed to deposit a second clutch during this period. There was no significant difference in the number of sperm on first clutch eggs of the females that oviposited twice and the females that failed to deposit the second clutch [generalized linear model (GLM: F1,309 = 0.458, P = 0.499). Hence, it is unlikely that sperm limitation was responsible for the failure to oviposit twice.
Statistical analyses were performed with SPSS, version 13.0. Prior to the analyses, each egg was assigned to one of the several sequential groups: egg numbers 1–12, 13–24, 25–36, 37–48, 49–58, and 59–80. The number of analysed eggs was not always completely balanced in a female's first and second clutch. We therefore performed a GLM analysis including female identity as a random factor, equivalent to a repeated-measures analysis. Egg sequence was used as a covariate in the model. Prior to analysis, the dependent variable ‘number of sperm per egg + 1’ was log-transformed. Residuals of the analysis did not deviate from normality (Kolmogorov–Smirnov test: P = 0.427). By contrast, the distribution of the sperm on the egg surface could not be normalized. We therefore applied a nonparametric test for two related samples.
RESULTS
Influence of clutch number and egg sequence on patterns of sperm release
The number of sperm attached to the eggs of first clutches deposited shortly after insemination was significantly larger (mean ± SE; 78.38 ± 2.95) than in second clutches deposited without an additional insemination (23.13 ± 1.361; GLM: F1,456 = 94.13, P < 0.001; Fig. 2A). The number of sperm per egg declined significantly during the course of oviposition of both clutches (GLM: F1,456 = 33.39, P < 0.001; Fig. 2A), from 103.44 ± 7.83 to 65.94 ± 12.94 sperm on first clutch eggs and from 28.15 ± 3.78 to approximately 14.37 ± 2.59 sperm on eggs of the second clutches (Fig. 2A). The interaction between clutch and egg sequence was not significant when using log-transformed data, indicating an overall asymptotic decline in sperm numbers (GLM: F1,456 = 2.43, P = 0.120; Fig. 2B). Because clutch sizes and hence the number of categories of egg sequences varied between females, we repeated our analysis, including only females that had produced more than 59 eggs (covering all six categories). The result obtained was essentially the same, indicating that our data are robust in this aspect.
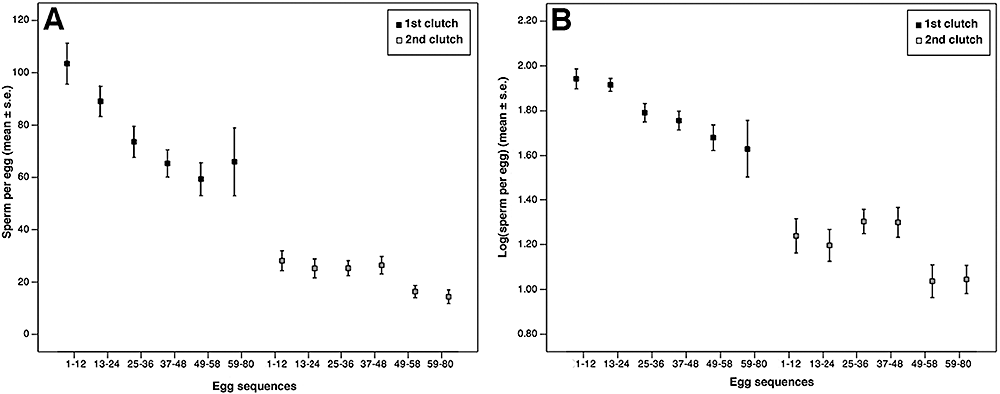
The decline of the number of sperm adhering to eggs of first and second clutches using untransformed (A) and log-transformed (B) data (N = 14 females).
The distribution of sperm on the egg surface
The cap covering the micropyle of the egg (Arthur et al., 2008) often persisted on deposited eggs, and sperm were entangled in the cap (Fig. 3A–C). In some preparations, the cap had collapsed, but sperm remained clumped in the remnant of the cap (Fig. 3D). However, in few eggs, the cap had fallen apart, so that the sperm could not be assigned unequivocally to the micropyle region (Fig. 3E). These eggs were excluded from this analysis. This procedure yielded a total of 164 eggs from first clutches and 194 eggs from second clutches included in the analysis.
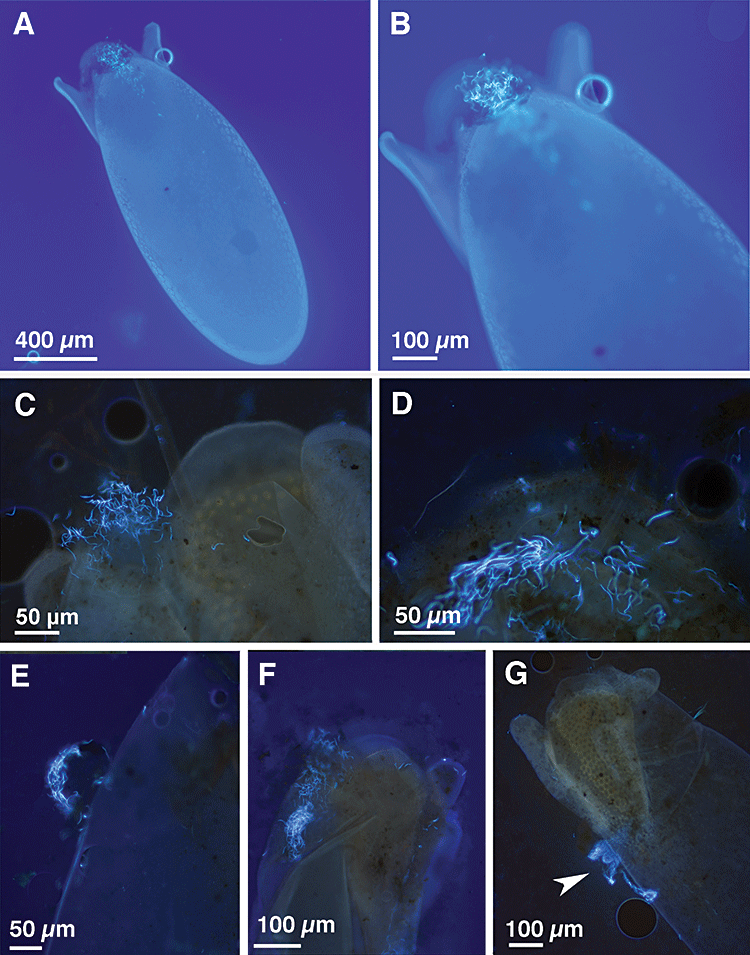
Fluorescence microscope images of deposited Scathophaga stercoraria eggs, where sperm heads are stained with 4,6-diamidino-2-phenylindole (DAPI). A, overview of the ventral part of an egg with sperm heads entangled in the acellular cap. B, C, views of the apex of an egg with sperm entangled in the micropylar cap. D, close-up view of the apex of an egg where the cap is collapsed but sperm heads are retained in the micropylar region. E, preparation of a second clutch egg where the cap has been displaced during handling. F, an egg with sperm heads entangled in the micropylar cap and an additional dense bulk of sperm heads beneath the micropyle. G, the egg with a dense sperm mass attached to the dorsal part.
In both clutches, sperm adhered mainly to the ventral side of the eggs and most sperm were located around the micropyle (Fig. 3A–D, F, Fig. 4). Occasionally, we observed sperm adhering to the dorsal part of the eggs below the plastron (Fig. 3G). Very dense aggregations with more than 100 sperm were restricted to the micropyle region (5.3% of the eggs) or to the ventral part below the micropylar region (2.5% of the eggs), except for one egg showing a large aggregation of sperm located dorsally (Fig. 3G). We exclusively observed such dense sperm aggregation on eggs of the females' first clutches.
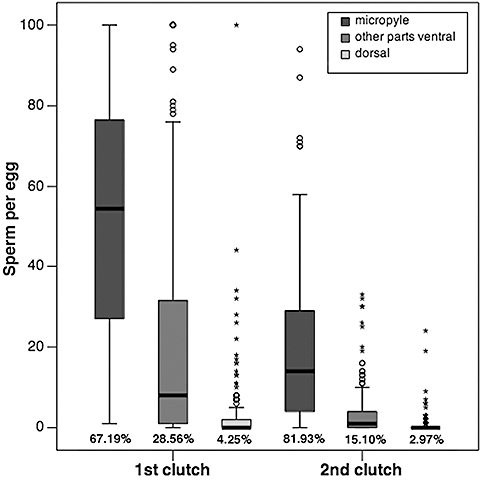
Number of sperm adhering to the micropyle region, beneath the micropyle region ventrally, and to the dorsal part of the eggs from first and second clutches (N = 14 females).
The number of sperm decreased significantly from the first to the second clutch in all three regions of the eggs (Wilcoxon signed ranks test: all N = 358, Z ≤ −8.13, P < 0.001; Fig. 4). The lower number of sperm on eggs of the second clutch was accompanied by a strong increase of the proportion of sperm adhering to the micropyle region (from 67.2% on first clutch eggs to 81.9% on eggs of the second clutch; Fig. 4).
Efficiency of sperm use and total number of sperm engaged in fertilization
The efficiency of sperm use can be calculated as the number of sperm used to fertilize an egg divided by the total number of sperm released from the sperm storages (Sakurai, 1998). On average, females deposited 58 eggs during each clutch (first clutch: 58.36 ± 3.21; second clutch: 58.43 ± 4.21 eggs). First clutch eggs had attached an average of 78.38 ± 2.95 sperm, whereas second clutches eggs contained an average of 23.13 ± 1.361 sperm. One insemination normally leads to hatching of 95% of the eggs during the first two clutches (Parker, 1970b). Data concerning the number of sperm entering the egg at fertilization are not yet available for S. stercoraria. For the calculation, we assumed that only a single sperm has entered the egg, although polyspermy might reduce the efficiency of fertilization (Parker, 1970a). This procedure avoids an overestimation of the number of sperm stored. Our estimate indicates that, on average, at least 4629 sperm contributed to the fertilization of the first clutch and 1406 sperm contributed to the fertilization of the second clutch. This translates into an efficiency for the use of released sperm of approximately 1.3% in first clutches and 4.2% in second clutches, respectively.
DISCUSSION
In our study on patterns of sperm utilization, we obtained three main results. First, the number of sperm adhering to the eggs strongly decreased during the oviposition of first clutches and, to a lesser degree, also during the oviposition of second clutches, reflecting an overall asymptotical decline in sperm number. Second, sperm were predominantly attached to the ventral micropyle region of the eggs, indicating that sperm release from the storage sites and ovulation are highly coordinated by the female. Finally, although we did not count the sperm within the spermathecae directly, we calculated that, on average, more than 6000 sperm were involved in fertilization during the first two oviposition bouts. This estimation significantly exceeds earlier estimates of a storage capacity of approximately 1000 sperm in this species (Parker et al., 1990; Ward, 1993; Otronen et al., 1997). Below, we first discuss our findings in the light of physiological mechanisms and then focus on the implications for post-copulatory sexual selection.
Physiological mechanisms of sperm release
We found that, in first clutches deposited shortly after copulation, the number of sperm per egg was very large at the beginning of oviposition and then rapidly declined. By contrast, eggs of the second clutches deposited without remating contained, on average, much fewer sperm and the decline in the absolute number of sperm was less steep. There are at least two, not mutually exclusive, explanations for these findings. First, passive sperm loss from the female reproductive tract causes the asymptotic decline in sperm numbers (Wishart, 1987; Birkhead, Pellatt & Fletcher, 1993; Birkhead & Fletcher, 1994; Birkhead et al., 1994). Early eggs of the second clutch contained, on average, much fewer sperm than late eggs of the first clutch (28 versus 65 sperm), showing that sperm supply decreases over time for reasons other than sperm use. Extra-spermathecal sperm may partially account for the large sperm numbers on first clutch eggs. The three spermathecae of the yellow dung fly have fixed volumes and are filled near to capacity even after a single mating (Parker et al., 1990; Parker & Simmons, 1991). In view of our double-mating setting, it is therefore very likely that significant amounts of sperm were left in the bursal cavity (Hosken et al., 1999). However, because the bursa stretches when eggs pass through, bursal sperm are likely to be extruded by the very first eggs laid (Simmons et al., 1999; S. H. Sbilordo personal observation). Indeed, we cannot rule out that, again, there was a significant amount of sperm in the long spermathecal ducts, which was squeezed out during the first bout of oviposition. This fits well with our observation that very dense sperm agglomerations were seen on eggs of the females' first clutches.
Sperm used to fertilize the eggs of the second clutch should originate entirely from the spermathecae, where gland cells maintained sperm viability during the inter-ovulation period (Davey, 1985). Sperm loss from the storage organs themselves (Bloch Qazi & Wolfner, 2006) and/or post-copulatory sperm mortality (Bernasconi et al., 2002) may further have reduced sperm supply before the second oviposition bout.
Second, females actively release fewer sperm when the sperm supply becomes smaller. This hypothesis is strongly supported by our morphological investigation showing that sperm were pre-dominantly attached to the ventral part of the eggs near the micropyle. Because the micropyle of the bursal egg is orientated away from the dorsal openings of the spermathecal ducts (Fig. 1; Arthur et al., 2008) this pattern can be expected only if sperm release and ovulation are coordinated. Interestingly, a greater proportion of sperm was precisely situated close to the micropyle in eggs of the second clutches compared to eggs of the first clutches. This suggests that sperm use is more efficient and sperm release better controlled when sperm supplies are reduced, and may explain why single-mated females do not show any reduction in fertility in up to four successive clutches, despite limited sperm reserves (Parker, 1970b). In line with this argument, Ward (2000) found that female effects on paternity were larger in second compared to first clutches when females had no opportunity to re-mate.
Access of the sperm to the micropyle may be achieved indirectly, as suggested by Arthur et al. (2008). During oviposition, the egg passes through the anterior part of the bursa copulatrix first and then arrests until sperm are placed at the ventral bursal wall. The egg is then pushed back into the anterior bursa copulatrix and sperm can reach the cap covering the micropyle (Arthur et al., 2008). A similar mechanism of fertilization has been reported for the house fly Musca domestica (Degrugillier & Leopold, 1973). However, although removal of the cap appears to be a prerequisite for sperm penetration in house flies (Leopold, Meola & Degrugillier, 1978), we observed that the cap often persisted on the deposited eggs, and that sperm were trapped in the cap or in its remnants. Hence, approaching the micropyle involves active movement of the sperm in S. stercoraria, raising the opportunity for selection on sperm traits affecting mobility (e.g. sperm length; Otronen et al., 1997). It would be interesting to determine whether only live sperm arrive at the micropyle cap of the egg, which could probably be addressed using viability assessment of sperm adhering to the eggs, if carried out immediately after oviposition (Hunter & Birkhead, 2002).
Implications for post-copulatory sexual selection
The female's efficiency of sperm storage and usage affects the degree of overlap between viable sperm of different males and hence sperm competition intensity (Parker, 1970a). The sperm storage capacity of yellow dung fly females has been repeatedly estimated to be limited to approximately 1000 sperm based on counts of spermathecal sperm (Parker et al., 1990; Ward, 1993; Otronen et al., 1997). By contrast, our estimation indicates that, on average, more than 6000 sperm were involved in fertilization of the first two clutches, suggesting that the storage capacity is larger than previously assumed. Although we do not know the reasons for this discrepancy, a higher sperm storage capacity should affect the efficiency of sperm displacement (Parker, 1984; Hellriegel & Ward, 1998; Parker et al., 1999). In the extreme, if the females' sperm storage capacity was infinitely large, there would be little possibility of displacing previous males' sperm.
A second implication of our study is that female yellow dung flies appear to control sperm release and utilization to a significant extent, which is one of the fundamental assumptions of sperm selection by the female. This result, together with previous findings indicating that the sperm of different males is stored nonrandomly (Hellriegel & Bernasconi, 2000), makes a ‘mate-now-choose-later’ strategy as proposed by Ward (2007) very plausible. Our study further highlights that, when females mate shortly before oviposition, which is the typical situation in the field (Parker, 1970a), the effects of sperm displacement are likely to overlay more subtle effects of cryptic female choice. Consequently, the potential for detecting the effects of cryptic female choice appears to be increased when investigating the female's second clutch deposited without re-mating.
ACKNOWLEDGEMENTS
We would like to thank Ursula Briegel for her great help during the data collection and Yves Choffat for providing technical support. We also would like to thank Tim Birkhead, Oliver Martin, Andrew Pemberton, Luc Bussière, Wolf Blanckenhorn, Tony Wilson, and three anonymous referees for their constructive comments on earlier versions of the manuscript. We are particularly grateful to Paul Ward for his significant support. Unfortunately, he died too early during the preparation of the manuscript. We hope that the presentation of the data fully reflects his scientific view.




