Molecular phylogenetic evidence for paraphyly of the genus Sooglossus, with the description of a new genus of Seychellean frogs
Abstract
The Seychelles harbour an endemic frog family, the Sooglossidae, currently containing two genera: Sooglossus, with three species, and Nesomantis, with one species. These unique frogs are generally considered to be basal neobatrachians, although their relationships to other neobatrachian taxa, except the Nasikabatrachidae, remain unresolved. Our molecular phylogeny based on a dataset consisting of fragments of the nuclear rag-1 and rag-2 genes, as well as mitochondrial 16S rRNA in representatives of the major neobatrachian lineages, confirmed the previously postulated Sooglossidae + Nasikabatrachidae clade and the placement of the South American Caudiverbera with the Australian Myobatrachidae, but did not further resolve the position of sooglossids. Our results do, however, unambiguously show sooglossids to be monophyletic but the genus Sooglossus to be paraphyletic, with the type species Sooglossus sechellensis being more closely related to Nesomantis thomasseti than to Sooglossus gardineri and Sooglossus pipilodryas, in agreement with morphological, karyological, and bioacoustic data. As a taxonomic consequence, we propose to consider the genus name Nesomantis as junior synonym of Sooglossus, and to transfer the species thomasseti to Sooglossus. For the clade composed of the species gardineri and pipilodryas, here, we propose the new generic name Leptosooglossus. A significant genetic differentiation of 3% was found between specimens of Sooglossus thomasseti from the Mahé and Silhouette Islands, highlighting the need for further studies on their possible taxonomic distinctness. © 2007 The Linnean Society of London, Biological Journal of the Linnean Society, 2007, 91, 347–359.
INTRODUCTION
The frogs of the basal neobatrachid family Sooglossidae are restricted to mossy mountain forests on two islands of the Seychelles archipelago: Mahé and Silhouette. These granitic islands on the Mascarene plateau are highly isolated Gondwanan fragments in the Indian Ocean, 1000 km south of India (Briggs, 2003). The Seychelles became isolated from other landmasses approximately 67–47 Mya, although the islands may have been connected to each other during the last glaciation (Badyukov, Demidenko & Kaplin, 1989). The family Sooglossidae consists of four species divided into two genera; Sooglossus, with three small-sized species, and Nesomantis, with a single medium-sized species. All four species are listed as ‘vulnerable’ on the IUCN Red List (http://www.globalamphibians.org; accessed 9 July 2005) due to their restricted ranges, being associated with moist upland rainforests. Sooglossus sechellensis (Boettger, 1896) and Sooglossus gardineri (Boulenger, 1911) are leaf-litter inhabiting species, whereas Sooglossus pipilodryas Gerlach & Willi, 2003 is arboreal and usually lives in the axils of endemic palms and banana trees. Nesomantis thomassetiBoulenger, 1909 is associated with rock overhangs. Sooglossus gardineri is one of world’s smallest tetrapods with a snout–vent length of only 9.8 mm in adult males.
The relationships of Sooglossidae to other frogs have been a subject of continuing debate. Except for their placement with the Pelobatidae (Griffiths, 1963), they usually were classified with a modern lineage of frogs that several authors (Nussbaum, 1982; Hay et al., 1995; Feller & Hedges, 1998; Van der Meijden, Vences, Hoegg & Meyer, 2005) referred to as Neobatrachia. Partly following the classification of Dubois (2005), who proposed discontinuing the use of this name as the formal name of a suborder of frogs, here, we use only the name ‘neobatrachians’ informally to refer to this clade. Within neobatrachians, Sooglossidae have been grouped with the ranoids (Savage, 1973), the Microhyloidea (Blommers-Schlösser, 1993), and in the hyloid superfamily sister to Myobatrachinae (Lynch, 1973; Duellman & Trueb, 1986; Ford & Cannatella, 1993). The last comprehensive review of the taxonomic position of this family was provided more than 20 years ago by Nussbaum (1984).
Recent molecular studies based on analysis of different markers have also failed to provide convincing evidence allying Sooglossidae with any other neobatrachian group, with the notable exception of their strongly-supported sister group relationship to the newly-discovered Nasikabatrachidae from India (Biju & Bossuyt, 2003). Phylogenies using DNA sequences coding for 12S and 16S rRNA (Hay et al., 1995), 12S, 16S and the valine tRNA, together with rhodopsin and single exon fragments of rag-1 and CXCR-4 (Biju & Bossuyt, 2003), rag-1 and rag-2 (Hoegg, Vences, Brinkmann & Meyer, 2004) and a larger fragment of rag-1 (San Mauro, Vences, Alcobendas, Zardoya & Meyer, 2005) placed the sooglossids, or sooglossids plus nasikabatrachids (Biju & Bossuyt, 2003), either as an isolated clade basal in the neobatrachians, or related to other neobatrachian clades with very low phylogenetic support.
A phylogenetic pattern that became obvious from recent molecular studies on deep anuran relationships (Biju & Bossuyt, 2003; Hoegg et al., 2004; Roelants & Bossuyt, 2005; San Mauro et al., 2005) is the existence of two well-defined and very species-rich neobatrachian clades, ranoids and hyloids, and of several other, more basal lineages of largely unclarified relationships. Besides the sooglossids and nasikabatrachids, these basal assemblages include the heleophrynids from South Africa, the myobatrachids from Australia, and the South American Caudiverbera which previously was believed to belong to the Leptodactylidae. Firmly established relationships among these taxa would be highly informative for the reconstruction of anuran biogeography and the effect of the fragmentation of Gondwana (Biju & Bossuyt, 2003). However, recent phylogenetic studies did not include sooglossids together with representatives of all other basal lineages, and all used only Nesomantis to represent the Sooglossidae clade. A molecular test for the monophyly of sooglossids is so far missing. At the level of intrafamilial relationships in the Sooglossidae, it has long been suspected that the genus Sooglossus might be paraphyletic with respect to Nesomantis (Noble, 1931). Additional morphological evidence for paraphyly of Sooglossus has since been accumulated (Griffiths, 1963; Gerlach & Willi, 2002).
Here, we provide the first intrafamilial molecular phylogenetic hypothesis of the Sooglossidae, based on two nuclear genes and one mitochondrial ribosomal RNA gene. Our study also includes representatives of all deep neobatrachian lineages identified to date to test for monophyly and relationships of the Sooglossidae.
MATERIAL AND METHODS
Selection of taxa
All four currently described species of the Sooglossidae were included in this study. We used the following specimens: S. gardineri (4-2003, collected by R. Boistel, collection of the Muséum National d’Histoire Naturelle, Paris, MNHN 2003.3410, Silhouette, Jardin Marron, 400 m a.s.l.); S. pipilodryas (4-2003, collected by R. Boistel, MNHN 2003.3411, Silhouette, Jardin Marron 350 m a.s.l.); two specimens of S. sechellensis (4-2003, collected by R. Boistel, MNHN 2003.3412–3413, Silhouette, Jardin Marron 450 m a.s.l.); and two specimens of N. thomasseti (5-7- 2001, collected by L. Chong Seng & A. Ohler, and MNHN 2001.0269, Mahé; 4-2003, collected by R. Boistel, MNHN 2003.3414, Silhouette, Jardin Marron 350 m a.s.l.). We also selected representatives of several hyloid and ranoid families available from GenBank (Table 1) to place the clade formed by the Sooglossidae and Nasikabatrachus with its closest taxon within the neobatrachians. We included Nasikabatrachus sahyadrensis, although no rag-2 sequence is available for this species. Of the ranoid superfamily, we selected Rana temporaria as a representative ranid, Kaloula pulchra representing the Microhylidae, and the hyperoliid Hyperolius viridiflavus. The superfamily Hyloidea was represented by the bufonid Bufo regularis, the hylid Agalychnis callidryas, and the leptodactylids Leptodactylus fuscus and Caudiverbera caudiverbera. Also included were the basal neobatrachians Heleophryne regis of the Heleophrynidae, and Lechriodus melanopyga representing the Myobatrachidae. The pipid frog Pipa parva was used as the outgroup.
| Species | Family | 16S | Rag-1 | Rag-2 |
|---|---|---|---|---|
| Agalychnis callidryas | Hylidae | AY330890 | AY323765 | AY323780 |
| Bufo regularis | Bufonidae | AY330891 | AY323763 | AY323784 |
| Heleophryne regis | Heleophrynidae | AF432230* | AY323764 | AY323786 |
| Hyperolius viridiflavus | Hyperoliidae | AF215441 | AY323769 | AY323789 |
| Kaloula pulchra | Microhylidae | AY330893 | AY323772 | AY323790 |
| Lechriodus melanopyga | Myobatrachidae | DQ872915 | AY583341 | DQ872908 |
| Caudiverbera caudiverbera | Leptodactylidae (?) | DQ872913 | AY583337 | DQ872909 |
| Leptodactylus fuscus | Leptodactylidae | AY263226 | AY323770 | AY323791 |
| Nasikabatrachus sahyadrensis | Nasikabatrachidae | AY364381 | AY364225 | NA |
| Pipa parva | Pipidae | AY333690 | AY323761 | AY323799 |
| Rana temporaria | Ranidae | AF249048 | AY323776 | AY323803 |
| Nesomantis thomasseti‘Mahé’ | Sooglossidae | AY330889 | AY323778 | AY323798 |
| Nesomantis thomasseti‘Mahé’ | Sooglossidae | DQ872914 | – | – |
| Nesomantis thomasseti | Sooglossidae | AY364373 | – | – |
| Nesomantis thomasseti‘Silhouette’ | Sooglossidae | DQ872920 | – | – |
| Nesomantis thomasseti‘Silhouette’ | Sooglossidae | X86288 | – | – |
| Sooglossus sechellensis | Sooglossidae | DQ872916 | DQ872921 | DQ872910 |
| Sooglossus sechellensis | Sooglossidae | DQ872917 | – | – |
| Sooglossus gardineri | Sooglossidae | DQ872919 | DQ872923 | DQ872911 |
| Sooglossus pipilodryas | Sooglossidae | DQ872918 | DQ872922 | DQ872912 |
- * Sequence of Heleophryne purcelli used.
- NA, not applicable.
Sequencing and alignment
DNA was extracted from toe clips fixed in 99% ethanol. Tissue samples were digested using proteinase K (final concentration 1 mg mL−1), homogenized, and subsequently purified following a high-salt extraction protocol (Bruford et al., 1992). Primers for rag-1 and rag-2 were from Hoegg et al. (2004) as reported in Chiari et al. (2004). Primers for the fragment of the 16S rRNA gene were 16SA-L and 16SB-H of Palumbi et al. (1991). Polymerase chain reaction (PCR) was performed in 25-µL reactions containing 0.5–1.0 units of REDTaq DNA Polymerase (Sigma), 50 ng genomic DNA, 10 pmol of each primer, 15 nmol of each dNTP, 50 nmol additional MgCl2 and the REDTaq PCR reaction buffer (in final reaction solution: 10 mm Tris-HCl, pH 8.3, 50 mm KCl, 1.1 mm MgCl2 and 0.01% gelatine). For rag-1 and rag-2, cycle conditions were adapted from a long range PCR protocol (Barnes, 1994), with an initial denaturation step at 94 °C for 5 min, followed by ten cycles with 94 °C for 30 s, annealing temperatures increasing by 0.5 °C per cycle from 52 to 57 °C and extending for 3 min at 68 °C. Additionally, 20 cycles were performed with 94 °C for 10 s, 57 °C for 40 s, and 68 °C for 3 min. The final extension was performed at 68 °C for 5 min. For 16S, the denaturation step was followed by 35 cycles of denaturation at 94° for 30 s, annealing at 50° for 30 s, and extension at 72° for 90 s.
PCR products were purified via spin columns (Qiagen). Sequencing was performed directly using the corresponding PCR primers (forward and reverse). DNA sequences of both strands were obtained using the BigDye Terminator cycle-sequencing ready reaction kit (Applied Biosystems Inc.) on an ABI 3100 capillary sequencer in accordance with the manufacturer’s instructions. New sequences were combined with existing sequences taken from GenBank in the final dataset. These sequences were deposited in GenBank; for accession numbers, see Table 1.
Chromatograms were checked by eye using Sequencher (Gene Codes Corp.) or Chromas, version 1.45 (Technelysium Pty Ltd) and the sequences were subsequently aligned. Rag-1 and rag-2 sequences were aligned by hand using the Mega3 alignment editor (Kumar, Tamura & Nei, 2004). The 16S sequences were aligned using Clustal W (Thompson, Higgins & Gibson, 1994) and subsequently edited by hand. Gapped and hypervariable sections of the 16S alignment were removed from the full alignment. Hypervariable regions were included in the dataset containing only Nasikabatrachus and the sooglossids.
Data analysis
A homogeneity partition test (Farris et al., 1994), as implemented in PAUP* (Swofford, 2002), rejected homogeneity of the different markers (P = 0.06). Besides a pooled analysis of the combined data set, we therefore also performed separate analyses of each of the various genes. Transitions and transversions were plotted against F84 distances (Felsenstein, 1984) for the separate gene alignments. None of the datasets showed signs of saturation.
Phylogeny reconstruction based on the separate and combined datasets was performed using maximum likelihood (ML) and Bayesian inference (BI) methods. The best fitting models of sequence evolution were determined by the AIC criterion in Modeltest, version 3.06 (Posada & Crandall, 1998). ML tree searches were performed using PhyML, version 2.4.4 (Guindon & Gascuel, 2003). Bootstrap branch support values were calculated with 500 replicates. The Bayesian analyses of the combined and separate datasets was conducted with MrBayes, version 2.0 (Huelsenbeck & Ronquist, 2001), using models estimated with Modeltest under the AIC criterion, with 250 000 generations, sampling trees every tenth generation (and calculating a consensus tree after omitting the first 3000 trees).
The paraphyly of the genus Sooglossus in our analysis theoretically could have been caused by introgression phenomena or sample contamination regarding the clade containing S. sechellensis and Nesomantis. To test for this possibility, and to assess the differentiation among individuals of Nesomantis from different populations, we sequenced the 16S rRNA gene from a second specimen of S. sechellensis and from a Nesomantis specimen from Silhouette, added three Nesomantis sequences from GenBank, and submitted this 16S data set to a separate ML analysis with Nasikabatrachus as outgroup.
Microtomography
Microtomographic analyses were carried out in the European Synchrotron Radiation Facility (ESRF) at Grenoble, France. We used the following adult specimens: four N. thomasseti, three S. sechellensis, four S. gardineri, and three S. pipilodryas. The animals were deposited in a small tube of polypropylene. Microtomography in absorption-based form (amplitude contrast and phase contrast), consists of recording several hundreds of radiographs, with the sample slightly rotated between exposures, and it uses a standard filtered back-projection algorithm to perform the three-dimensional (3D) reconstruction. The detector uses a FReLoN 1024 × 1024 and 2048 × 2048 camera (Labiche et al., 1996), and involves an optical microscope assembly between the X-ray sensitive converter and the CCD. The effective pixel size is varied by altering the visible-light part of the assembly. Three different sets of experiments were performed on the imaging beamline ID19 of the ESRF, with pixel sizes, respectively, of 7.5 µm at a sample-to-detector distance of 40 mm, 10 µm at 40 mm specimen-to-detector distance and 30 µm at 40 mm specimen-to-detector distance. For phase contrast, samples were scanned at a sample-detector distance of 300 mm. The experiment was performed to obtain a high resolution 3D image of the skeleton and soft tissue. It was performed with the synchrotron radiation monochromatized to 17, 20, and 20.5 keV by a double-crystal silicon monochromator operating in the vertical plane. The flux at the level of the sample for a beam current of 60–180 mA and a wiggler as X-ray source is approximately 8 × 109 photons s−1 mm−2. Image visualization was performed using Amira software, version 3.1, from TGS and the public domain ImageJ program developed at the United States National Institute of Health (http://rsb.info.nih.gov/ij/). Supplementary data on osteology such as colour plates are available on the web site (http://indigene.ibaic.u-psud.fr/rubrique.php3?id_rubrique=41) of the Université d’Orsay.
RESULTS
The final combined dataset consisted of 1447 bp of rag-1, 810 bp of rag-2, and 435 bp of 16S rRNA, resulting in a combined alignment of 2692 bp. The 16S rRNA alignment had 118 parsimony informative sites, and 256 conserved sites. Rag-1 and rag-2 had 445 and 325 parsimony informative, and 815 and 337 conserved sites, respectively.
Basal neobatrachian relationships were poorly resolved with all datasets. In both the ML and BI analyses, the rag-1 and rag-2 datasets resolved the hyloid and ranoid clades and a separate myobatrachid clade, whereas the 16S rRNA dataset provided poor resolution at this level. All separate and combined analyses were congruent in the resolution of a clade representing the Sooglossidae with high support. All datasets using both phylogeny reconstruction methods resolved identical relationships within the Sooglossidae with high support. Sooglossids were split in two clades: S. gardineri and S. pipilodryas were sister taxa, as were N. thomasseti and S. sechellensis.
Sooglossids were placed sister to Nasikabatrachus in all analyses, and Caudiverbera clustered with the myobatrachid Lechriodus based on both nuclear datasets. This sister relationship of Caudiverbera and Lechriodus was not supported by the 16S dataset, which grouped Caudiverbera with Heleophryne, albeit with only low support values (68% Bayesian posterior probability and 66% ML bootstrap support). Heleophryne was weakly associated with the clade formed by Caudiverbera and Lechriodus in the combined analyses (Fig. 1).
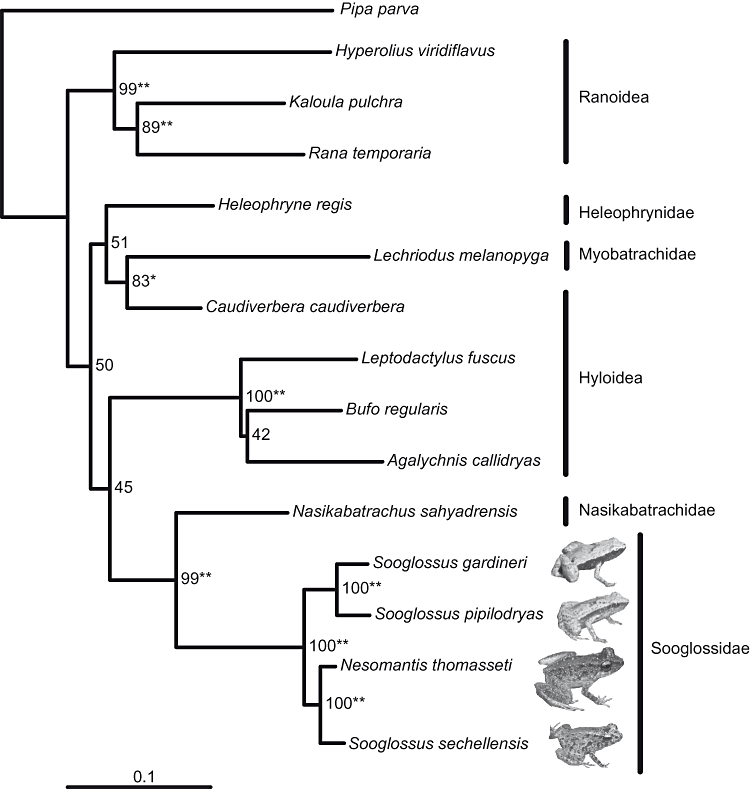
Maximum likelihood phylogram of the combined dataset of rag-1, rag-2, and 16S rRNA. Numbers indicate bootstrap support percentages of 500 replicates. A single asterisk indicates a Bayesian posterior probability of over 0.97. Two asterisks indicate a Bayesian posterior probability of 1.0. Sizes of insets are not shown to scale.
To be able to include published data on additional sooglossid individuals, we performed a second analysis based on the 16S rRNA gene only. This dataset included the two N. thomasseti samples (from Mahé and Silhouette) available to us, as well as three further sequences of this species from GenBank, two individuals of S. sechellensis, single individuals of S. gardineri and S. pipilodryas, and Nasikabatrachus as the outgroup. The obtained tree (Fig. 2) provided the same arrangement of taxa as the combined analysis and furthermore placed sequences of N. thomasseti from Mahé and Silhouette, respectively, in two separate subclades.
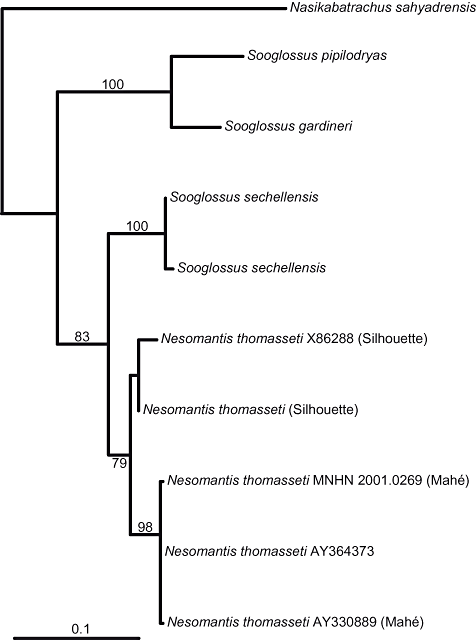
Maximum likelihood phylogram based on a reduced dataset (16S rDNA only). Numbers indicate bootstrap percentages of 500 replicates. Different 16S rDNA sequences of Nesomantis available through GenBank were included: X86288 (Hay et al., 1995), AY364373 (Biju & Bossuyt, 2003), and AY330889 (Hoegg et al., 2004).
Genetic differentiation between sooglossid taxa was of similar levels as known for other amphibians. Pairwise Jukes–Cantor (JC) corrected distance (Jukes & Cantor, 1969) between S. gardineri and S. pipilodryas based on 16S was 5.7%, whereas the distance between S. sechellensis and N. thomasseti was 4.4%. The rag-1 and rag-2 fragments showed a similar pattern; the JC corrected pairwise distances between S. gardineri and S. pipilodryas were 4.0% for both rag-1 and rag-2, and 2.3% and 2.5% between S. sechellensis and N. thomasseti, respectively. The mean 16S rRNA-based JC distance between the Silhouette and Mahé specimens of N. thomasseti was 3.0%. The mean 16S rRNA based distance between the S. gardineri/S. pipilodryas clade and the N. thomasseti/S. sechellensis clade was 16.6%.
DISCUSSION
Relationships among basal neobatrachians
The phylogenetic relationship of the clade formed by the Sooglossidae and Nasikabatrachus to other neobatrachians could not be resolved in our analyses. Although correct assignment of most species to the larger superfamilies Ranoidea and Hyloidea is usually unproblematic, the current work, similar to previous studies using molecular characters (Hay et al., 1995; Biju & Bossuyt, 2003; Hoegg et al., 2004; San Mauro et al., 2005), failed to provide resolution among the more basal neobatrachian taxa. Like the sooglossids, most of these basal taxa are species-poor, and have only relictal distributions. Sooglossids are restricted to the Seychelles, Nasikabatrachus is highly localized in India as are heleophrynids in South Africa, and Caudiverbera, which is possibly closely related to the Australian myobatrachids, in South America. The global scattering of their small distributions, suggests that these taxa might be remnants of an ancient neobatrachian radiation predating the breakup of Gondwana (San Mauro et al., 2005). Lack of basal resolution among neobatrachian groups might be because inadequate markers were used in these studies. Alternatively, the initial radiation of neobatrachians could have occurred too fast to be reconstructable from present DNA sequences. Nevertheless, the data published previously and those that are included in the present work allow for three conclusions regarding the relationships of these frogs: (1) the Sooglossidae are a monophyletic group, which (2) is confirmed to be sister to the Indian Nasikabatrachus, thereby validating the biogeographical scenario of Biju & Bossuyt (2003); (3) the placement of Caudiverbera, which is typically included with the Leptodactylidae, sister to the myobatrachid Lechriodus rather than with the leptodactylid Leptodactylus, receives further support in addition to the phylogeny of San Mauro et al. (2005) that was based on only rag-1 sequences. By contrast to the other lineages of basal neobatrachians, Myobatrachidae is a species-rich taxon with 124 species (including Rheobatrachidae and Limnodynastidae; AmphibiaWeb.org, as of August 2005), and a more comprehensive sampling of these Australian taxa in the future will be crucial to fully understand the phylogenetic and biogeographical pattern of the various clades in the initial neobatrachian radiation.
A striking character of sooglossids is their lack of extensible external vocal sacs (R. Boistel, pers. observ.) and the absence of middle ear ossicles (Parker, 1934). A single internal vocal sac with very small vocal slits (1 mm length, Tyler, 1985) is present. The middle ear is considered an adaptation to hearing in air (Allen, 1985) and the external vocal sacs are a common solution for the need for communication over greater distances in anurans. It has been assumed that frogs lacking such structures are deaf and voiceless, which is contradicted by the fact that at least some sooglossids emit advertisement calls (Gerlach & Willi, 2002). The closely related Nasikabatrachus shares the absence of the tympanum (see absence of bone columella in Biju & Bossuyt, 2003: fig. 1e, f; Dutta et al., 2004). Of the 5157 described species of anurans placed in 32 families (AmphibiaWeb.org), approximately 6% are earless (Boistel, 2003). Although the Sooglossidae and Nasikabatrachidae form one of the basal clades of the neobatrachians, the majority of neobatrachians do have a middle ear and its loss is most probably secondary (R. Boistel, unpubl. data).
Intrafamilial phylogeny and the classification of sooglossids
The paraphyly of the genus Sooglossus as shown by our data corroborates the earlier findings based on morphology (Noble, 1931; Griffiths, 1963; Gerlach & Willi, 2002), vocalizations (Nussbaum, Jaslow & Watson, 1982), genetic distance data (Green, Nussbaum & Datong, 1988), and karyology (Nussbaum, 1979). The high support that this placement receives (based on the separate and combined molecular datasets irrespective of phylogeny reconstruction method) and the low genetic distance between N. thomasseti and S. sechellensis, suggests a need for further taxonomic reconsideration of the genus Nesomantis. This robust arrangement may also provide a basis for the study of the evolution of reproductive modes and other features of the biology of these genetically highly distinctive frogs.
Originally, Sooglossus was described by Boulenger (1906) and assigned to the family Ranidae in order to separate Arthroleptis sechellensis Boettger, 1896 from the African Arthroleptis. The new genus was defined by the presence of an entire, elliptical tongue which Boulenger (1906) lists as the sole distinctive character. When he discovered a second species (N. thomasseti) from the Seychelles Islands, Boulenger (1909) described it as a member of a new ranid genus mainly distinguished by the presence of vomerine teeth and the shape of the digits. The characters used by Boulenger (1882) to redefine Nectophryne, particularly the presence of a fleshy web, allow us to understand his generic allocation of a new species Nectophryne gardineriBoulenger (1911) as a member of the Bufonidae. Thus, the frogs from the Seychelles were historically classified as members of two distinct families, grouping S. sechellensis with N. thomasseti as corroborated by the morphological study of Gerlach & Willi (2002). Available information on reproductive modes also shows some differences within the genus Sooglossus: S. sechellensis deposits eggs in a terrestrial nest. These hatch into nonfeeding tadpoles that are transported on the back of the male until metamorphosis (Nussbaum, 1984). Also, eggs of N. thomasseti are deposited in a terrestrial nest, and hatch into nonfeeding tadpoles (R. Boistel, unpublished data). By contrast, S. gardineri lays terrestrial eggs that hatch into froglets without a free tadpole stage. The breeding habits of S. pipilodryas are unknown.
Summarizing, except for body size, there is no convincing morphological difference supporting the recognition of a separate genus Nesomantis to place the species thomasseti separate from sechellensis. The genetic distance between these species is relatively low for frogs (4.4% in the 16S rRNA gene). In contrast, the species gardineri and pipilodryas form a genetically highly distinct clade, supported by molecular data and morphological and behavioural evidence. We suggest that these findings should be reflected in the taxonomy by including both sechellensis and thomasseti in a single genus Sooglossus, with the generic name Nesomantis being a junior synonym, and by describing a new genus Leptosooglossus to accommodate the two remaining sooglossid species, gardineri and pipilodryas.
SOOGLOSSIDAE NOBLE, 1931
Sooglossus Boulenger, 1906
SooglossusBoulenger, 1906: Type species by monotypy: Arthroleptis sechellensis Boettger, 1896.
NesomantisBoulenger, 1909: Type species by monotypy: Nesomantis thomasseti Boulenger, 1909. New synonym.
Species included: Sooglossus sechellensis (Boettger, 1896); Sooglossus thomasseti (Boulenger, 1909).
Diagnosis: Small to medium sized sooglossids (16–45 mm snout–vent length) with protruding nostrils, widely separated metacarpal tubercles, fingers with pointed toe pads, and tubercular skin on dorsum; toes free, without web.
Cranium (3): Dorsum of braincase with frontoparietals narrowly separated anteriorly and suturated through greater most of their lengths, and overlapping otic capsule (exoccipital), but not fused to it, extended laterally with medial margins of epiotic eminence and posteriorly at anterior margin of exoccipital; exotosis producing a pair of spines on frontoparietals. Nasals narrowly separated medially and close to preorbital process of maxilla. Presence of neopalatine, its lateral end distinctly wider than medial end, expanded or not on sphenethmoid, absence of anterolateral ossification of sphenethmoid; two halves of sphenethmoid separated dorsomedialy and fused ventromedialy in their posterior part on 4/5 of their length or not, but forming posterior nasal cavity and olfactory formina. Maxillary arcade complete bearing teeth, composed of maxilla articulated anteriorly with premaxilla and posteriorly with quadratojugal. Pars facialis of maxilla broad, teething starting just after terminus of zygomatic ramus of squamosal; anterior end of maxilla with pointed process overlaping premaxilla at superior part of pars dentalis; jaw articulation largely posterior of operculum. Squamosals T-shaped, otic ramus of squamosal forming a slender plate, incurved posteromedially, otic plate absent; zygomtic ramus well developed and deflected medially; otic ramus shorter than zygomatic ramus; ventral ramus inclined anteriorly, investing lateral surface of palatoquadrate and distinctly separated from quadratojugal. Pterygoid triradiate slender and gracile; anterior ramus of perygoid extends anterolaterally from otic capsule to articulation with groove of maxillae formed by the partes palatina and facialis; posterior ramus investing medial surface of palatoquadrate; medial ramus terminating vertically on anterior margin of otic capsule and not in contact with prootic and parasphenoid alary process; medial ramus longer than posterior ramus. Medio-ventral part of braincase closed by bone; parasphenoid T-shaped with alary process, orientated laterally but slanted slightly posterolaterally; cultriform process of parasphenoid extending anteriorly to level of antero-ventral margins of sphenethmoid; anterior terminus of cultriform process of irregular shape and non-acuminate; parasphenoid not fused with sphenethmoid and otic capsule; posterior process of parasphenoid acuminate. Operculum entirely ossified. Denticulate serration on dentary present; a sesamoid at maxillo-mandibular articulation present.
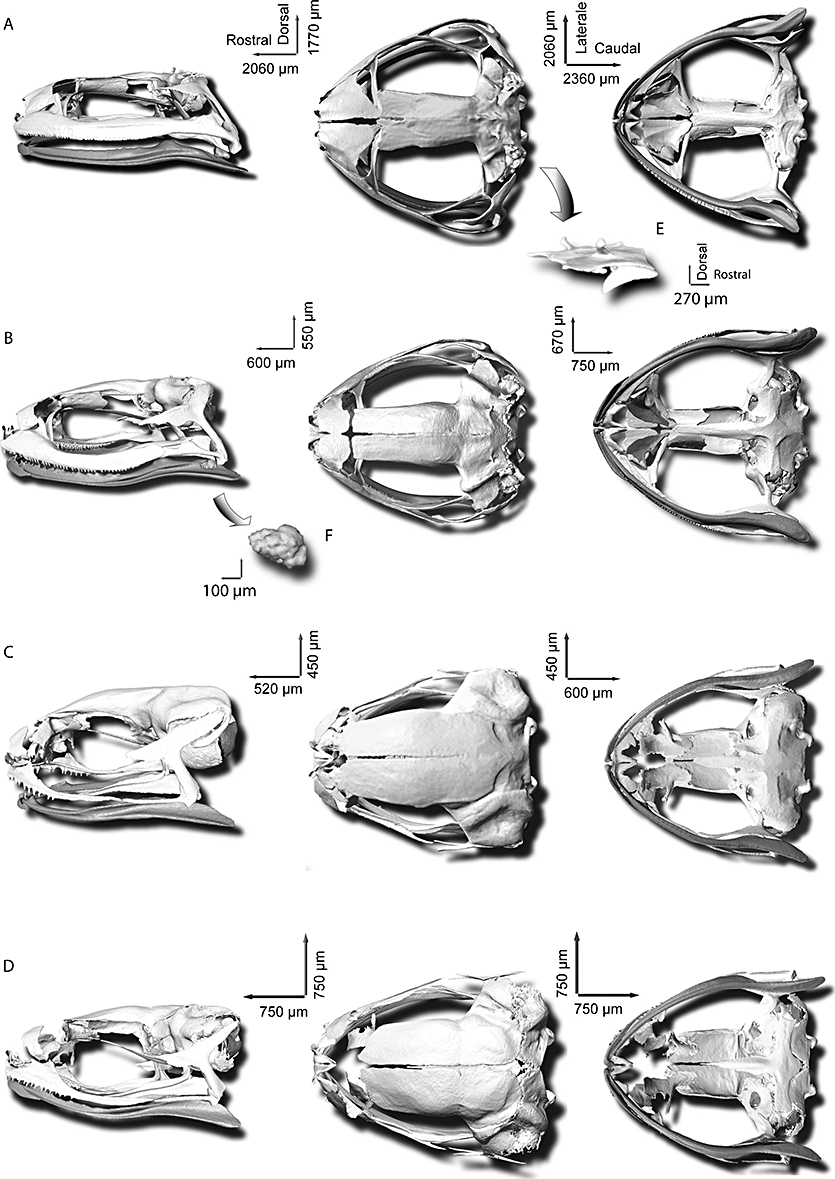
Volume rendering of X-ray microtomography of skulls of Sooglossidae species. A, Sooglossus thomasseti, MNHN 2001.0269, Mahé, resolution 30 µm; B, Sooglossus sechellensis, MNHN 1984.2371, Mahé, resolution 7.5 µm; C, Leptosooglossus gardineri, MNHN 1984.2369, Mahé, resolution 7.5 µm; D, Leptosooglossus pipilodryas, RBSS 2003.0007, Silhouette, resolution 7.5 µm; E, detailed latero-posterior view of otoccipital region with exostosis producing a pair of processes in S. thomasseti; F, detail of lateral view of sesamoid bone of articulation of maxillary mandibular of S. sechellensis. Left, lateral view from left; middle, dorsal view; right, ventral view.
Postcranium (4): Eight procoelous, not imbricate presacral vertebrae; presacral I and II fused or not; atlantal condylar type I (Lynch, 1971), widely separated; neural arches of vertebrae II–VIII bearing a single, posteriorly directed spinous process, overlaping succeeding vertebra or not. Sacro-coccygeal articulation monocondylar; sacral diapophyses dilated. Anterior end of urostyle bearing a pair of vestigial processes; transverse processes of third and fourth presacral vertebrae much wider than width of sacral diapophyses.
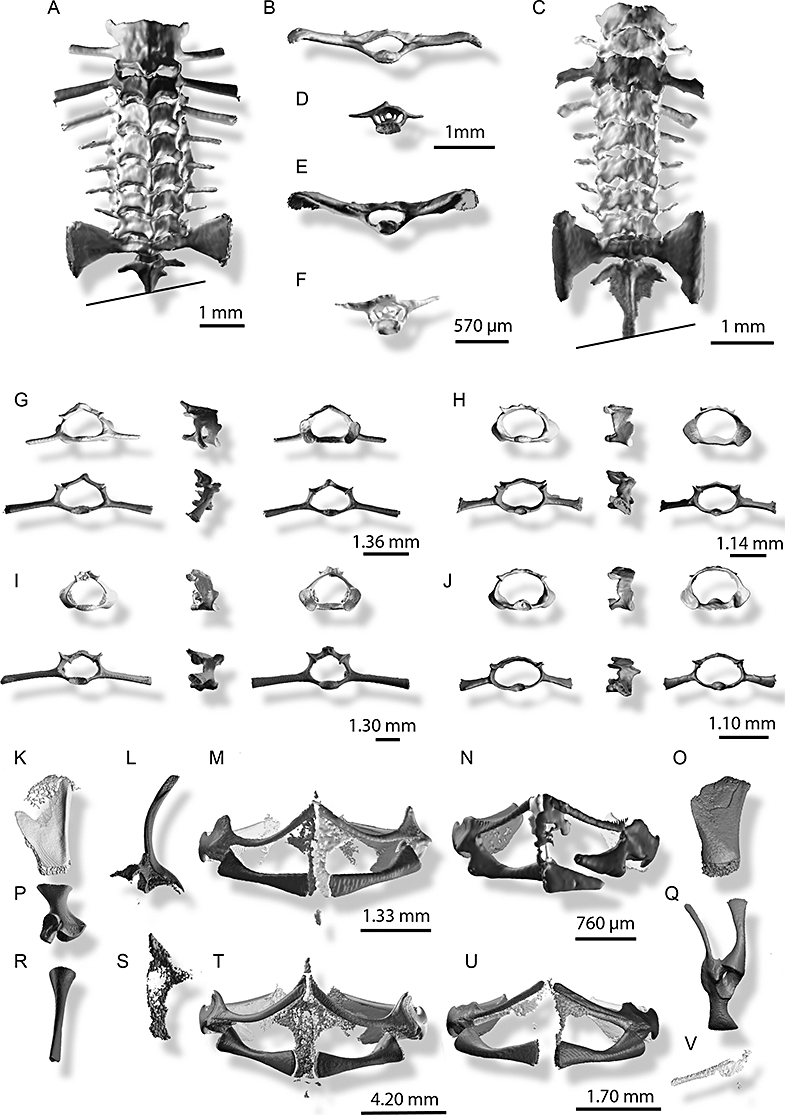
Volume rendering of X-ray microtomography of postcranial skeleton of Sooglossidae species. A, B, D, G, M, Sooglossus sechellensis, MNHN 1984.2371, Mahé, resolution 7.5 µm; C, E, F, J, N, Leptosooglossus gardineri, MNHN 1984.2369, Mahé, resolution 7.5 µm; H, O, Q, W, X, Leptosooglossus pipilodryas, RBSS 2003.0007, Silhouette, resolution 7.5 µm; I, K, L, P, R, S, T, Sooglossus thomasseti, RBSS 2003.0001, Silhouette, resolution 7.50 µm. A, C, vertebral column, dorsal view; B, E, Sacrum, posterior view; D, F, urostyle, anterior view; G, H, I, J (upper row), atlas vertebrae, posterior (left), lateral (middle), and anterior (right) view; G, H, I, J (lower row), third presacral vertebrae (left), lateral (middle), and anterior (right) view; M, N, T, W, pectoral girdle, ventral view; K, O, suprascapula, dorsal view; P, scapula, lateral view; L, clavicle, dorsal view; R, coracoid; anterior view; S, X, ossification of epi- and precoracoid cartilage; Q, scapula, clavicle, coracoid and procoracoid, synosteotically united, ventro-lateral view. N, the coracoid of L. gardineri is fractured.
Arciferal pectoral girdle: Cleithrum and suprascapula distinguishable; scuprascapula with proximal section ossified, Y-shaped; cleithrum partially ossified distally. Coracoid, scapula, procoracoids and clavicle not synosteotically united but linked by cartilage, partially ossified. Omosternum and sternum ossified or partially ossified.
Terminal phalanges simple, sharply pointed, ending in a very small knob, no intercalary cartilage between penultimate and ultimate phalanges of fingers and toes.
Phylogenetic definition: The clade stemming from the most recent common ancestor of S. sechellensis (Boettger, 1896) and S. thomasseti (Boulenger, 1909).
Reproductive behaviour: In S. sechellensis, eggs are laid on the ground; after hatching, nonfeeding (endotrophic) tadpoles will climb on the back of adult and are carried until metamorphosis. Females of S. thomasseti are known to have large and pigmentless ovarian eggs, and they hatch into endotrophic tadpoles (R. Boistel, pers. observ.).
Etymology: Composed of the Classical Greek terms soos, safe, sound, unscathed, unwounded; glossa, tongue.
Leptosooglossusgen. nov.
Type species by present designation: Nectophryne gardineri Boulenger, 1911.
Species included: Leptosooglossus gardineri (Boulenger, 1911); L. pipilodryas (Gerlach & Willi, 2002).
Diagnosis: Small sized sooglossids (9.3–16.4 mm snout–vent length) with nonprotruding nostrils; reduced metacarpal tubercles, reduced toe pads (pointed on feet only or on digit III only), and a smooth skin except for rows of well-defined tubercles on dorsum; toes with fleshy webs.
Cranium (3): Dorsum of braincase with frontoparietals narrowly separated anteriorly and fused through posterior half of their lengths or not and overlapping otic capsule (exoccipital), but fused or not, extended laterally with medial margins of epiotic eminence and posteriorly at anterior margin of exoccipital; no exostosis producing a pair of spine on frontoparietals. Nasals widely separated medially and not in contact with preorbital process of maxilla. Neopalatine reduced or absent; if absent, presence of anterolateral ossification of sphenethmoid in ventral region of planum antorbitale; two halves of sphenethmoid separeted dorsomedialy and fused ventromedialy on 1/6 of length or not, but forming posterior nasal cavity and olfactory formina. Maxillary arcade incomplete, bearing teeth, composed of maxilla articulated anteriorly with premaxilla but posteriorly not articulated with quadratojugal. Pars facialis of maxilla slender, teething beginning at level of posteroventral margin of sphenethmoid; anterior part of maxilla with pointed process, overlaping premaxilla at inferior part of pars dentalis; jaw articulation at level of operculum or just posterior. Squamosals T-shaped, otic ramus of squamosal forming slender plate, parallel or deflected laterally to medial plan; otic plate absent; zygomatic ramus well developed and deflected laterally; length of otic ramus larger than zygomatic ramus; ventral ramus inclined anteriorly, investing lateral surface of palatoquadrate, close but separated from quadratojugal. Pterygoids triradiate, slender and gracile; anterior ramus of perygoid extending anterolaterally from otic capsule to articulation with maxillae formed by pars palatine; posterior ramus investing medial surface of palatoquadrate; medial ramus terminating vertically on anterior margin of otic capsule and not in contact with prootic and parasphenoid alary process; medial ramus longer or smaller than posterior ramus; medial ramus of pterygoid not expended, articulating vertically with anterodorsal edge of optic capsule. Medioventral part of braincase not closed by bone; parasphenoid T-shaped with alary process, orientated slightly posterolateral; cultriform process of parasphenoid extending anteriorly to level of postero-ventral margins of sphenethmoid; anterior terminus of cultriform process pointed and acuminate; parasphenoid fused or not with sphenethmoid and otic capsule; posterior process of parasphenoid is truncate. Operculum partly ossified. Denticulate serration on dentary absent, but a single toothlike process on each dentary; sesamoid at maxillo-mandibular articulation absent.
Postcranium (4): Eight procoelous vertebrae or vertebrae III to VIII procoelous and vertebra I with posteriorly concave centrum and vertebra II with biconvex centra; non-imbricate presacral vertebrae; presacral I and II not fused; atlantal condylar type I (Lynch, 1971), widely separated; neural arches of vertebra II–VIII without neural spine. Sacro-coccygeal articulation monocondylar; sacral diapophyses further dilated. Anterior end of urostyle bearing a pair of vestigial processes; transverse processes of third and fourth presacral vertebrae wider than the width of sacral diapophyses.
Pseudo-arciferal pectoral girdle: Cleithrum and suprascapula entirely ossified. Coracoid, scapula, procoracoids and clavicle synosteotically united. Omosternum and sternum cartilaginous.
Terminal phalanges simple, sharply pointed, ending in a very small knob, no intercalary cartilage between penultimate and ultimate phalanges of fingers and toes.
Phylogenetic definition: The clade stemming from the most recent common ancestor of L. gardineri (Boulenger, 1911) and L. pipilodryas (Gerlach & Willi, 2002).
Reproductive behaviour: Females of L. gardineri sit on top of eggs laid in hidden terrestrial sites. Fully metamorphosed froglets of 3–4 mm will hatch out of these eggs. No tadpole carrying. Reproduction of L. pipilodryas is not yet known.
Etymology: Composed by the Classical Greek terms lepton, small, fine; soos, safe, sound, unscatted, unwounded; glossa, tongue.
NOTE ADDED IN PROOF
While the current paper was in press, several relevant papers on amphibian phylogeny have been published. Most relevant of these is the paper by Frost et al. (2006), in which new classifications were proposed for some of the taxa included in the current study. Although the classification of the Sooglossidae was not changed, the unique position of the genus Caudiverbera was acknowledged by Frost et al. (2006) by placing it in the family Batrachophrynidae together with Telmatobufo. The removal of Batrachophrynus from this family subsequently required the renaming of the family containing the remaining taxa to Calyptocephalellidae. Despite the inclusion by Frost et al. (2006) of a large molecular as well as morphological dataset in their analysis, the branching order in the basal part of the neobatrachian group remains largely unsupported also in their study.
ACKNOWLEDGEMENTS
We are very grateful to the Seychelles Bureau of Standards for permission (SBS) to work at the Silhouette Island, Republic of Seychelles, the R. Gerlach family (Nature Protection Trust of Seychelles), and L. Chong Seng for assisting during fieldwork. Also, many thanks to M. Géze and M. Dellinger, for facilitating and accommodating the use of the graphic workstation at CEMIM (MNHN, Paris). Many thanks to the Ministry of Environment of Seychelles and the Directory General of Conservation, Mr D. Dogley, for delivering collecting permits used for the specimens mentioned in this paper. Finally, thanks to A. Dubois, J. M. Boistel, M. Boistel, M. Goyon, T. Aubin, A. Mazabraud, P. Cloetens, E. Boller, P. Tafforeau, J.-F. Aubry, J.-Y. Tinevez, and the teams of id19 in ESRF and Laboratoire des Reptiles et Amphibiens (MNHN) for their help. The European Synchrotron Radiation Facility (ESRF) is acknowledged for providing beamtime in the framework of proposal SC-1593 and MD179. This work was financially supported by the Deutsche Forschungsgemeinschaft (grant ME1725/10-1).




