Inhibition of auditory cortical responses to ipsilateral stimuli during dichotic listening: evidence from magnetoencephalography
Abstract
The present magnetoencephalography (MEG) study on auditory evoked magnetic fields (AEFs) was aimed at verifying whether during dichotic listening the contralateral auditory pathway inhibits the ipsilateral one, as suggested by behavioural and patient studies. Ten healthy subjects were given a randomized series of three complex tones (261, 293 and 391 Hz, 500 ms duration), which were delivered monotically and dichotically with different intensities [60, 70 or 80 dBA (audio decibels)]. MEG data were recorded from the right auditory cortex. Results showed that the M100 amplitude over the right auditory cortex increased progressively when tones of increasing intensity were provided at the ipsilateral (right) ear. This effect on M100 was abolished when a concurrent tone of constant intensity was delivered dichotically at the contralateral (left) ear, suggesting that the contralateral pathway inhibited the ipsilateral one. The ipsilateral inhibition was present only when the contralateral tone fundamental frequency was similar to the ipsilateral tone. It was proposed that the occlusion mechanism would be exerted in cortical auditory areas as the dichotic effects were observed at M100 but not M50 component. This is the first evidence showing a neurophysiological inhibition driven by the contralateral auditory pathway over the ipsilateral one during dichotic listening.
Introduction
The dichotic listening (DL) technique allows testing separately left and right auditory cortices in a noninvasive manner (Bryden, 1988). It consists of presenting to the subject two different simultaneous auditory stimuli to either ear. DL has been broadly used in the study of hemispherical asymmetries and the main result that it yielded is that, in general, subjects with left-hemispheric language lateralization are faster and more accurate in reporting verbal items presented at the right ear (Kimura, 1961; Studdert-Kennedy & Shankweiler, 1970). Conversely, they exhibit a left ear advantage for tasks involving the recognition of musical or environmental sounds (Kallman & Corballis, 1975; Boucher & Bryden, 1997; Brancucci & San Martini, 1999, 2003).
Despite the number of studies that utilized DL and the amount of theory concerning this topic, the neurophysiological basis of DL remains unclear. It is known that monaural input to each ear is represented in both cerebral hemispheres, with an advantage for contralateral over ipsilateral pathways (Rosenzweig, 1951; Hall & Goldstein, 1968; Fujiki et al., 2002). Indeed, auditory evoked potentials or magnetic fields (AEFs) have shown that N/M100 response to monaural stimuli starts earlier and is more sustained contralaterally than ipsilaterally (Haaland, 1974; Reite et al., 1981; Pantev et al., 1986; Papanicolaou et al., 1990). However, when different stimuli are presented simultaneously (i.e. dichotically) to the two ears, interactions between auditory pathways make the situation more complex. On the basis of neuropsychological results, the so-called ‘structural theory’ (Kimura, 1967) suggests that during DL the contralateral pathway suppresses the ipsilateral one. Evidence for this comes from studies testing commisurotomized patients with DL tasks (Milner et al., 1968; Sparks & Geschwind, 1968; Springer & Gazzaniga, 1975). These patients have no difficulty reporting words or consonant–vowel syllables presented monaurally to either ear. However, when the same stimuli are presented dichotically, they fail to report items presented to the left ear. The lesion of the posterior part of the corpus callosum (splenium) prevents the input to the left ear from reaching the left hemisphere via the indirect contralateral route that goes initially to the right hemisphere and then crosses the callosal pathways (Pollmann et al., 2002). This indirect contralateral route going through the splenium would permit normal subjects to hear dichotic items in both ears, with a residual small advantage favouring the ear contralateral to the dominant hemisphere. In line with the structural theory, a recent neuroimaging study using positron emission tomography has demonstrated that verbal dichotic stimuli induce stronger cortical responses in the left temporal lobe, whereas nonverbal stimuli induce a stronger activity in the right temporal lobe (Hugdahl et al., 1999).
As an alternative view, the ‘attentional theory’ attributes ear advantages to the priming effect of attending to a particular type of auditory stimulus rather than to a structural advantage (Kinsbourne, 1970). However, growing evidence indicates that both structural and attentional factors play a role in DL (Asbjornsen & Hugdahl, 1995; Hugdahl et al., 2000; Jäncke et al., 2003).
It should be mentioned that in a recent MEG study on M100 (Yvert et al., 1998) using dichotically presented pure tones, no strong inhibition of the ipsilateral pathway during DL was found. This may be due to the fact that pure but not complex tones were used. Indeed, it has been shown that the magnitude of DL effects is related to the number of harmonics of stimuli and that dichotic ear advantage is weaker with pure tones (Sidtis, 1988). Furthermore, the frequency interval between the stimuli was very large, as the smaller one was equal to a musical fifth interval. This may have attenuated the dichotic effects, since evidence from behavioural studies has shown that the dichotic effect is strong when the spectral overlap among right and left ear inputs is high (Sidtis, 1981).
The aim of the present study was to test neurophysiologically the structural theory (Kimura, 1967), i.e. to investigate whether, once two complex tones are presented dichotically, the contralateral auditory pathway inhibits to some extent the ipsilateral one. Amplitudes of the cortical AEFs (M50, M100) were considered in the data analysis. The working hypothesis was that, following presentation of monotic and dichotic tone pairs composed by one tone with increased intensities and one tone with constant intensity, inhibition of the ipsilateral pathway would be reflected by a minor AEF amplitude increment in the dichotic (with the tone of constant intensity being delivered contralaterally) compared to monotic condition. Furthermore, the analysis of cortical responses at M50 and M100 latencies could give some indications about the site (cortical or sub–cortical?) of interaction between ipsilateral and contralateral pathways, as the sources of these AEF components have been localized in the planum temporale of the Heschl's gyrus, containing the primary auditory cortex (Pelizzone et al., 1987; Reite et al., 1988, 1994; Pantev et al., 1990; Yoshiura et al., 1996; Huotilainen et al., 1998).
Materials and methods
Subjects
Ten healthy subjects (4 females and 6 males) aged between 26 and 30 years (mean age, 28.3), volunteered and gave informed written consent to take part in the experiment. They were right-handed (Edinburgh Inventory). None of them had auditory impairments as shown by auditory functional assessment. No differences (± 5 dB) of hearing threshold at 250 and 400 Hz were found between left and right ears.
MEG recordings
The MEG recordings were performed with a 28-channel system, operating inside a magnetically shielded room (Vacuumschmelze GMBH). The system features 16 first-order axial gradiometers (1.8 cm coil diameter and 8 cm baseline), nine peripheral magnetometers (pick-up area, 81 mm2), and three balancing magnetometers for noise cancellation. All pick-up coils were coupled to low noise dc-SQUIDs with an overall sensitivity of about 5–7 fT/(Hz)1/2. The 25 sensors were regularly distributed on a segment of a sphere covering an area of about 180 cm2 (Fig. 1). Brain magnetic fields were recorded from the right temporal cortex, as the right hemisphere is dominant for the nonverbal material used in this study (Sidtis, 1981; Bryden, 1988). A single sensor position centred 1.5 cm anterior to T4 scalp site (10–20 EEG International System) was used: such a position, in fact, is able to map out the two extrema of the brain wave under examination (M100, Pantev et al., 1995; Tecchio et al., 2000). Five small magnetic coils were firmly taped on subject nasion, preauricular points, vertex, and inion and their positions were digitized (Polhemus Isotrak, Colchester, VT, USA). To monitor blinking or eye movements, electrooculogram (EOG) was also collected throughout the experiments. MEG and EOG data were recorded (0.1–60 Hz bandpass; 256 Hz sampling frequency) and processed off-line.
Positions of the magnetic sensors over right temporal cortex. The array is centred 1.5 cm anterior to T4 scalp site (10–20 EEG International System).
Stimuli
Twenty auditory stimuli were built up using eight basic tones (A1, A2, B1, B2, B3, E1, E2, and E3), which were synthesized on a PC Pentium 166 with audio card Sound Blaster AWE 32, by means of the CSound language (Vercoe, 1992) for sound synthesis. Sampling rate was 44100 Hz and amplitude resolution 16 bit. The tones A1 and A2 had a fundamental frequency of 261 Hz (middle C tone) and an intensity of 60 and 80 dBA (audio decibels), respectively. The tones B1, B2 and B3 had a fundamental frequency of 293 Hz (middle D tone) and an intensity of 60, 70 and 80 dBA, respectively. The tones E1, E2 and E3 had a fundamental frequency of 391 Hz (middle G tone) and an intensity of 60, 70 and 80 dBA, respectively (see Table 1, left). Spectral composition and amplitude envelope were the same for all basic tones. Spectrum was composed by eight harmonic components with the following relative amplitudes: 1, 0.8, 0.6, 0.5, 0.4, 0.3, 0.2, and 0.1. The tones lasted 500 ms and had a rise and fall-time of 50 ms.
| The basic complex tones | Combinations used | ||||
|---|---|---|---|---|---|
| Tone | Hz | dBA | Stimulus | Left ear | Right ear |
| A1 | 261 | 60 | 1 | – | B1 |
| A2 | 261 | 80 | 2 | – | B2 |
| B1 | 293 | 60 | 3 | – | B3 |
| B2 | 293 | 70 | 4 | – | A1+B1 |
| B3 | 293 | 80 | 5 | – | A1+B2 |
| E1 | 391 | 60 | 6 | – | A1+B3 |
| E2 | 391 | 70 | 7 | – | A1+E1 |
| E3 | 391 | 80 | 8 9 10 11 12 13 14 15 16 17 18 19 20 | –––––A1 A1 A1 A1 A1 A1 A1 – | A1+E2 A1+E3 A2+B1 A2+B2 A2+B3 B1 B2 B3 E1 E2 E3 –A1 |
- Left, fundamental frequencies and intensities of the eight basic tones used to build up the 20 stimuli. Duration is 500 ms for all tones, including 50 ms rise and 50 ms fall time. Relative amplitudes of the harmonic components are: 1, 0.8, 0.6, 0.5, 0.4, 0.3, 0.2 and 0.1. Right, the eight (basic) tones are delivered in different combinations to the left and/or right ear to constitute the 20 stimuli.
The 20 stimuli were assembled by delivering the eight basic tones to the left or right ear in different combinations (see Table 1, right). The stimuli 1, 2 and 3 were, respectively, the tones B1, B2 and B3 presented to the right (ipsilateral) ear. The stimuli 4, 5 and 6 were built up, respectively, by conveying monotically the tones A1 with B1, A1 with B2 and A1 with B3 to the right auditory channel. The stimuli 7, 8 and 9 were built up, respectively, by conveying monotically the tones A1 with E1, A1 with E2 and A1 with E3 to the right ear. The stimuli 10, 11 and 12 were built up, respectively, by conveying monotically the tones A2 with B1, A2 with B2 and A2 with B3 to the right auditory channel. The stimuli 13, 14 and 15 were built up, respectively, by conveying dichotically the tone A1 to the contralateral left ear with B1 to the ipsilateral right ear, the tone A1 to the left ear with B2 to the right ear, and the tone A1 to the left ear with B3 to the right ipsilateral ear. The stimuli 16, 17 and 18 were built up, respectively, by conveying dichotically the tone A1 to the contralateral left ear with E1 to the ipsilateral right ear, the tone A1 to the left ear with E2 to the right ear, and the tone A1 to the left ear with E3 to the right ipsilateral ear. Finally, stimuli number 19 and 20 consisted of tone A1 presented to the left or right ear, respectively. These stimuli were utilized as a general control as the M100 auditory cortical response is known to be stronger and earlier for contralateral than ipsilateral stimuli (Pantev et al., 1986). Stimuli were delivered over nonmagnetic tube-phones. To ensure that no transients or undesired alterations were present in the stimuli, they were recorded from the tube-phones and re-analysed.
Experimental procedure
Subjects lay on a bed and listened passively to a randomized sequence of the stimuli. No task was required. Each of the 20 stimuli was presented 80 times for a total of 1600 presentations. Inter-stimulus interval varied randomly between 3500 and 4500 ms. The recording session was segmented in 16 blocks of 100 stimuli (1 min interblock pause). The whole experiment lasted about 2 h.
Data analysis
Continuously collected MEG data were segmented in single trials each spanning from −1000 ms to + 4000 ms, the zero time being the onset of the auditory stimulus. MEG single trials were discarded when associated with eye movements, saccades or blinking. The evoked magnetic field was filtered off-line between 1 and 30 Hz. Artifact-free evoked magnetic fields were averaged to obtain AEFs. M100 and M50 AEF components were then considered for the data analysis. The absolute value of the amplitudes of each AEF component's minima and maxima were calculated with respect to a baseline level chosen between −500 ms and zero time. These AEF amplitudes were measured at individual peaks of the M50 and M100 for each channel (i.e. both gradiometers and magnetometers). The M50 or M100 amplitudes at all channels were then averaged for each condition separately (Yabe et al., 2001). Statistical analyses of amplitudes were performed by anova and Duncan post hoc analysis. For sake of brevity, the anova designs were described only in the Results section.
Results
Spatiotemporal distribution of AEFs
Figure 2 illustrates superimposed AEF waveforms (all sensors) induced in the right hemisphere of a representative subject by monotic ipsilateral (right ear) stimulation with tone B having increased intensities (B1 = 60, B2 = 70, and B3 = 80 dBA). Waveforms show the typical polarity reversal of the AEFs recorded over the temporal cortex. There was a clear increment of the amplitude as a function of the stimulus intensity.
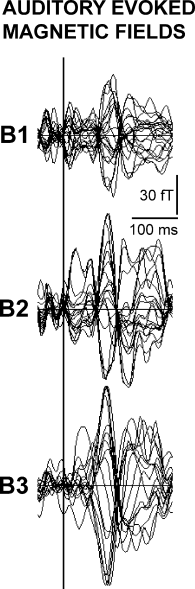
Superimposed AEF waveforms (representative subject) from all sensors induced by monotic ipsilateral stimulation with the tones B1 (60 dBA), B2 (70 dBA) and B3 (80 dBA).
Figure 3 shows AEF waveforms (most responsive sensor) induced in the representative subject by monotic ipsilateral and dichotic stimulations. The AEFs showed an amplitude increment of the M100 (Fig. 3B) when a tone with constant intensity (A1) was presented monotically (right ear) together with the tones B close as fundamental frequencies (B1, B2, B3). In contrast, the M100 amplitude did not increase when the tones B (right ear) were dichotically presented with the tone A1 (left ear) according to the working hypothesis on the ipsilateral ear suppression during DL (Fig. 3C). Spatial topography of this effect is displayed in Fig. 4.
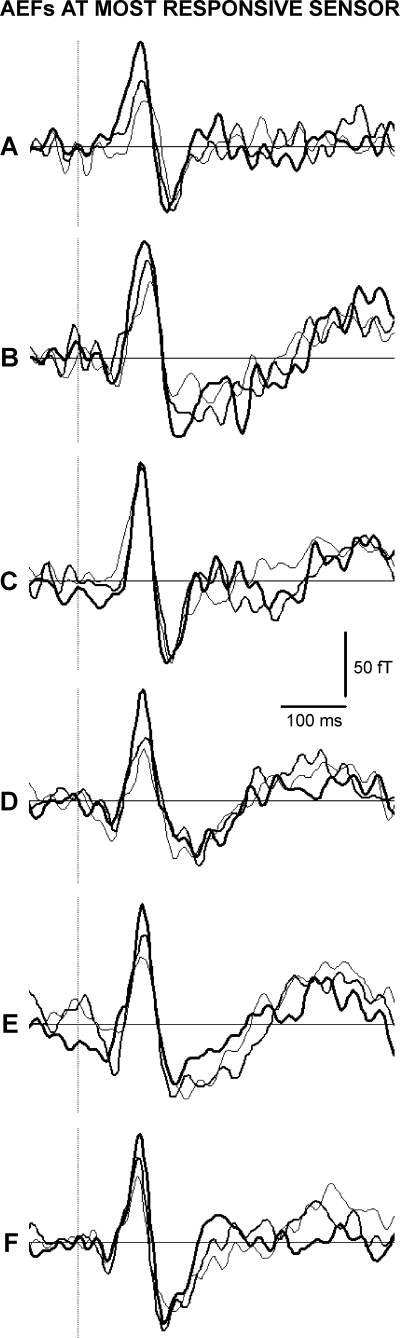
Representative AEF waveforms at the most responsive sensor from one representative subject. Vertical lines indicate stimulus onset. (A) Auditory evoked magnetic fields (AEFs) to tones B1 (60 dBA), thin trace; B2 (70 dBA), medium trace; B3 (80 dBA), thick trace. Tones were delivered monaurally at the right (ipsilateral) ear. (B) AEFs to tone pairs A1 (60 dBA) + B1 (60 dBA), thin trace; A1 + B2 (70 dBA), medium trace; A1 + B3 (80 dBA), thick trace. Tones were delivered monotically at the right (ipsilateral) ear. (C) AEFs to tone pairs A1 (60 dBA) + B1 (60 dBA), thin trace; A1 + B2 (70 dBA), medium trace; A1 + B3 (80 dBA), thick trace. Tones were delivered dichotically with tone A1 at the left (contralateral) ear and tones B at the right (ipsilateral) ear. (D) AEFs to tone pairs A2 (80 dBA) + B1 (60 dBA), thin trace; A2 + B2 (70 dBA), medium trace; A2 + B3 (80 dBA), thick trace. Tones were delivered monotically at the right (ipsilateral) ear. (E) AEFs to tone pairs A1 (60 dBA) + E1 (60 dBA), thin trace; A1 + E2 (70 dBA), medium trace; A1 + E3 (80 dBA), thick trace. Tones were delivered monotically at the right (ipsilateral) ear. (F) AEFs to tone pairs A1 (60 dBA) + E1 (60 dBA), thin trace; A1 + E2 (70 dBA), medium trace; A1 + E3 (80 dBA), thick trace. Tones were delivered dichotically with tone A1 at the left (contralateral) ear and tones E at the right (ipsilateral) ear.
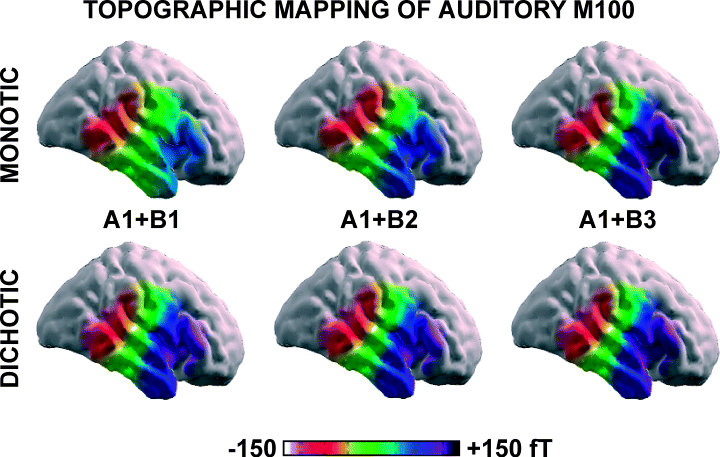
Topographic distribution of M100 amplitude over right temporal cortex. First row shows topographic maps elicited by ipsilateral (right ear) stimulations with tone pairs A1 + B1, A1 + B2 and A1 + B3. Second row shows topographic maps elicited by dichotic stimulations with tone pairs A1 + B1, A1 + B2 and A1 + B3; tone A1 is delivered to the contralateral (left) ear and tones B are delivered to the ipsilateral (right) ear.
This dichotic effect (i.e. no increase of the M100 amplitude with tones A1 + B1, A1 + B2 and A1 + B3 when A1 is presented contralaterally) is substantiated by the fact that the M100 amplitude increased after monotic stimulation including A2 + B1, A2 + B2 and A2 + B3 given at the right ear (Fig. 3D). Furthermore, the M100 amplitude augmented after monotic stimulation with A1 presented together with tones E (E1 = 60, E2 = 70 and E3 = 80 dBA; Fig. 3E), which are distant from A1 as fundamental frequencies. Finally, the M100 amplitude enhanced also after stimulation with tone A1 (left ear) dichotically presented together with the tones E1, E2 and E3 at the right ear (Fig. 3F).
Statistical results
The first step of the statistical data analysis was to evaluate whether the intensity of the tone B (right ear) significantly affected the amplitude of the AEFs (M50, M100) over the ipsilateral temporal cortex. Two one-way anovas for repeated measures had ‘Intensity of the tone B’ as a factor and the AEF amplitude as a dependent variable (M50, M100). Results for the M50 showed that the AEF amplitude significantly increased (F2,9 = 8.66; P = 0.006) with the intensity of the complex tone. The same was true for the M100 amplitude (F2,9 = 11.04; P = 0.001). Figure 5A shows a graph of the mean (± standard error) of the M100 amplitude relative to this effect.
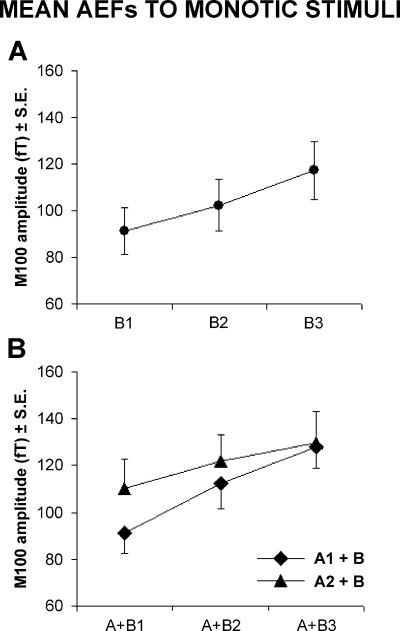
Mean ± standard error (n = 10) of M100 amplitude to tones delivered monotically to the right (ipsilateral) ear. (A) M100 amplitude to tones B1 (60 dBA), B2 (70 dBA) and B3 (80 dBA). (B) M100 amplitude to tone pairs A1 (60 dBA) + B1 (60 dBA), A1 + B2 (70 dBA), A1 + B3 (80 dBA) and A2 (80 dBA) + B1, A2 + B2, A1 + B3.
Further steps of the statistical evaluation were addressed by two three-way anovas for repeated measures using ‘Frequency distance’ (A1 + B, A1 + E), ‘Type of stimulation’ (monotic, dichotic), and ‘Intensity of ipsilateral tone’ (B1 or E1, B2 or E2, B3 or E3) as factors. The dependent variable was the AEF amplitude (M50 for the first anova, M100 for the second one). The results for the M50 showed only a main effect of ‘Intensity of ipsilateral tone’ (F2,9 = 5.51; P = 0.014) indicating a global increase of the M50 due to the increment of the stimulus intensity (i.e. regardless the kind of stimuli). In contrast, the results for the M100 showed a significant triple interaction (F2,18 = 3.70; P = 0.045). Figure 6 shows a plot of the mean (± standard error) of the M100 amplitude relative to this statistical effect. Post hoc comparisons showed a significant increase of the M100 amplitude from the tone A1 + B1 to the tone A1 + B3 in the monotic (P < 0.01) but not dichotic condition, pointing to an inhibition (in the dichotic condition) of the ipsilateral pathway conveying tones B. On the contrary, post hoc comparisons between tones A1 + E1 and A1 + E3 were significant for both monotic (P < 0.01) and dichotic (P < 0.02) conditions, indicating that the inhibitory effect was not present when the tones have distant fundamental frequencies. Of note, there was a significant difference (P < 0.03) between the AEF amplitude of A1 + B1 vs. A1 + E1 in the dichotic condition.
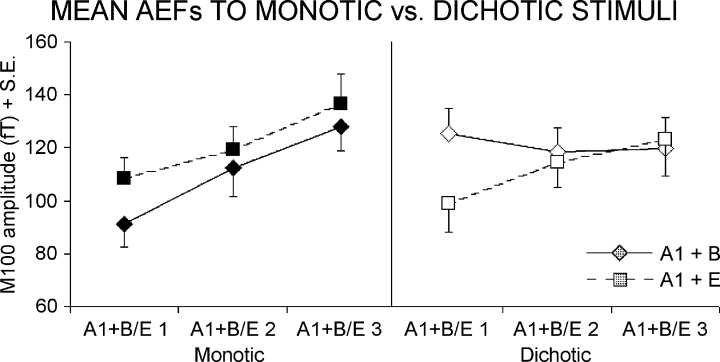
Mean ± standard error (n = 10) of M100 amplitude for tone pairs A1 (60 dBA) + B1 (60 dBA), A1 + B2 (70 dBA), A1 + B3 (80 dBA) and A1 + E1 (60 dBA), A1 + E2 (70 dBA), A1 + E3 (80 dBA). Tones were delivered monotically (left panel) at the right ipsilateral ear and dichotically (right panel) with tone A1 at the left contralateral ear and tones B or E at the right ipsilateral ear.
It can be argued that the lack of the M100 increment observed in the dichotic condition with tone A1 (contralateral left ear) and tones B1, B2 and B3 (ipsilateral right ear) merely depended on the preponderance of the contralateral pathway rather than to an actual inhibition of the ipsilateral pathway. To control for this alternative explanation, we simulated the preponderance of the contralateral pathway in the ipsilateral right channel. We compared monotic ipsilateral tone pairs A1 + B1, A1 + B3 and A1 + B3 with a monotic ipsilateral condition in which the intensity of the tone A was 20 dBA stronger (A2 + B1, A2 + B3 and A2 + B3). Statistical evaluation was performed by an anova analysis for repeated measures with ‘Intensity of tone A’ and ‘Intensity of tone B’ as factors and the M100 amplitude as a dependent variable. The results showed only a main effect of ‘Intensity of tone B’ (F2,9 = 10.39, P = 0 < 001) indicating an increase of the M100 amplitude due to the intensity increment of the tone B (i.e. regardless the intensity of the tone A). The absence of significant interaction points to the fact that the lack of M100 increment observed in the dichotic condition with tone A1 contralateral and tones B ipsilateral was an effect of the inhibition of the ipsilateral pathway.
Control experiment
We compared AEF amplitudes and latencies produced by contralateral and ipsilateral stimulation with tone A1, to have a general control of our experiment. Existing literature shows that contralateral auditory stimulation produces stronger M50 and M100 amplitudes and shorter latencies than ipsilateral stimulation (Pantev et al., 1986). Accordingly, the present data showed a significant stronger M50 amplitude (F1,9 = 3.21; P < 0.04) and shorter latency (F1,9 = 13.85; P < 0.01) for the contra- compared to ipsilateral stimulation with tone A1. Regarding M100, contralateral stimulation produced stronger amplitude (F1,9 = 11.48; P < 0.01) and shorter latency (F1,9 = 16.25; P < 0.01). Table 2 shows M50 and M100 latencies of other stimuli.
| Stimulus | M50 latency (ms) | M100 latency (ms) |
|---|---|---|
| B1 (monotic ipsilateral) | 64.87 ± 2.39 | 115.91 ± 3.08 |
| B2 (monotic ipsilateral) | 64.29 ± 2.64 | 114.39 ± 3.90 |
| B3 (monotic ipsilateral) | 59.14 ± 3.14 | 110.53 ± 3.89 |
| A1+B1 (monotic ipsilateral) | 63.53 ± 2.59 | 118.17 ± 9.85 |
| A1+B2 (monotic ipsilateral) | 61.01 ± 3.22 | 118.56 ± 8.18 |
| A1+B3 (monotic ipsilateral) | 60.77 ± 1.19 | 111.28 ± 8.78 |
| A1+B1 (dichotic) | 58.76 ± 2.16 | 109.36 ± 10.04 |
| A1+B2 (dichotic) | 55.23 ± 4.13 | 107.43 ± 9.43 |
| A1+B3 (dichotic) | 53.15 ± 2.07 | 106.37 ± 9.23 |
| A1+E1 (monotic ipsilateral) | 64.88 ± 3.31 | 116.17 ± 5.29 |
| A1+E2 (monotic ipsilateral) | 62.21 ± 3.33 | 114.17 ± 5.04 |
| A1+E3 (monotic ipsilateral) | 62.11 ± 4.02 | 112.18 ± 5.93 |
| A1+E1 (dichotic) | 55.12 ± 2.45 | 105.17 ± 6.91 |
| A1+E2 (dichotic) | 54.43 ± 3.39 | 105.02 ± 7.19 |
| A1+E3 (dichotic) | 55.18 ± 5.21 | 103.87 ± 6.39 |
- Data are presented as means ± SEM. Monotic ipsilateral stimuli were delivered at the right ear. In the dichotic stimuli tone A1 was always delivered at the left (contralateral) ear.
Discussion
Ipsilateral inhibition
The aim of this study was to test whether the amplitude of the M50 and M100 AEF components induced by nonverbal dichotic stimuli reveals an inhibition of the ipsilateral right auditory pathway. AEFs were recorded from the right auditory cortex that is supposed to be strictly involved in the processing of nonverbal material. The study was carried out in a sample of 6 males and 4 females and no effects related to sex were found. Results showed that the M100 amplitude over the right auditory cortex increased progressively when tones of increasing intensity were provided at the ipsilateral (right) ear side. This effect on M100 was abolished when a concurrent tone of constant intensity was added dichotically at the contralateral (left) ear. That contralateral tone was effective on the inhibition of the M100 amplitude increase only when it had a fundamental frequency similar to the one of the ipsilateral tone. These results point to an inhibition driven by the contralateral auditory pathway over the ipsilateral one during DL.
The interaction between contralateral and ipsilateral pathways generating this inhibition would occur at the level of auditory cortex, as the above effects were observed at M100 but not at M50 component. The M50 component is the earlier auditory response whose source has been localized at cortical level. It has been localized in the planum temporale of the Heschl's gyrus, containing the primary auditory cortex (Pelizzone et al., 1987; Reite et al., 1988; Mäkeläet al., 1994; Yoshiura et al., 1996; Huotilainen et al., 1998). Thus, the fact that at the M50 AEF component no interaction effects were found, suggests that the inhibition of the ipsilateral pathway does not occur at subcortical nor at earliest cortical levels. Indeed, an inhibition effect at subcortical level would have reduced the amplitude increment of all cortical AEF components, including both M50 and M100. As an alternative explanation, it could be argued that the M50, which is generated in the earliest stages of cortical auditory input processing, is more strongly influenced by specific thalamic inputs than the M100. This could explain why M100 is more affected by cortical inhibitory networks during DL. On the whole, it can be speculated that the dichotic inhibition is associated with the information processing of primary and secondary auditory cortex, which is mainly reflected by M100 (Scherg & Von Cramon, 1985; Pantev et al., 1990; Reite et al., 1994; Yoshiura et al., 1996; Huotilainen et al., 1998).
An intriguing result of the present study was that the AEF amplitude of A1 + B1 was stronger than A1 + E1 in the dichotic condition. This result only apparently contradicts the above-mentioned ‘structural theory’ (Kimura, 1967). Indeed, this theory is clearly substantiated by the present findings that there is no increment of the M100 amplitude in the dichotic condition for the increasing intensity of the ipsilateral stimuli. As a tentative explanation of the apparently ‘odd’ result, it can be speculated that it reflects a sort of ‘enhanced’ representation of the contralateral tone coupled with an ipsilateral tone having a near frequency. The enhanced representation of the contralateral tone would be independent from the intensity of the ipsilateral stimulus. Further behavioural experiments are needed to test this hypothesis.
The present findings validate the structural theory proposed by Kimura (1967), which explained dichotic ear advantage by reference to the anatomy and physiology of the auditory system. This model emphasizes the notion that the contralateral auditory pathways are dominant, more numerous and more rapidly conducting than the ipsilateral ones. Kimura further suggested that such differences between contralateral and ipsilateral pathways were exaggerated by an occlusion mechanism during dichotic stimulus application, whereby input from the contralateral ear would block the ipsilateral pathways and prevent information from reaching the auditory cortex via direct ipsilateral route. It should be mentioned that the present results cannot exclude the possibility that the contralateral pathway, in addition to the ipsilateral one, undergoes an inhibition during DL. Indeed, a partial inhibition of the contralateral pathway has been demonstrated by recent MEG studies on binaural hearing of simple tones (Fujiki et al., 2002; Kaneko et al., 2003).
Frequency-dependence of the inhibition
Regarding the role of the interval between tone's fundamental frequencies in the occlusion mechanism, the present study gives a physiological basis to previous reports using tonal (Sidtis, 1981) or verbal (Springer et al., 1978) material. Sidtis (1981) demonstrated that a nearly threefold difference in the magnitude of the laterality measure could be obtained by delivering dichotically tones with similar vs. different fundamental frequencies. The fifth interval (i.e. the interval between tones A and E in the present study) yielded minimal laterality effects, whereas intervals of a second (i.e. the interval between tones A and B in the present study), a minor third, or an octave as a special case, yielded maximal laterality effects. Springer et al. (1978) have shown that while report of consonant–vowel syllables presented to the left ear during dichotic testing was at chance, report of left ear digits under the same conditions was greater than 80% in four out of five commisurotomized patients tested. As the acoustic overlap between consonant–vowel syllables is greater than the overlap between digits, they concluded that the availability of information from the ipsilateral auditory pathway is a function of the spectral acoustic overlap between competing dichotic stimuli. Similar results have been obtained with subjects who have undergone temporal lobectomy and hemispherectomy (Berlin et al., 1973). Thus, the present study and these previous reports indicate the existence of a strong relationship between the degree of spectral competition and the magnitude of the laterality effect. As the spectral overlap increases (i.e. frequency separation decreases), stimulus competition increases and laterality effects are maximized by favouring the contralateral pathways in each hemisphere. This suggests that the effects of stimulus competition have to be adequately considered in the interpretation of laterality effects, as not only different types of auditory materials (i.e. verbal vs. tonal) but also small changes in the degree of competition between ipsilateral and contralateral information of the same type might significantly affect the magnitude of perceptual asymmetry.
The evaluation of the neural interactions underlying DL at the cellular level would be an interesting challenge. Forthcoming studies using stereo-electroencephalography in epileptic patients (presurgical functional monitoring) or single unit recordings in animals, may disclose the respective role of neuronal populations in primary auditory cortex having ipsilateral, contralateral and bilateral receptive fields. Relevant literature (reviewed by Bear et al., 2001) shows that, beyond the main tonotopic organizational principle of the auditory cortex, there is a second organizational principle based on ear preference. In cat primary auditory cortex, there are alternating patches of neurons, which respond preferentially to binaural stimulation (being inhibited by monaural stimulation) or monaural stimulation (being inhibited by binaural stimulation). These neuronal populations may be a suitable substrate for the DL effects reported in the present study.
Conclusions
The results of the present study support the hypothesis of an inhibition driven by the contralateral auditory pathway over the ipsilateral one during DL, as revealed by M100 auditory cortical responses. This inhibition seems to be dependent on the frequency interval between ipsilateral and contralateral stimuli existing only for stimuli having similar fundamental frequencies. The ipsilateral–contralateral interaction would be exerted at cortical level since the above effects were observed at the M100 but not M50 component. The present conclusions should be considered as a first step towards the understanding of pathway interactions during DL. Future research should test the generalization of the present findings with respect to the selection of sounds (i.e. language sounds, vocalizations, noise), possible functional asymmetry of left and right pathways, and whether they extend to the left hemisphere.
Acknowledgements
We thank Matilde Ercolani and Dr Febo Cincotti and Dr Pietro San Martini for their helpful technical assist and Professor Fabrizio Eusebi for his continuous support.