Sublittoral abundance and food consumption of Baltic gobies
Abstract
Two small demersal fishes, the sand goby Pomatoschistus minutus and the common goby Pomatoschistus microps, were quantified on soft bottoms at 20–40 m depth in the Baltic Sea, using a camera placed above the bottom. The largest numbers of gobies were seen following the settlement of young in late summer and autumn. Most recorded fishes were sand gobies. An annual average of 4·7 individuals m−2(0·24 g dry mass m−2) was recorded in 1983–1985 and 2·5 individuals m−2(0·13 g m−2) in 1997–1998. Using these densities, the annual goby food consumption was estimated to 100 kJ m−2 in 1983–1985 and 50 kJ m−2 in 1997–1998, corresponding to most of the annual macrobenthos production available to the gobies. The resulting goby production, assumed equal to 25% of the food consumed, must have been an important food source for the larger fishes occasionally recorded in the photographs.
Introduction
The ecology of small, demersal fish species that are not of direct commercial interest is often poorly known, even though they may be important components of the food web. Such species are often missed by the gears used to quantify commercially important fishes. Visual observation, either direct or from remote recordings, can be the only means of obtaining abundance data.
The present study covers two such species, the sand goby Pomatoschistus minutus(Pallas) and the common goby Pomatoschistus microps(Krøyer), in the Baltic Sea. Both species live on soft bottoms, are potentially important predators on benthos, and occur over large geographical areas, with a wide range of abiotic and biotic conditions (Bouchereau & Guelorget, 1998). Their lifespan is 1 to 3 years, but varies among cohorts and areas (Fonds, 1973; Miller, 1975; Hesthagen, 1977; Moreira et al., 1991; Arruda et al., 1993). In the Baltic Sea, the sand goby seldom exceeds 60 mm in total length (LT)(Schmidt-Moser & Westphal, 1981; Curry-Lindahl, 1985), while the common goby reaches 30–35 mm LT(Schmidt-Moser & Westphal, 1981).
The temperature preference of the sand goby changes with season (Hesthagen, 1979), which may result in migrations between habitats. According to Jones & Miller (1966), migrations occur in areas where the sea temperature normally falls to <5° C, but not where the minimum stays >7° C. In the Baltic, the two gobies are known to move deeper in winter and migrate to shallow waters for spawning in spring (Morawski, 1978; Sundell, 1994). Both goby species arrive to spawn during April-June in shallow waters, where their peak in density is reached in the autumn, followed by a decrease due to mortality and migration to deeper waters (Wahlberg, 1969; Miller, 1975; Nellbring, 1985; Mattila, 1992).
The biology of sand and common gobies in shallow coastal waters of the Baltic Sea is well documented (Wahlberg, 1969; Aneer & Nellbring, 1977; Thorman & Wiederholm, 1983, 1984; Jansson et al., 1985; Nellbring, 1985, 1986, 1988, 1993; Wiederholm, 1986; Aarnio et al., 1991), but little is known of their ecology at depths below 15–20 m, the depth of the summer thermocline. A camera mounted over the bottom was used to quantify the size and abundance of gobies below 20 m depth. Using bioenergetic assumptions based on literature data, their food consumption on these bottoms was then estimated and their possible predatory effect on benthic invertebrates evaluated. Even though gobies are common worldwide and the Baltic is one of the most studied seas, this aspect of goby ecology has not been investigated before.
Methods
Goby densities on deep bottoms, below the summer thermocline, were estimated based on two series of photographs each covering two stations. The older data (1983–1985) were collected at c. 21 and 40 m depth, while the more recent data (1997–1998) were from 21 m (same station) and 31 m (Fig. 1). The stations are referred to and analysed as two different depths: the 21 m station being below but relatively close to the thermocline, and the ‘deeper station’ at 31–40 m. The bottom at the 21 m station was sandy, with stones covered with algae and blue mussels Mytilus sp. The deeper stations had soft and muddy bottoms. The deeper station was moved in 1997 because of intense boat traffic and poor near-bottom visibility. Photographs from several additional stations below 10 m, taken in 1982–1983 were also available (von Euler, 1983; referred to as supplementary stations, Fig. 1).
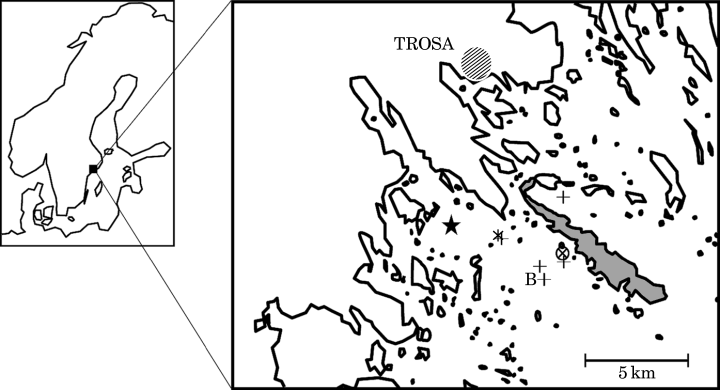
Study area and stations [1997–1998, 31 m (★); 1983–1985, 40 m (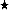 ); 1997–1998 and 1983–1985, 21 m (⊗)]. Supplementary stations (+) were sampled only occasionally in 1982–1983 (von Euler, 1983). The field station is situated on the island of Askö(
); 1997–1998 and 1983–1985, 21 m (⊗)]. Supplementary stations (+) were sampled only occasionally in 1982–1983 (von Euler, 1983). The field station is situated on the island of Askö( ). B, Askö-B1 (temperature data collected).
). B, Askö-B1 (temperature data collected).
Photographic Recordings in the Field
A Minolta X-700 camera with a Tamron lens (f = 24 mm, I = 2·5) was mounted in a Dyfo Sub 35 proslr underwater housing equipped with two Dyfo SL32 flashes. The camera was equipped with a 2+ correction lens that minimized optical distortion. A Minolta Auto-winder G and a multi-function back, programmed to take pictures at intervals, allowed 36 exposures in 24–30 h. Sometimes fewer pictures were obtained, due to weather and boat availability. Black-and-white film was used in 1983, but colour was used thereafter to enhance recognition of the animals.
The camera equipment was attached to a rack, and rested on the bottom with a square frame, with one open side. The rack was lowered to the bottom by a line, attached to a surface buoy. A float on the line kept it away from the rack, and an anchor in the middle of the line prevented the rack being moved by waves acting on the surface buoy. The recorded area was 0·33 m2 in 1983–1985 and 0·75 m2 in 1997–1998.
Analysis of Photographs
Each recorded fish was counted and identified to the lowest possible taxon. The LT was measured as accurately as possible, given the difficulties sometimes caused by suspended sediment limiting visibility. Measurements deemed overly uncertain were omitted from analysis. Fish dry mass (MD; g) was estimated from the measured LT(cm), using the empirical relationship MD = 4·34 × 10−4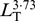 (S.Z. Ehrenberg, unpubl. data). From 1984 onwards (when colour film was used), a distinction was made between: (1) definite gobies, (2) probable gobies, (3) probable non-gobies and (4) other species. Some definite gobies could be further classified as sand gobies by the closer setting of the eyes and a more elongated body and head than in the common goby. Estimates of density, biomass and consumption are based on the sum of definite and probable gobies.
(S.Z. Ehrenberg, unpubl. data). From 1984 onwards (when colour film was used), a distinction was made between: (1) definite gobies, (2) probable gobies, (3) probable non-gobies and (4) other species. Some definite gobies could be further classified as sand gobies by the closer setting of the eyes and a more elongated body and head than in the common goby. Estimates of density, biomass and consumption are based on the sum of definite and probable gobies.
Goby Food Consumption
Food consumption was calculated from respiration and growth. Respiration was calculated as routine respiration (Rr) plus specific dynamic action (SDA), the energy cost of processing consumed food. The Rr(ml O2 h−1) was calculated for individual fish according to Healey (1972) for P. minutus:
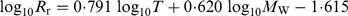 (1)
(1)where T is temperature in ° C and MW is wet mass in g (the proportion of MD to MW is 0·2; Healey, 1972). Temperature data were from a monitoring station in the study area (station B1, Fig. 1; U. Larsson, unpubl. data). The temperature was typically close to 0–2·5° C during January to April and 5–12° C during June to October. Oxygen consumed was converted to energy assuming an oxycalorific constant of 21 kJ ml−1 O2(Winberg, 1960; Healey, 1972). It was assumed that SDA required 16% of the total consumption (well within the range reported by Hanson et al., 1997), and that 20% of consumed food was egested and excreted (Winberg, 1960; Healey, 1972; Hanson et al., 1997). The energy budget then became:
 (2)
(2)where C is consumption and G is growth. This relationship can be further rewritten by incorporating the growth efficiency z, the proportion of consumed food that is transferred into growth:
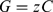 (3)
(3)Combining equations 2 and 3 and rearranging:
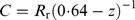 (4)
(4)Equation 1 and different z values were used to predict the growth of 193 gobies that were measured on 22 July 1997, and their day-by-day growth predicted until 9 December (the midpoint of the data collection period in this month). The resulting size distribution was compared to that of 230 fish measured in December 1997. When z was set to 0·25, the predicted and observed size distribution agreed well. When recalculating goby mass to energy units, the fish energy density was set to 20 kJ g−1 dry mass (Healey, 1972). From equation 4, the daily energy consumption then becomes: C ≈ 2·6Rr.
The total annual consumption by gobies was then calculated by multiplying estimates of individual consumption and abundance, integrating over the months. Missing LT or abundance data excluded two periods (July 1983–1985 and June 1997–1998) from the analysis, but as the benthic density of gobies was very low in these months the excluded consumption was negligible.
Results
Almost all fishes seen on the pictures were gobies, with sand gobies constituting 71 and 16% of the fish classified as ‘definite gobies’ in 1984–1985 and 1997–1998 respectively. The lower fraction in the latter period is explained by a lower resolution in the pictures due to the larger distance between the camera and the bottom. Besides gobies, a few individuals of other species were identified: flounder Platichthys flesus (L.) or turbot Psetta maxima(L.), eelpout Zoarces viviparus(L.), fourhorn sculpin Triglopsis quadricornis(L.), perch Perca fluviatilis L., unidentified percids, a small cod Gadus morhua L. and a presumed eel Anguilla anguilla(L.). All these species are known as potential predators on gobies (Wahlberg, 1969; Aneer, 1975).
As there were no significant differences in goby density between day and night (Sign tests, P > 0·05), all available data were used in the analyses. Furthermore, as no significant density differences were found between depths (Kruskal–Wallis ANOVA by ranks, P > 0·05); data from different stations were merged. The goby density varied with season and was higher in autumn than in spring [August to December v. January to March; Fig. 2(a)]. The autumn goby density was higher in the 1980s than in 1997, with estimated annual mean densities of 4·7 and 2·5 m−2 respectively.
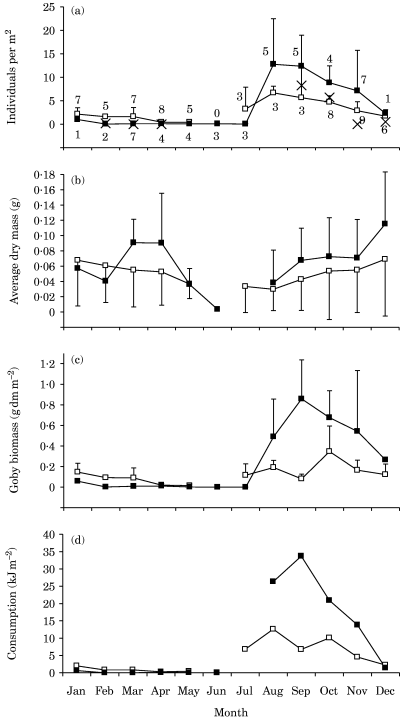
Monthly estimates (means + or − s.d.) for benthic gobies below 20 m in 1983–1985 (▪) and 1997–1998 (□). Numbers at data points show the number of films used to calculate each point. (a) Density, estimated from the average numbers of individuals m−2 for each film, with data from different depths and years merged. Data from supplementary stations studied by von Euler (1983) in 1982–1983 are indicated ( ). Goby density was significantly higher in autumn than in spring (August to December v. January to March, Kruskal–Wallis ANOVA by ranks: P < 0·001 in 1983–1985 and P < 0·05 in 1997–1998). Goby density at the 21 m station was significantly higher in autumn in the 1980s than in 1997 (Kruskal–Wallis ANOVA by ranks, P < 0·001). (b) Biomass of individual gobies calculated from total length data. During both the 1980s and 1990s, the total length differed significantly among months (Kruskal–Wallis ANOVA by ranks, P < 0·001). (c) Goby dry biomass, combining data from all stations. (d) Estimated food consumption, assuming a growth efficiency of 0·25. Changing the assumed growth efficiency by ±0·05 altered the estimated consumption by +10 or −12%.
). Goby density was significantly higher in autumn than in spring (August to December v. January to March, Kruskal–Wallis ANOVA by ranks: P < 0·001 in 1983–1985 and P < 0·05 in 1997–1998). Goby density at the 21 m station was significantly higher in autumn in the 1980s than in 1997 (Kruskal–Wallis ANOVA by ranks, P < 0·001). (b) Biomass of individual gobies calculated from total length data. During both the 1980s and 1990s, the total length differed significantly among months (Kruskal–Wallis ANOVA by ranks, P < 0·001). (c) Goby dry biomass, combining data from all stations. (d) Estimated food consumption, assuming a growth efficiency of 0·25. Changing the assumed growth efficiency by ±0·05 altered the estimated consumption by +10 or −12%.
The size of the gobies varied seasonally, with smaller individuals in summer than during the rest of the year [Fig. 2(b)]. They were also generally larger at the deeper stations, both when comparing different months and when comparing annual averages (Table I). The estimated annual average dry biomass of gobies was 0·24 g m−2 in 1983–1985, and 0·13 g m−2 in 1997–1998, and showed substantial variation within the year [Fig. 2(c)]. Food consumption estimates correlated to the fish biomass, with marked peaks in late summer to autumn. The calculated annual consumption was c. 100 kJ m−2 in 1983–1985 and 50 kJ m−2 in 1997–1998 [Fig. 2(d)], with the resulting goby production assumed to be 25% of the consumption.
| Mean ± s.d. LT 1983–1985 (n) | Mean ± s.d. LT 1997–1998 (n) | |||||
|---|---|---|---|---|---|---|
| Month | 21 m | 40 m | Total | 21 m | 31 m | Total |
| January | 3·7 ± 0·2 (6) | – | 3·7 ± 0·2 (6) | 3·4 ± 0·7 (171)*** | 3·9 ± 0·8 (149)*** | 3·6 ± 0·8 (320) |
| February | – | 3·4 (1) | 3·4 (1) | 3·4 ± 0·7 (105)** | 3·8 ± 0·6 (61)** | 3·6 ± 0·7 (166) |
| March | 4·1 ± 0·4 (8) | – | 4·1 ± 0·4 (8) | 3·4 ± 0·8 (253)** | 3·1 ± 0·8 (42)** | 3·4 ± 0·8 (295) |
| April | 4·1 ± 0·9 (7) | 3·8 ± 0·6 (3) | 4·0 ± 0·8 (10) | 3·4 ± 0·7 (51) | – | 3·4 ± 0·7 (51) |
| May | 3·2 ± 0·6 (3) | – | 3·2 ± 0·6 (3) | 3·2 ± 0·4 (47) | 3·0 (1) | 3·2 ± 0·4 (48) |
| June | – | 1·8 (1) | 1·8 (1) | – | – | – |
| July | – | – | – | 2·9 ± 0·8 (213) | – | 2·9 ± 0·8 (213) |
| August | 3·1 ± 0·7 (467) | 3·5 ± 0·4 (7) | 3·1 ± 0·7 (474) | 2·9 ± 0·6 (214) | – | 2·9 ± 0·6 (214) |
| September | 3·6 ± 0·5 (335)*** | 4·0 ± 0·6 (169)*** | 3·8 ± 0·6 (504) | 3·2 ± 0·7 (31) | – | 3·2 ± 0·7 (31) |
| October | 3·8 ± 0·6 (213) | 3·9 ± 0·7 (39) | 3·8 ± 0·6 (252) | 3·2 ± 0·7 (511)*** | 4·3 ± 0·8 (80)*** | 3·4 ± 0·8 (591) |
| November | 3·8 ± 0·6 (336) | 4·1 ± 0·6 (18) | 3·8 ± 0·6 (354) | 3·2 ± 0·6 (454)*** | 4·0 ± 0·9 (162)*** | 3·4 ± 0·7 (616) |
| December | 4·3 ± 0·7 (24) | – | 4·3 ± 0·7 (24) | 3·4 ± 0·9 (121) | 3·8 ± 0·8 (109) | 3·6 ± 0·9 (230) |
| All | 3·5 ± 0·7 (1399)*** | 3·9 ± 0·6 (238)*** | 3·6 ± 0·7 (1637) | 3·2 ± 0·7 (2171)*** | 3·9 ± 0·9 (604)*** | 3·4 ± 0·8 (2775) |
- ** , P < 0·01; ***, P < 0·001; –, no data.
Discussion
It is not known whether fishes were repelled or attracted to the camera rack, but fewer gobies than average were often seen for the first few hours after positioning the rack on the bottom. The results may also be influenced by gobies swimming in the water column at night (Rumohr, 1979; Gibson & Hesthagen, 1981; Maes et al., 1999; S.Z. Ehrenberg & G. Ejdung, unpubl. data) and hiding in the sediment during day (laboratory observations; S.Z. Ehrenberg & G. Ejdung, unpubl. data). Although no significant differences between day and night recordings were detected, these factors should result in underestimates of abundance, biomass and food consumption.
Turbidity sometimes made it difficult to detect fishes in the photographs. Resolution was reduced when the distance from the camera to the bottom was increased in 1997–1998 which may have resulted in some fishes being overlooked. Since more small fishes (<3 cm) were recorded in 1997–1998, a lower rate of detection is not, however, a probable explanation for the lower density in the latter period. Consequently, the difference in goby density between the two series is probably due to true inter-annual variation. Large variation among years in goby density has also been reported from the Gulf of Riga in the Baltic Sea (Ojaveer et al., 1999) and the coast of England (Rogers & Millner, 1996).
The food consumption estimates presented in this study are based mainly on bioenergetic data from sand gobies, which may have biased the results. Sand gobies, however, constituted at least 70% of identified gobies and may constitute as much as 95% of the goby population in the study area (Jansson et al., 1985). The bias caused by the presence of some common gobies is thus likely to be less important than errors caused by biases in the bioenergetics assumptions, such as growth rates and energy densities of the gobies and their prey. In spite of these uncertainties, the consumption estimates presented here are of considerable interest, as the first of their kind from sub-thermocline areas in the Baltic Sea.
The goby biomass estimated in this paper is similar but lower than the 0·3 g m−2 dry biomass on shallow bottoms (<1 m) in the study area in 1976–1978 reported by Nellbring (1985). Given the hypsography of the area (Ankar & Elmgren 1976), and the density estimates presented here for January to March, migration from deeper bottoms to shallow areas (0–10 m) during spawning time should result in four to five gobies per m2. This is consistent with the two to five individuals per m2 found on shallow bottoms by Nellbring (1985).
The length of gobies differed among months and depths. Some variation may be caused by a variable proportion of the larger sand goby and the smaller common goby, and possibly by the presence of a second year class in the sand goby. Generally, larger fishes were found deeper, except in spring (March 1998 and April 1984; Table I), suggesting that larger fishes are the first to migrate to shallow areas for spawning. This is consistent with the earlier arrival in shallow water of the larger sand goby, as observed by Nellbring (1985). Seasonal migrations have been reported for both sand and common gobies from other geographical areas (Fonds, 1973; Hesthagen, 1977; Pihl & Rosenberg, 1982; Claridge et al., 1985).
Few gobies were detected in early summer, possibly because the adults were in shallow waters (Nellbring, 1985). While abundance was still low by the end of July, juvenile gobies had started to settle on sub-thermocline bottoms, as shown by their small average size (Table I). Similar massive settlement of juveniles has been reported from the North Sea by Miller (1975) for the common goby, and by Fonds (1973) for the sand goby. From settlement of the young and throughout autumn, the gobies grew, as shown by individual increase of biomass in Fig. 2(b).
The estimates of goby food consumption (c. 100 kJ m−2 year−1 in the 1980s and 50 kJ m−2 year−1 in the 1990s) are close to those derived for sand gobies in shallow waters in Lübeck Bight, western Baltic (84 kJ m−2 between May and November, recalculated from Zander & Hagemann, 1987, using 20 kJ g−1 dry mass of prey). Healey (1972) estimated the consumption of an average sand goby to be 15 kJ from July to November in the Ythan Estuary, Scotland. Taking into account that those fish were larger, the goby consumption estimates presented here, 9 kJ per fish from July to November, are reasonable.
Amphipods and other crustaceans are among the preferred prey of sand and common gobies, along with other benthic fauna such as insect larvae and meiofauna (Baltic Sea: Wahlberg, 1969; Morawski, 1978; Thorman & Wiederholm, 1984; Zander, 1990; Aarnio et al., 1991; Antholtz et al., 1991; other areas: Hesthagen, 1971; Edlund & Magnhagen, 1981; Evans, 1983; Thorman, 1983; Claridge et al., 1985; Pihl, 1985; del Norte-Campos & Temming, 1994). For the present study area, Ankar & Elmgren (1976) estimated the annual benthic production at 9–50 m depth to be 337 kJ m−2. For the macrofauna alone, the value was 225 kJ m−2, of which c. 120 kJ m−2 was bivalves, which are probably seldom eaten by gobies. The goby consumption of 50–100 kJ m−2 thus constitutes 15–30% of the total benthos production, 25–50% of the macrofauna production and reaches 50–100% of the macrofaunal non-bivalve production. Although approximate, these calculations show that gobies are potentially important and hitherto underestimated predators on soft bottom benthos in the Baltic Sea. The benthos production data used were from the 1970s, and the biomass and density of benthic macrofauna other than bivalves have since decreased in the study area (Cederwall et al., 1998; Cederwall, 1999). The predatory impact by the gobies is therefore likely to have been high also in 1997–1998.
The substantial benthos predation by gobies on 20–40 m deep bottom also imply that these fishes are important links for transferring benthic invertebrate production to higher trophic levels, e.g. piscivorous fishes. The occurrence of such species in the photographs and the presence of gobies in the stomach content of e.g. Baltic G. morhua(Kondratovics, 1997) shows that this food web connection is real. Similar results were reported by Kritzer (2002) from a very different environment, a coral reef, where small gobies vary greatly seasonally, yet are a significant trophic link between benthos and higher trophic levels. The present study also shows that while reproduction of gobies takes place in shallow water, much of their benthic growth and production in this area (and probably in the Baltic Sea in general) takes place on bottoms below 20 m depth, during late summer and autumn.
Special thanks to J.-E. Hägerroth for building the camera rack and crucial help and advice, particularly on underwater photography. E. Ericsson, S. Eriksson and others also gave valuable help in the field. The Stockholm Marine Research Centre provided funding, boat time, assistance and space at the Askö Laboratory. Funding was also provided by the Alice and Lars Silén and Hierta-Retzius Foundations to SZE, by the Swedish Natural Science Research Council and the Swedish Research Council to RE, and by the Swedish Council for Forestry and Agricultural Research to SH. We thank O. Hjerne for advice on programming, K. Limburg for enthusiastic support, E. Flach and C. Savage for valuable comments on the manuscript.




