When an epithelium ceases to exist – an ultrastructural study on the fate of the embryonic coelom in Epiperipatus biolleyi (Onychophora, Peripatidae)
Abstract
It is an accepted fact that fusion between the coelomic cavities and the primary body cavity occurs during development in the Arthropoda. However, such a fusion is much disputed in the Onychophora. In order to clarify this subject, the fate of embryonic coelomic cavities has been studied in an onychophoran. Ultrastructural investigations in this paper provide evidence that embryonic coelomic cavities fuse with spaces of the primary body cavity in Epiperipatus biolleyi. During embryogenesis, the somatic and splanchnic portions of the mesoderm separate and the former coelomic linings are transformed into mesenchymatic tissue. The resulting body cavity therefore represents a mixture of primary and secondary (coelomic) body cavities, i.e. the ‘mixocoel’. The nephridial anlage is already present, when the ‘mixocoel’ is formed, although there is no trace of a sacculus yet. The lumen of the nephridial anlage, thus, communicates with the newly formed ‘mixocoel’. Accordingly, the lumen of the nephridial sacculus cannot be regarded as a kind of ‘persisting coelomic cavity’ in E. biolleyi. Our findings support the hypothesis that the ‘mixocoel’ was already present in the common stem species of the Onychophora and Euarthropoda.
Introduction
Traditionally, Arthropoda and Annelida are considered to be sister taxa comprising the Articulata (e.g. Cuvier 1817; Dohle 1979; Ax 2000; Nielsen 2001; Scholtz 2002). The presence of paired coelomic cavities per segment is one of the major synapomorphies of the Articulata. According to the Articulata hypothesis, coelomic cavities have been modified in the stem lineage of the Arthropoda by the fusion of secondary (coelom) and primary body cavities (blood vascular system). In order to take this process into account, the term ‘mixocoel’ is sometimes used (Daiber 1913; Weygoldt 1986; Seifert 1995; Nielsen 2001; Scholtz 2002). Serially arranged embryonic coelomic cavities have been described in representatives of every higher euarthropod taxon (see Anderson 1973; Gilbert 1997; Schwalm 1997). Transitory coelomic cavities also occur during embryogenesis in the Onychophora (Kennel 1888; Sedgwick 1888; Evans 1901; Glen 1918; Pflugfelder 1948; Anderson 1973; Walker 1995; Bartolomaeus and Ruhberg 1999). However, a fusion between embryonic coelomic cavities and the primary body cavity was never mentioned by the authors who worked on the differentiation of the mesoderm in the Onychophora (see Kennel 1885, 1888; Sedgwick 1885, 1886, 1887, 1888; Sheldon 1887, 1888; Sclater 1888; Willey 1898; Evans 1901; Pflugfelder 1948, 1962; Manton 1949; Pflugfelder 1980; Bartolomaeus and Ruhberg 1999). Therefore, the existence of such a fusion in onychophorans has been denied or seriously doubted (Kennel 1888; Sedgwick 1888; Pflugfelder 1948, 1962, 1980; Bartolomaeus and Ruhberg 1999). Consequently, it remains unclear, whether a ‘mixocoel’ is a characteristic of all arthropods, or represents an apomorphy of the Euarthropoda only.
In order to clarify this subject, the fate of embryonic coelomic cavities has been studied in Epiperipatus biolleyi, an onychophoran species of the Peripatidae from the neotropics. This is the first time that ultrastructural data are provided on late differentiation of embryonic coelomic linings in an arthropod. Special reference is given to early nephridiogenesis. This study is part of a larger project to unravel the organogenesis in onychophorans.
Materials and Methods
Specimens of Epiperipatus biolleyi (bouvier, 1902) were collected in Las Nubes-Cascajal area, de Coronado, near San José, Costa Rica (83°57′37″ W, 10°00′18″ N, 1750–1800 m altitude), in February 1997 and maintained in captivity by H. Ruhberg. A gravid female was dissected in March 1997 at room temperature within a fixative consisting of 2.5% glutaraldehyde buffered in 0.1 m sodium cacodylate (pH 7.0). Ruthenium red was added to the fixative before fixation in order to stain components of the extracellular matrix. The embryos were removed from the uterus and kept in the same fixative for 1 h, then washed several times in 0.1 m sodium cacodylate buffer and kept therein for 1 day at 4 °C. The embryos were then postfixed in 0.2% osmium tetroxide buffered in 0.1 m sodium cacodylate, dehydrated in an acetone series, and embedded in araldite. Several embryos or certain parts of them were cut into series of silver interference-coloured sections (65–75 nm thick) with a diamond knife in a Reichert Ultracut ultramicrotome. The sections were mounted on formvar-covered single-slot copper grids and automatically stained with uranyl acetate and lead citrate in a Leica EM ultrostainer. The sections were examined with a Philips CM 100 transmission electron microscope. One significant embryo (‘coiled stage’) was selected for the present study. The diagrammatic illustration, montage of electron micrographs, and final plates were produced using the Adobe Photoshop 7.0 and Adobe Illustrator 10 software.
Characterization of Body Cavities. – Histologically, two types of body cavities can be distinguished. The coelomic (or secondary) body cavities are generally recognized by their epithelial linings. The coelomic lining cells are interconnected by apical junctions and rest on an extracellular matrix (ECM, basal lamina, lamina densa). The apico-basal polarity of coelomic lining cells might also be indicated by (rudimentary) ciliary structures situated at the apical cellular surfaces. In contrast to secondary body cavities, the lumen of a primary body cavity is never lined with epithelial cells but is merely bordered by ECM (see Rieger 1986; Ruppert 1991; Bartolomaeus 1993; Ax 1996; Bartolomaeus and Ruhberg 1999). This characterization is applied to ultrastructural data presented herein.
Results
A coiled stage-embryo is investigated in the present study (Fig. 1A). Apart from one pair of presumptive antennae no further body appendages are seen. The embryo bears 32 external swellings which decrease in size towards the posterior end. Inside the embryo, pairs of serially arranged coelomic cavities are found in front of a posterior proliferating region. They flank vacuolated walls of the presumptive gut (Fig. 1E–G). The coelomic cavities coincide in position with the external segmental swellings. Their linings are composed of polarized cells which are connected by apical junctions and rest on a basal lamina. Rudimentary cilia barely protrude from the surface of the coelomic lining cells into the coelomic cavities (Fig. 1B).
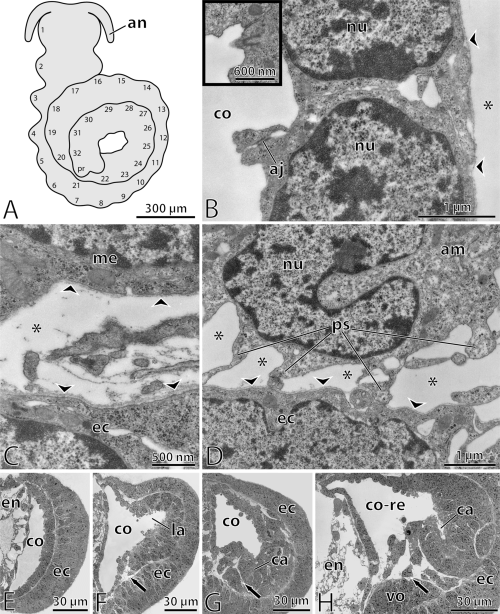
—Epiperipatus biolleyi, coiled stage embryo. —A. Schematic overview of the embryo. Segmental swellings are numbered. —B. Details of coelomic lining cells in the posterior-most body segments. Lining cells are connected by apical junctions and rest on a basal lamina (arrowheads). Rudimentary cilia scarcely protrude into the coelomic cavity (black-framed inset). Asterisk indicates primary body cavity situated beneath the coelomic lining. —C. High magnification of slit-like spaces between ectoderm and mesoderm which represent constituents of the primary body cavity (asterisk). The spaces are clearly lined by lamina densa (arrowheads). —D. Amoeboid cell which is attached to basal lamina of ectoderm (arrowheads). Although the amoeboid cell is situated within a primary body cavity (asterisks), its surface is not covered with lamina densa. —E. Transverse section of the 29th hemisegment. Coelomic cavity is crescent-shaped and lined by a monolayered epithelium. —F. Transverse section of the 14th hemisegment. A lateral protrusion of the coelomic cavity is formed. The somatic wall becomes thicker. Its medioventral part begins to fold inwards (arrow). —G. Transverse section of the 10th hemisegment. The somatic wall has thickened further and the medioventral folds get stronger (arrow). Within the thickened lateral part, a ciliated canal of the nephridial anlage is formed. —H. Transverse section of the 7th hemisegment. The folds of medioventral mesoderm have become more prominent (arrow). Splanchnic and somatic portions of the mesoderm have already separated. The former coelomic cavity has fused with spaces of the primary body cavity. Abbreviations: aj, apical junction; am, amoeboid cell; an, presumptive antenna; ca, ciliated canal of nephridial anlage; co, coelomic cavity; co-re, remnant of coelomic cavity; ec, ectoderm; en, endoderm; la, lateral protrusion of coelomic cavity; me, mesoderm; nu, nucleus; ps, pseudopodia of amoeboid cell; vo, ventral organ (precursor of central nervous system).
Except for the lumen of the presumptive gut and the coelomic cavities, all other hollow spaces within the embryo are lined with lamina densa (1-3). Between different cellular layers within the embryo, these hollow spaces are often reduced to narrow spaces. They are, thus, situated between the basal laminae of the corresponding epithelia (Fig. 1C). Since all these spaces are confluent with each other, and since they are lined by electron-dense matrix, they are regarded as constituents of the primary body cavity. Every cell facing the primary body cavity is lined with lamina densa. The only exception is represented by amoeboid cells. These solitary cells are found in different regions of the embryonic body (1, 2). Amoeboid cells are recognized by pseudopodia, by which they can be attached to the lamina densa of other cells (Fig. 1D).

—Epiperipatus biolleyi, coiled stage embryo. —A. Transverse section of the 7th hemisegment (montage of several TEM micrographs). Splanchnic and somatic portions of mesoderm are separated ventrally. Lumen of former coelomic cavity has become confluent with spaces of primary body cavity (marked by asterisks). Arrows indicate the borders, up to which lamina densa extends into the remnant of coelomic cavity. Note the former splanchnic lining which extends into the ventral sinus situated beneath the presumptive gut.High magnifications of regions indicated in A by encircled capitals–B–D. —B. Border between epithelial and nonepithelial mesodermal cells. Non-epithelial or mesenchymatic cells are covered with lamina densa (arrowhead), whereas epithelial cells are, instead, connected by apical junctions. —C. Dorsally, the remnant of coelomic cavity is exclusively lined by epithelial cells. In contrast to ventral mesoderm, the cells of dorsal mesoderm are still connected by apical junctions and lack any apical lamina densa. —D. Cilium and microvilli from canal of nephridial anlage. The canal cells are connected by apical junctions. Abbreviations: aj, apical junction; am, amoeboid cell; bb, basal body; ca, ciliated canal of nephridial anlage; ci, cilium; cl, coelomic lining; co-re, remnant of coelomic cavity; ds, dorsal sinus; ec, ectoderm; en, endoderm; gl, lumen of presumptive gut; me, mesoderm; mt, mitochondria; mv, microvilli; nu, nucleus; vo, ventral organ; vs, ventral sinus.
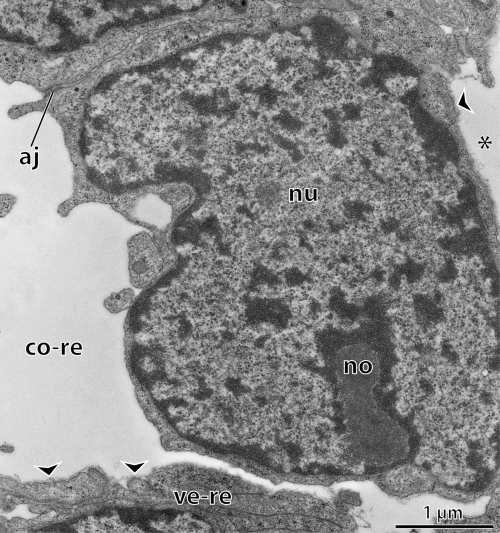
—Epiperipatus biolleyi, coiled stage embryo. Oblique transverse section of the 3rd body segment: high magnification of remnants of coelomic linings. The former coelomic cavity is lined by epithelial and nonepithelial cells at the same time. Some of the lining cells are still interconnected by apical junctions, whereas the cells of the former ventral coelomic wall lack apical contacts and are already covered with lamina densa (arrowheads). Asterisk indicates primary body cavity situated beneath the former coelomic lining. Abbreviations: aj, apical junction; co-re, remnant of coelomic cavity; no, nucleolus; nu, nucleus; ve-re, remnant of ventral coelomic wall.
Within the embryo, an anterior-to-posterior developmental gradient is evident with respect to the differentiation of the mesoderm (Fig. 1E–H). The posterior segments of the embryo are the least differentiated. The coelomic linings are composed of simple monolayered epithelia which represent the only mesodermal structures (Fig. 1E). The coelomic lining cells lack myofilaments and the cellular volume is largely occupied by the nucleus (Fig. 1B). Mitotic stages rarely occur within the coelomic linings. Apart from apical junctions, no other mechanical cell-cell or cell-matrix contacts are seen among coelomic lining cells. The basal lamina of the coelomic linings is thinner than that of the ectoderm (Fig. 1C). The coelomic cavities of the posterior segments appear crescent-shaped in cross-sections. Accordingly, two contiguous portions of mesoderm are distinguished: (1) the splanchnic wall which is adjacent to the endoderm, and (2) the somatic wall which lies beneath the ectoderm. The splanchnic wall is thinner than the somatic wall (Fig. 1E).
The structure of the mesoderm is more developed in the anterior segments of the embryo. The somatic wall becomes thicker, and a lateral protrusion of the coelomic cavity is formed (Fig. 1F). The ventromedian part of the somatic wall begins to migrate inwards. Here, the coelomic lining loses its contact to the ectoderm. At the same time, the dorsal and ventral walls of the presumptive gut (endoderm) begin to lose their contact with the dorsal and ventral ectoderm. Two hollow spaces are thus formed, the dorsal and the ventral sinuses, which enlarge further and extend anteriorly (Fig. 2A). The dorsal and ventral sinuses constitute the largest spaces of the primary body cavity within the embryo.
Along with the enlargement of the neuroectoderm, several folds are formed in the ventromedian part of the mesoderm which migrates further inwards (Fig. 1G). The cells of the somatic part still undergo mitoses indicating a further increase in thickness. Within the thickened, ventrolateral area of the mesoderm, a narrow canal is formed. The lumen of this canal bears numerous microvilli and cilia. Within the canal, ciliogenesis is indicated by several vestigial cilia which are still enclosed by vesicles. The shortest of the cilia project into a small vesicle which lies within the cytoplasm of canal cells. The longer the cilium the closer it is to the cellular surface (Fig. 2D). Several cilia protrude from cellular surfaces into the lumen of the canal. Only one cilium per cell is present. The lumen of the canal is continuous with the remaining coelomic cavity distally. Proximally, the canal ends blindly, its cells being overlain by ectoderm. In contrast to the somatic wall, the splanchnic coelomic lining is still a monolayer of cells (Fig. 1G). In all posterior and most of the mid-body segments (from the 8th segment backwards), the splanchnic and somatic coelomic linings are continuous.
The continuity between the splanchnic and somatic linings is lost, however, further anteriorly (within the segments 1–7). While the dorsolateral portions of the coelomic wall are still continuous, the ventral parts have become separated (Fig. 1H). The splanchnic wall extends to the ventral sinus. That part of the somatic wall which is adjacent to the ciliated canal disintegrates into several folds (1, 2). Owing to the separation of the splanchnic and somatic walls, the coelomic cavity becomes confluent with spaces of the primary body cavity. This fusion is accompanied by changes at the cellular level. The dorsal and lateral portions of the coelomic wall (including the ciliated canal) are still composed of epithelia which bear polarized cells connected by apical junctions (Fig. 2A–D). In contrast, the remnants of the ventral coelomic wall have lost their epithelial character. Their cells are unpolarized, since they lack apical junctions and are, instead, covered with lamina densa (2, 3). Accordingly, the remnants of the coelomic cavities are surrounded by epithelial and nonepithelial cells at the same time, since apical junctions and lamina densa are met within the same hollow space (2, 3).
Discussion
The presence of a ‘mixocoel’
Fusion between the embryonic coelomic cavities and the primary body cavity is thought to be a common feature in the development of arthropods (Anderson 1973; Weygoldt 1986; Seifert 1995). Such a fusion, however, has never been mentioned in classical papers on embryogenesis in the Onychophora (see Sedgwick 1887, 1895; Kennel 1888; Evans 1901; Pflugfelder 1948, 1962, 1980). Instead, it has explicitly been denied to occur in onychophoran development (Kennel 1888; Pflugfelder 1948; Bartolomaeus and Ruhberg 1999). Despite this fact, Anderson (1973: 114) described the occurrence of fusion between coelomic cavities and the primary body cavity during onychophoran embryogenesis, although he did not provide his own data on this subject. Nevertheless, the present work corroborates the assumption of Anderson (1973). According to our data, the resulting body cavity of E. biolleyi represents a mixture of secondary (coelomic) and primary body cavities. This finding is in contrast to previous assumptions of authors who worked on late mesoderm differentiation in the onychophorans. These authors generally questioned the occurrence of a ‘mixocoel’ in representatives of the Onychophora (see Kennel 1885, 1888; Sedgwick 1887, 1888, 1895; Pflugfelder 1948, 1962, 1980; Bartolomaeus and Ruhberg 1999). However, fusion between the embryonic coelomic cavities and the primary body cavity does take place during development in at least one onychophoran species. Regardless of what condition is found in other onychophoran species, the presence of the ‘mixocoel’ in E. biolleyi represents a plesiomorphy, since it also occurs during the development in representatives of the outgroup, i.e. the Euarthropoda (Anderson 1973; Seifert 1995; Nielsen 2001). Our findings therefore support the hypothesis that a ‘mixocoel’ existed in the common stem species of the Onychophora and Euarthropoda. Accordingly, it represents a synapomorphy of both taxa (Weygoldt 1986; Nielsen 2001).
The sacculus of nephridial organs
Segmentally arranged nephridial organs which bear a terminal sacculus are assumed to be an apomorphy of the Arthropoda (Weygoldt 1986; Ax 2000; Nielsen 2001). In representatives of the Euarthropoda, derivatives of the nephridial organs only occur in a few anterior segments. They are modified into organs with glandular function: coxal glands in chelicerates, antennal and maxillary glands in crustaceans, labial glands in myriapods and apterygote insects (Kümmel 1964; Altner 1968; Groepler 1969; Haupt 1969a,b; Alberti and Storch 1977; Seifert 1979, 1995). Only representatives of the Onychophora bear nephridial organs in almost every body segment (Gabe 1957; Storch et al. 1978; Lavallard 1981; Storch and Ruhberg 1993). Despite functional differences, the sacculi of nephridial organs are organized similarly in representatives of different arthropodan taxa (Altner 1968; Haupt 1969a,b; Seifert and Rosenberg 1976; Storch et al. 1978; Seifert 1979). Embryologically, they are assumed to be derivatives of transitory coelomic cavities which persist as sacculi in adults (Sedgwick 1887, 1888, 1895; Evans 1901; Daiber 1913; Pflugfelder 1948, 1962, 1968, 1980; Anderson 1973; Dohle 1979; Storch and Ruhberg 1993; Bartolomaeus and Ruhberg 1999; Ax 2000; Nielsen 2001).
According to our data, however, the situation is more complicated in E. biolleyi. The anlage of the nephridial organ is already present, when the corresponding coelomic cavity begins to fuse with the primary body cavity. There is no trace of a sacculus yet. The lumen of the nephridial anlage, thus, opens freely into the newly formed ‘mixocoel’. Therefore, in contrast to previous assumptions, the lumen of the nephridial sacculus cannot simply be regarded as a kind of ‘persisting coelomic cavity’ (see Sedgwick 1887, 1888, 1895; Evans 1901; Pflugfelder 1948, 1962, 1968, 1980; Anderson 1973; Dohle 1979; Bartolomaeus and Ruhberg 1999). At present, no answer can be given to the question of how the nephridial sacculus originates in E. biolleyi. However, assumptions can be made about the course of further development. At the embryonic stage in this study, the lumen of the nephridial anlage communicates with the ‘mixocoel’. Therefore, it has to become distally closed at a later stage. It is unlikely that the already transformed median portion of the coelomic wall reverts back into an epithelium. We assume therefore that the still existing epithelial linings at the distal end of the ciliated canal fuse together, thus closing up the lumen of the nephridial anlage from the ‘mixocoel’.
It is important to investigate the formation of the sacculus in representatives of the Peripatidae from the neotropics in the near future, since differentiation of the mesoderm in these species deviates from the pattern that is usually generalized for the Onychophora (see, e.g. Sedgwick 1895; Evans 1901; Daiber 1913; Glen 1918; Anderson 1973; Dohle 1979). In contrast to other onychophoran species, the embryonic coelomic cavity is not subdivided into two or three compartments in E. biolleyi. Instead, it consists of one large compartment, the lumen of which communicates with the lumen of the nephridial anlage. This situation resembles that described by Kennel (1888) in two other neotropical species of the Peripatidae. In contrast to the uniform organization of adults in the Onychophora, mesodermal development seems to be highly variable within this group. Further study on this subject therefore might contribute to (1) a better resolution of the phylogeny within the Onychophora (2) our general knowledge of the organogenesis in arthropods, and (3) the systematic placement of the Arthropoda which is still controversial.
Acknowledgements
We are indebted to Ina Vrodoulis for all her patience and helpful discussions and to Carol Schoening for linguistic improvement of the manuscript. We thank Graham Budd and two anonymous referees for their useful suggestions on the manuscript. Uwe Mayer kindly helped with the time-consuming montage of the electron micrographs for the Figure 2A. This work was supported by grants from the Studienstiftung des deutschen Volkes to G. Mayer (D/2002 0033) and from the Deutsche Forschungsgemeinschaft to T. Bartolomaeus (BA 1520/8–1, 8–2) and H. Ruhberg (RU 358/4–1, 4–2).




