Pathophysiology of Wound Development and Chronicity in Renal Disease: A Narrative Review
ABSTRACT
Renal disease, including chronic kidney disease (CKD) and end-stage renal disease (ESRD), has a profound impact on wound healing. Multiple studies have demonstrated that renal disease leads to an increased risk of diabetic foot ulcers, the formation of unique wounds like calciphylaxis, slower wound healing and a higher risk of amputation. This review details the interrelated mechanisms by which renal disease impacts wound healing. Motor and sensory neuropathies contribute to wound formation via foot deformities and decreased sensation. Neuropathies also decrease neuropeptide release, impairing angiogenesis and inflammatory regulation. Accumulation of uremic toxins in renal disease leads to vessel wall calcification, impairing blood supply and predisposing patients to calciphylaxis. Vitamin and mineral deficiencies lead to impaired clotting, development of a chronic inflammatory state and decreased collagen production. Renal disease and its comorbidities are also associated with immune dysregulation, increasing the risk of wound infections and promoting the persistence of pro-inflammatory macrophages. While hypoxia-inducible factor-1α (HIF-1α) promotes angiogenesis under hypoxic conditions in normal wound healing, oxidative stress and chronic hypoxia in renal disease generate an environment that compromises the activity of HIF-1α. Inadequate erythropoietin response to hypoxia also leads to anaemia, further impairing oxygen delivery to wound sites. Clinically, these factors result in increased 10-year mortality for patients with DFU and CKD compared to those with DFU alone, both with and without amputation. We must utilise our understanding of the pathophysiology of impaired wound healing in renal disease to target preventative measures, optimise treatment and improve overall outcomes.
Summary
- CKD and ESRD profoundly impair wound healing and increase risk of amputation due to chronic wounds.
- This review highlights the pathophysiology of aberrant wound healing in patients with renal disease, including neuropathies, uremia, nutrient deficiencies, immune dysregulation, and anemia.
1 Introduction
Renal failure casts a long shadow over global health, emerging as one of the leading causes of death worldwide. Chronic kidney disease (CKD) occurs when renal function is diminished for over three months, characterised by either a glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 along with the presence of kidney damage markers, such as albuminuria or other electrolyte imbalances [1]. Progression of CKD or acute kidney injury leading to GFR below 15 mL/min/1.73 m2 can be characterised as end-stage renal disease (ESRD), a highly lethal and burdensome condition [1].
CKD impacts 14.9% of the US population, with a higher prevalence for females (16%), those over 65 years old (38.6%), Black people (17%) and Hispanic people (15.3%) [2]. The global prevalence of CKD suggests the current total number of individuals affected by CKD is over 800 million, approximately 10% of the world's population [3]. This figure is poised to rise further, driven by a constellation of risk factors such as diabetes, hypertension and an aging demographic. The prevalence of CKD was 11.8% from 1988 to 1994 and has since increased to 14.2% in 2015 and 2016.
Renal failure is well regarded to impact wound healing. Animal model data suggest that CKD impacts epithelialization kinetics and causes a delayed rate of granulation tissue formation, leading to impaired wound healing. Chronic inflammatory state, low vascularization and cell proliferation rates may also exist as potential mechanisms of disrupted wound healing [4]. Uremia also has adverse effects on wound healing [5].
CKD and ESRD patients often have unique development of wounds. Calcific uremic arteriolopathy (CUA), also known as calciphylaxis, is the calcification of the medial layer of small vessels. Mainly seen in patients with ESRD, calciphylaxis is rare and causes necrosis of the skin and painful wounds. Lesions appear on the buttocks, thighs and abdomen and are a major opportunity for infection and eventually sepsis [6]. Although the pathogenesis of calciphylaxis is not fully understood, hyperparathyroidism and the resulting calcium imbalance in ESRD are thought to play a role [7, 8]. In early CKD, elevated parathyroid hormone (PTH) secretion compensates for reduced calcium absorption and allows for the maintenance of a normal serum calcium concentration. However, chronically high PTH in patients with ESRD stimulates bone resorption, leading to increased serum calcium concentration and increased calciphylaxis risk [9].
Diabetic foot ulcer (DFU) is another complication that appears to be linked with renal failure and the incidence of ESRD in patients with diabetes severely impacts the ability of wounds to heal. Mortality of DFU is highest in patients with ESRD, and both share multiple pathophysiological elements such as peripheral arterial disease (PAD) and neuropathic aggravation through the hyperuricemia [10]. Pressure ulcers are also a significant risk factor for mortality in patients with renal failure; however, the understanding of the pathophysiology between the two remains limited [11]. The increased incidence of complex wounds in patients with renal failure underscores the importance of comprehensive care that addresses not only kidney function but also the associated comorbidities and risk factors that contribute to wound development.
Multiple converging studies demonstrate renal failure patients have worse wound healing outcomes [4, 12-14]. One retrospective analysis of national surgical databases found that CKD stage predicts higher complication rates [15]. Uremic pruritus, a symptom of ESRD, is also a risk factor for wound development and poor wound healing because of the repetitive trauma it induces [16]. The loss of protein and the ensuing protein-deficient state resulting from renal replacement therapy (RRT) are also predicted to have a significant negative impact on wound healing, with patients losing 6–8 g of amino acids per haemodialysis procedure and 8–20 g per peritoneal dialysis procedure.
Furthermore, the detrimental impact of ESRD on limb salvage rates in critical limb ischemia has been extensively demonstrated in prior literature. A recent meta-analysis investigating outcomes in critical limb ischemia one year after angioplasty or bypass found that patients with ESRD had significantly lower rates of limb salvage (OR: 0.33 for angioplasty and 0.54 for bypass) and amputation-free survival (OR: 0.48 for angioplasty and 0.28 for bypass) compared to non-ESRD patients [17]. A systematic review looking into risk factors for amputation in diabetic patients with non-healing lower extremity wounds identified ESRD as a very strong independent risk factor (OR: 30.7) [18].
Ultimately, impaired renal function has multiple implications on wound healing, and studies suggest CKD and ESRD patients have worse outcomes and greater complications. Here, we aim to provide a comprehensive review of the pathophysiology of impaired wound healing in patients with CKD and ESRD to facilitate the implementation of preventative measures, optimisation of treatment and improvement of overall outcomes.
2 Brief Overview of Renal Failure
CKD involves progressive kidney function loss that may eventually lead to the need for RRT in the form of dialysis or transplantation. Classification of CKD occurs based on GFR and albuminuria. The six categories are as follows: G1: GFR 90 mL/min per 1.73 m2 and above, G2: GFR 60–89 mL/min per 1.73 m2, G3a: GFR 45–59 mL/min per 1.73 m2, G3b: GFR 30–44 mL/min per 1.73 m2, G4: GFR 15–29 mL/min per 1.73 m2, G5: GFR less than 15 mL/min per 1.73 m2 or treatment by dialysis [19]. Estimating GFR levels based on filtration markers such as creatinine has now become a common method of determining staging [20]. Albuminuria is classified as A1 (urine ACR < 30 mg/g), A2 (30–300 mg/g) and A3 (> 300 mg/g). Compared with urine protein-to-creatinine ratio, urine ACR is a more sensitive marker of glomerular pathology [21]. The various etiologies of CKD and the roles of comorbidities such as diabetes mellitus (DM) and hypertension are summarised in Table 1.
| Category of injury | Examples | How it leads to CKD | Impact of diabetes and hypertension |
|---|---|---|---|
| Pre-renal injuries |
|
Reduced blood flow to the kidneys leads to ischemia and damage to nephrons. If not addressed, this can lead to progressive decline in kidney function. |
Diabetes: Can contribute to dehydration due to increased urination and autonomic neuropathy. Hypertension: Worsens pre-existing conditions that can lead to hypovolemia and can further damage blood vessels leading to the kidneys. |
| Intra-renal injuries |
|
Direct damage to the glomeruli, tubules, or other kidney structures can impair filtration and waste removal, leading to CKD. |
Diabetes: A major cause of intrarenal injury through diabetic nephropathy. High blood sugar damages the glomeruli and promotes inflammation. Hypertension: Can contribute to glomerulosclerosis and further damage to the kidneys. |
| Post-renal injuries |
|
Blockage of urine flow creates back pressure on the kidneys, which can damage nephrons over time. |
Diabetes: Can increase the risk of urinary tract infections that can lead to scarring and blockage. Hypertension: May contribute to an enlarged prostate, which can obstruct urine flow. |
CKD patients experience a significantly higher prevalence of mortality related to cardiovascular disease (CVD). The US Renal Data System shows a higher prevalence of CVD at higher CKD stages, and the CKD Prognosis Consortium found an odds ratio of 2.5 for CVD mortality in people with eGFR of 45–59 mL/min/1.73 m2 and urine ACR of 10–29 mg/g compared to those with normal kidney function [22]. The Atherosclerosis Risk in Communities Study found similar results with a 38% increase in the risk of CVD in patients with a GFR of 15–59 mL per minute per 1.73 m2 at baseline, as compared with an estimated GFR of 90–150 mL per minute per 1.73 m2. At the heart of this increased risk lies endothelial dysfunction. In CKD, these cells malfunction, contributing to reduced nitric oxide (NO) production. NO is essential for vasodilation, but in CKD, NO production is hampered, leading to vasoconstriction and increased blood pressure [23]. Deficiencies in l-arginine are also seen in CKD, further limiting NO production and worsening endothelial dysfunction [24]. Reactive oxygen species (ROS) also damage endothelial cells and impair their function, and CKD patients often exhibit elevated levels of ROS [25]. Studies have shown that dysfunction occurs in the peripheral vasculature of patients with both moderate and severe stages of the disease [26].
Several studies have demonstrated a strong relationship between nutritional status and CKD, with rates of malnutrition ranging from 28% to 65% depending on the stage of CKD [27]. Main mechanisms of malnutrition include vitamin dysregulation of B-group vitamins and of C and D vitamins and oxidative stress [28]. In haemodialysis adult patients, an impairment of nutritional status has been observed, induced not only by the procedures but also due to numerous CKD-related comorbidities [29]. The alteration of nutritional assessment induces systemic manifestations including slow healing of wounds related to hypovitaminosis C [30]. Elevated catabolic hormones like PTH and inflammatory cytokines promote muscle protein breakdown, leading to loss of lean body mass and malnutrition [31]. CKD can also be associated with GI mucosal abnormalities and decreased gastric acid secretion, leading to impaired absorption of various nutrients. Uremia and subsequent management can also trigger nausea, vomiting and anorexia, significantly decreasing appetite and dietary intake [32].
3 Effects of Renal Failure on Wound Healing and Chronicity
3.1 Uremia
The accumulation of uremic toxins in ESRD is a well-known risk factor for worsened cardiovascular outcomes. It can contribute to calcification and stiffening of peripheral arteries, in turn predisposing patients to calciphylaxis and leading to overall impaired wound healing. Uremia induces differentiation of vascular smooth muscle cells into an osteogenic phenotype which, in combination with excess calcium and phosphate, accelerates calcification of vessel walls [33, 34]. This then leads to inflammation and oxidative stress via activation of NADPH oxidase, impairing endothelial cell function as outlined below in section 4.3. Uremic toxins can also induce vasoconstriction by modulating the expression of endothelin-1 and downregulating the production of nitric oxide [34]. Altogether, these effects of uremia result in narrow and stiff vessels, resulting in impaired oxygenation to wound sites. The impacts of PAD on wound healing are detailed further in section 3.7.
3.2 Calcium and Phosphate Imbalances
Hypercalcemia due to hyperparathyroidism, in combination with uremia, is thought to increase their risk for calciphylaxis. Although the pathogenesis is not well understood, calciphylaxis is believed to be caused by circumferentially layered deposits of calcium apatite in small- to medium-sized vessels [35]. Proposed mechanisms include increased expression of osteogenic markers [36], ectopic expression of bone morphogenic protein at wound sites [37, 38] and a chronic inflammatory state leading to increased activation of nuclear factor kappa B (NFkB) and overexpression of receptor activator of NFkB ligand (RANK-L) [39].
Phosphate excretion in CKD patients is maintained by increased fibroblast growth factor 23 (FGF23) and PTH secretion. However, as CKD progresses, the kidneys become less efficient at excreting phosphorus, leading to hyperphosphatemia, promoting inflammation and further delaying healing. While less studied, CKD might also lead to magnesium depletion [5]. Normally, over 60% of filtered magnesium is reabsorbed in the kidneys. In CKD, mechanisms involving the calcium-sensing receptor may lead to increased magnesium excretion. This potential magnesium deficiency can impair various cellular processes crucial for wound healing, such as enzyme function and protein synthesis.
3.3 Nutrient Imbalances
Patients with CKD and ESRD, especially those undergoing haemodialysis, often have deficiencies in several vitamins and minerals, including vitamins B6, B9 and C, selenium and zinc [40]. Additionally, the chronic inflammatory state leads to decreased proteins, amino acids and fats [41]. In combination, these nutrient imbalances leave patients with renal disease vulnerable to chronic wounds and impaired wound healing.
Vitamin C and arginine are both vital to collagen formation, which is an essential step in wound healing. Thus, patients with these deficiencies experience delayed wound healing. Arginine supplementation has been shown to enhance healing of surgical wounds and pressure ulcers [42]. However, there is little research on the specific role of supplementation for chronic wounds in patients with renal disease. A recent randomised-controlled trial found that compared to glucosamine supplementation, vitamin C supplementation in patients with chronic foot ulcers led to accelerated wound healing and higher rates of wound closure [43].
Vitamin B6 is thought to play a role in collagen formation, while vitamin B9 is essential to cell division and may contribute to fibroblast proliferation. An in vitro study found that supplementation with vitamin B complex improves wound closure rates by 25%–30% [44].
Zinc is a cofactor in the enzymatic activity of matrix metalloproteinases (MMPs), which play a role throughout the stages of wound healing, including inflammation, angiogenesis, re-epithelialisation and tissue remodelling [45]. Zinc also independently plays a role in differentiating proinflammatory (M1) and anti-inflammatory (M2) macrophages, which is essential to the transition from pro-inflammatory to anti-inflammatory states of wound healing [46]. Finally, it has been shown to accelerate haemostasis, although the exact mechanism is not well understood [44]. Although zinc supplementation has been shown to improve immune response [47, 48] and stabilise haemoglobin levels [48] in patients with CKD, its role in enhancing wound healing is not well studied.
3.3.1 Contribution of Haemodialysis to Nutrient Imbalance
Haemodialysis leads to a decreased concentration of circulating calcification inhibitors, likely due to vitamin K deficiency. Vitamin K plays a role in the gamma-carboxylation of matrix Gla protein, which helps protect against vessel wall calcification [49, 50]. Vitamin K deficiency in patients with ESRD may be precipitated either by warfarin therapy or restricted intake of leafy greens. A 2017 retrospective study of a large cohort of calciphylaxis patients found that 52% of them were receiving vitamin K antagonists prior to the incidence of the wound [51].
3.4 Chronic Inflammation and Immune Dysregulation
CKD and ESRD are associated with chronic inflammation, which significantly impairs wound healing. Factors such as uremia, oxidative stress and comorbidities like diabetes and CVD are key players in driving this inflammation. Uremia, the accumulation of waste products in the blood due to impaired kidney function, leads to increased levels of proinflammatory cytokines, including interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α) and C-reactive protein (CRP) [5].
In addition to chronic inflammation, patients with CKD and ESRD exhibit significant alterations to immune cell functions. Neutrophils, the first responders in the inflammatory phase of wound healing, show impaired chemotaxis, phagocytosis and bactericidal activities while under uremic conditions. Studies have also shown that CKD patients have impaired function of T-cells and dendritic cells. These dysfunctions lead to a delayed and inadequate inflammatory response, which makes wounds more susceptible to infections [5, 52]. Additionally, macrophages in these patients often remain in a pro-inflammatory (M1) state longer and fail to transition to the anti-inflammatory (M2) state necessary for tissue repair and resolution of inflammation [5].
Oxidative stress is a hallmark of renal disease. Resulting from an imbalance between the production of ROS and the body's ability to detoxify these reactive intermediates, elevated ROS levels damage cellular components, including DNA, proteins and lipids. This exacerbates the inflammatory response, further impairing wound healing. The persistent oxidative stress also hinders the function of endothelial cells, which are crucial for angiogenesis and tissue repair [26, 53].
Hypoxia-inducible factor-1α (HIF-1α) is a critical transcription factor that responds to low oxygen levels and plays a vital role in wound healing. Under acute hypoxic conditions, HIF-1α promotes the expression of genes involved in angiogenesis, such as vascular endothelial growth factor (VEGF), and helps mediate cellular responses to hypoxia. However, in the chronic hypoxic environment created by impaired renal function, the stability and activity of HIF-1α are often compromised due to the high oxidative stress and inflammation, which leads to inadequate angiogenic responses and impaired wound healing [25]. Studies indicate that HIF-1α's role in promoting glycolysis and reducing mitochondrial respiration is crucial for adapting to the hypoxic conditions prevalent in CKD [54].
3.5 Anaemia and Neuropathies
Under hypoxic conditions, HIF-1α also leads to activation of the erythropoietin (EPO) gene. However, as the kidneys are a major site for EPO production, patients with chronic renal impairment have an inadequate EPO response to hypoxia [55]. Decreased production of EPO, along with chronic inflammation and iron deficiency, leads to anaemia in patients with CKD and ESRD, thus causing impaired oxygen delivery to wound sites. Multiple studies have demonstrated the negative impact of anaemia on wound healing outcomes. In a prospective study of 336 patients with DFUs, anaemia led to significantly increased rates of amputation (OR: 1.67, p = 0.036) and increased risk of death (OR: 1.82, p = 0.034) [56]. A recent retrospective study also found that anaemia led to decreased healing rates and increased recurrence rates of DFUs [57].
Vascular damage and the neurotoxic effects of uremia also lead to peripheral and autonomic neuropathies in CKD and ESRD patients, contributing to both wound pathogenesis and impaired healing [58]. Motor neuropathies lead to imbalances between muscle bulk and tone, causing claw foot deformities and areas of increased pressure. In parallel, sensory neuropathies impair pain and pressure perception, predisposing patients to the development and delayed detection of wounds [59].
Damage to cutaneous autonomic nerve fibres, which typically release neuropeptides as part of the healing response, leads to dysregulation of the immune response [60]. Key neuropeptides involved in this phenomenon include neuropeptide Y, substance P and calcitonin gene-related peptide (CRGP). Neuropeptide Y has angiogenic effects and modulates cell migration, cytokine release and antigen presentation. Substance P contributes to a pro-inflammatory environment and acts as a chemoattractant to immune cells. Substance P and CGRP also promote vasodilation via the production of nitric oxide. CGRP enhances neovascularization via increased VEGF secretion at wound sites. Thus, the decreased release of neuropeptides in autonomic neuropathy leads to an impaired wound-healing response [60]. The various pathways through which renal failure leads to impaired wound healing are summarised in Figure 1.
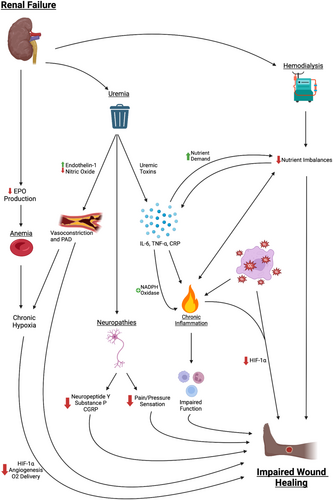
3.6 DM
There are many risk factors that can contribute to poor wound healing such as poorly controlled DM, peripheral vascular disease, aging, neuropathy and chronic venous insufficiency [5]. These factors are commonly seen in those with renal failure. Diabetes is the most common cause of CKD, with one third to one half of all DM patients developing kidney damage in their lifetime and 2.5 million incident cases of CKD attributed to diabetes in 2019 [5, 61-64]. As one of the most common renal failure comorbidities, DM also independently leads to microvascular ischemia and peripheral neuropathy, leading to impaired wound healing as outlined in Section 3.4.
3.7 PAD
Patients with renal disease have a higher incidence of PAD compared to the general population [65], which leads to poor healing of limb wounds due to decreased vascularity. PAD can be broadly categorised into macrovascular disease, which affects large blood vessels, and microvascular disease, which involves smaller vessels. These two forms of PAD differ in their pathophysiology and impact on wound healing, with microvascular disease often leading to more severe tissue hypoxia and delayed healing due to the involvement of capillary networks.
Oxygen, and by extension vascularity, is essential to wound healing. Oxygen is critical for phagocytosis, cell proliferation and collagen deposition. Patients with peripheral vascular disease, particularly microvascular PAD, are at increased risk for hypoxia at wound sites, leading to delayed healing [66]. A retrospective study of DFUs treated with revascularization found that patients on dialysis were more likely to experience revascularization failure compared to those with diabetes alone. This difference was attributed to a higher incidence of below-the-ankle arterial disease, which predominantly reflects microvascular PAD, in patients on dialysis [67].
However, kidney disease also leads to worse outcomes within a cohort of PAD patients. A study of nearly 40 000 PAD patients hospitalised for revascularisation found that patients with renal disease were more likely to experience a major adverse cardiac event, require amputation, or be readmitted after revascularization [68]. This may be explained by the decreased capacity for angiogenesis among patients with renal disease. While many patients with macrovascular PAD are able to form collateral vessels to combat limb ischaemia, CKD patients experience impaired angiogenesis due to deficiencies in modulators such as VEGF, angiopoietin and MMP-9 [65, 69]. Due to the role of hypoxia in impaired wound healing, hyperbaric oxygen therapy has been studied for the treatment of chronic wounds and has shown to increase healing rates and decrease the risk of amputation [70]. However, its impact on wound healing in patients with renal disease has not been fully discerned. The interplay of common comorbidities associated with renal failure and wound healing is summarised in Figure 2.
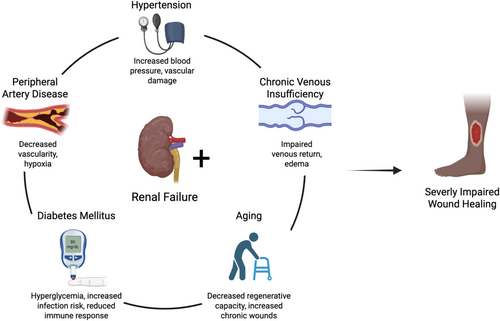
4 Clinical Implications: Mortality and Amputation Outcomes in Humans
It is important to understand the above pathophysiological factors in the context of clinical implications. Several studies have demonstrated increased mortality and risk of amputation among those with chronic wounds and renal disease.
4.1 Amputation Rates and Survival After Amputation
Renal failure increases the likelihood of amputation surgery [71]. Using both a univariate and multivariate analysis, Otte et al. found a significantly higher risk of major amputation for the CKD Stages 4–5 and the dialysis treatment groups relative to the CKD 3 group. A study on transmetatarsal amputations showed that ESRD was a significant predictor of failure to heal at the amputation site [72]. Other comorbid conditions such as coronary artery disease, hypertension and diabetes were also evaluated for their effect on non-healing, but none of these factors proved to be statistically significant. Malyar et al. found a hazard ratio of 1.30 for limb amputation in diabetic patients with ESRD compared to the overall DFU population [73]. Figure 3 shows the yearly survival rates of diabetic patients following amputation across groups with no CKD, CKD and on haemodialysis, which was adapted from data reported by Lavery et al. [74]. Long-term survival after amputation is significantly lower in patients who are on dialysis relative to the reference group with no renal disease. The 1-year survival rates dramatically drop from 85.6% for amputees without CKD to 50.8% for amputees on dialysis, indicating the clear effects of ESRD on chances of survival.
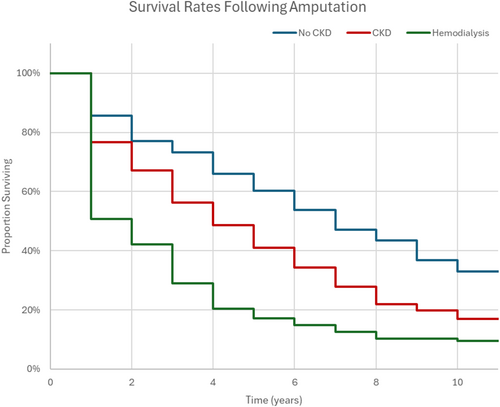
4.2 DFU Mortality
Ghanassia et al. found that renal impairment was a significant risk factor in mortality in diabetic patients, with a hazard ratio of 4.57 [75]. In DFU patients, a prior study has shown that mortality rates at 1, 3, 5 and 7 years after admission for DFU are significantly higher in patients with ESRD on dialysis [76]. Similarly, a meta-analysis found a pooled hazard ratio for DFU mortality of 1.535 in CKD patients and 3.568 in ESRD patients [77]. Figure 4 shows the difference in survival rates between all DFU patients and DFU patients who also have CKD. This figure was adapted from data reported by Jeyaraman et al. [78] and Holman et al. [79]. Chances of survival for the DFU and CKD population are visibly decreased relative to the larger DFU population, especially on a longer-term timeline of 10 years.
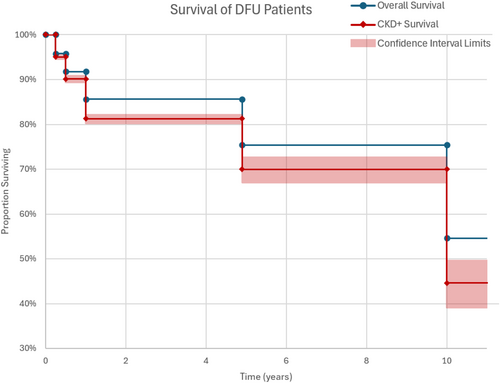
5 Conclusions
CKD and ESRD affect a large portion of the American and global populations and are highly associated with other adverse health outcomes, including uremia, nutritional imbalances, immune dysregulation, anaemia, neuropathies, arterial calcifications and plaques and diabetes mellitus. These factors lead to decreased collagen formation and deposition, tissue hypoxia and impaired inflammatory responses, putting patients with CKD and ESRD at elevated risk for chronic wounds, delayed wound healing and ultimately increased mortality.
Acknowledgements
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




