The Effect of Platelet Rich Plasma on Wound Healing in Pressure Ulcer Patient Grade III and IV
ABSTRACT
Pressure injury is soft tissue damage caused by persistent mechanical pressure on bony prominences, with incidence rates continuing to rise. To address this issue, platelet-rich plasma (PRP) is being researched and developed. This study aims to compare the effects of injectable and topical PRP administration on pressure injury healing, using the Pressure Ulcer Scale for Healing (PUSH) score and collagen density as measures. This experimental study was conducted with grade III and IV pressure injury patients treated at a tertiary referral hospital in Bandung. The subjects were divided into three groups: control, PRP injection and topical PRP. Wound treatment and observation were conducted over 21 days, with evaluations based on the PUSH score (wound area, exudate amount and tissue type) and collagen density. This study involved 22 patients with 30 pressure ulcers. Significant differences were observed in the mean Δ PUSH score (p = 0.047) and mean Δ collagen density (p = 0.011) in the PRP injection group compared to the control group. No significant differences were found between the PRP injection and topical PRP groups regarding PUSH score (p = 0.162) and collagen density (p = 0.862). In the PRP injection group, a significant decrease in the PUSH score was observed on day 12 (p = 0.045), whilst in the topical PRP group, a significant decrease occurred on day 21 (p = 0.034). PRP injection significantly reduces PUSH scores and increases collagen density compared to the control group. By day 21, no significant differences were observed between PRP injection and topical PRP. However, PRP injection demonstrated faster clinical improvement, with significant changes in PUSH score evident by day 12, compared to day 21 for topical PRP.
Summary
- Analyse the effect of PRP injection and topical PRP on wound healing in pressure ulcer patients based on Pressure Ulcer Scale for Healing (PUSH) score compared to the control group.
- Compare the effect of PRP injection and topical PRP in reducing PUSH score in pressure ulcer patients.
- Analyse the effect of PRP injection and topical PRP on wound healing in pressure ulcer patients based on collagen density compared to the control group.
- Compare the effect of PRP injection and topical PRP in increasing collagen density in pressure ulcer patients.
1 Introduction
Pressure ulcer is defined as soft tissue damage caused by continuous mechanical pressure on prominent bones, such as the sacrum, ischium, heel and trochanter. When external pressure exceeds capillary pressure (32 mmHg), it disrupts tissue perfusion, leading to tissue ischemia and the development of pressure ulcers. Other factors contributing to the occurrence of pressure ulcers include shearing, friction, high humidity and infection [1].
Annually, a total of 2.5 million pressure ulcer patients are treated in the United States [1]. In Indonesia, the incidence of pressure ulcers reaches 33% in Intensive Care Unit (ICU) patients, which is notably high compared to the incidence rates in Southeast Asia, ranging from 2.1% to 31.3% [2-4]. At Dr. Hasan Sadikin Hospital in Bandung, 264 patients with grade 1–4 pressure ulcers were treated by the Division of Plastic Reconstructive and Aesthetic Surgery from 2014 to 2017. The breakdown of patients per year was as follows: 69 patients in 2014, 59 patients in 2015, 76 patients in 2016, and 60 patients in 2017. Grade III and IV pressure ulcers represented the highest proportions, at 54.8% and 22.5%, respectively [5, 6]. Data from the hospital's medical records indicate an increase in the number of pressure ulcer patients, with an average hospitalisation period of more than 3 months [5, 6]. This also leads to increased treatment costs. In the United States, the cost of treating pressure ulcers exceeds $11 billion annually, presenting a significant burden on the healthcare system [1]. Furthermore, after the patient undergoes outpatient treatment, it is not impossible that the family will also spend money on homecare from trained nurses. Certain patient populations are at higher risk of developing pressure ulcers, including those who have been bedridden for long periods: patients with pelvic fractures, spinal cord injuries, trauma to the lower extremities requiring fixation and casting, elderly patients with immobilisation and/or cachexia [1], patients treated in the Intensive Care Unit (ICU), and, more recently, COVID-19 patients with Acute Respiratory Distress Syndrome (ARDS) undergoing mechanical ventilation, who are also at high risk for developing pressure ulcers [7].
Pressure ulcers can impair the skin's ability to protect underlying organs. One of the primary functions of the skin is to act as a barrier against germs, bacteria, and to maintain moisture. If not properly managed, pressure ulcers can lead to various complications, such as delayed wound healing, cellulitis, sepsis and even death [2].
Wound healing is a complex process. The four phases of wound healing—haemostasis, inflammation, proliferation and remodelling—do not occur in a simple, linear sequence but rather overlap and interconnect [8]. To support wound healing in pressure ulcers, biological factors are essential, particularly those involved in cells and signal transduction pathways influenced by growth factors. Additionally, intrinsic extracellular matrix proteins, extrinsic mechanical forces and biophysical forces (mechanotransduction) play a crucial role during the proliferation phase [8].
Various management approaches have been employed to address pressure ulcers. Wound care options such as hydrocolloids, alginates, silver sulfadiazine patches, honey gauze and ointments are commonly used to promote the healing of these chronic wounds. However, recent studies have suggested that it is unclear whether these interventions significantly improve the likelihood of wound healing. Other treatments, such as hyperbaric oxygen (HBO) therapy and negative pressure wound therapy (NPWT), are also believed to accelerate wound healing. However, these methods have drawbacks, including limited availability, patient intolerance and high costs [9].
Another alternative approach being researched and developed in recent years is platelet-rich plasma (PRP). PRP contains various growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor-A, epidermal growth factor, transforming growth factor (TGF)-β1, and insulin-like growth factor 1, all of which are present at higher concentrations compared to those in blood. The elevated levels of growth factors in PRP act during the haemostasis, inflammation and proliferation phases, thereby accelerating the regeneration of endothelial, epithelial and epidermal cells, stimulating angiogenesis and collagen synthesis, and enhancing the haemostasis response [9-11].
However, the effectiveness of PRP in chronic wounds, including pressure ulcers, remains controversial, as some studies with high evidence of significance have produced contradictory results [9]. A study by Carter et al. demonstrated that PRP therapy had a positive effect on wound healing and related factors, such as pain and infection, in both chronic and acute wounds [12]. On the other hand, Zapata et al. concluded that the effect of PRP on the healing of chronic wounds, other than diabetic foot ulcers, remains unclear. Their meta-analysis showed no improvement in wound healing compared to standard therapy, likely due to inconsistencies in the assessment of chronic wounds amongst healthcare providers [13].
It has been reported that PRP is administered to chronic wounds through various routes, such as intradermal, subcutaneous and topical injections, but the effectiveness of each route remains unclear. Liu et al. stated that using PRP gel in pressure ulcer cases can accelerate wound healing, reduce pain, shorten the therapy cycle, and improve patients' quality of life without increasing complications [14]. In contrast, a study by Elbarbary et al. found that PRP injection is superior to topical PRP in treating chronic venous leg ulcers. PRP injection can deliver adequate platelet concentrations to the wound edge and depth, maintaining the release of growth factors for a longer duration compared to topical PRP used as a local dressing [15]. Topical PRP is considered less effective because the PRP fluid tends to spread to the wound edge or adhere to the secondary dressing [15]. However, a study by Ibraheem et al. comparing the effects of PRP injection and topical PRP in venous ulcers found no statistically significant differences in wound healing between the two routes of administration, with both showing similar healing outcomes [16].
An objective modality is needed to evaluate wound healing. Several scores are used to assess pressure ulcer healing, including the Pressure Ulcer Scale for Healing (PUSH) score, Bates-Jensen Wound Assessment Tool (BWAT), and Sussman Wound Healing Tool (SWHT) [17-19]. Amongst these, the PUSH score is commonly used, which is a fast and accurate method created by the National Pressure Ulcer Advisory Panel (NPUAP). It evaluates three parameters: wound size (length × width in cm2), exudate amount, and tissue type [17]. Additionally, collagen density can be used to assess wound healing. Collagen density can be examined using haematoxylin and eosin (HE) staining or Masson's trichrome staining. A study by Abuaf et al. found that the increase in collagen density before and after therapy in wounds treated with PRP was more significant than in those treated with a placebo [18].
Karina et al. conducted a study on intravenous PRP administration and intralesional injection in patients with grade IV pressure ulcers [20]. However, there has been no recent study in Indonesia comparing the modalities of PRP use in pressure ulcers, particularly regarding the effects of PRP injection versus topical PRP on the healing of pressure ulcer patients, and observing wound development week by week. Additionally, the characteristics of pressure ulcers in four general hospitals in Indonesia differ from those found in studies from other countries. Nearly half of the pressure ulcers in Indonesia are grade III and IV (42.3%) [21], whereas a systematic review by Li et al., which included data from six geographic regions (Asia, Australia, Europe, the Middle East, North America and South America), found that grade I and II pressure ulcers are most common amongst patients treated [22]. The author is encouraged to conduct further research, which could serve as a reference for the management of pressure ulcers at tertiary referral hospitals in Bandung and across Indonesia. This study aims to analyse the effect of PRP injection and topical PRP on wound healing in pressure ulcer patients based on the Pressure Ulcer Scale for Healing (PUSH) score, compare the effects of PRP injection and topical PRP in reducing PUSH scores in pressure ulcer patients, analyse the effect of PRP injection and topical PRP on wound healing based on collagen density compared to the control group, and compare the effects of PRP injection and topical PRP in increasing collagen density in pressure ulcer patients.
2 Materials and Methods
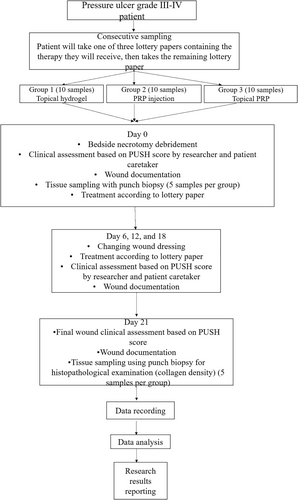
This experimental study involved pressure ulcer patients with grade III and IV ulcers, treated at a tertiary referral hospital in Bandung from July 2023 to January 2024. The inclusion criteria for this study were: grade III and IV pressure ulcers at any location in patients aged 18–59 years; pressure ulcers of any size with controlled exudate (moist), as indicated by non-saturated and non-dry gauze; and patients who were willing to participate in the study until completion by signing an informed consent. The exclusion criteria included: patients with diabetes mellitus (DM), hypertension, malnutrition, sepsis, or shock; patients with Hb < 10.0 g/dL, albumin < 2.5 g/dL, or random blood sugar > 200 mg/dL; blood perfusion disorders, blood coagulation disorders, or vascular disorders (atherosclerosis, stenosis and blood vessel occlusion); immunosuppressed conditions (HIV/AIDS, history of radiation, or use of cytotoxic drugs); a history of corticosteroid use for more than 1 week; a history of malignancy; infections extending deeper than the subcutaneous layer (for example necrotizing fasciitis, osteomyelitis); inability to mobilise every 2 h, as indicated by the mobilisation table; and other conditions such as being on a ventilator or worsening clinical condition when tilted left and right; pressure ulcer patients who had undergone defect closure surgery. The dropout criteria included: patients who did not complete the follow-up procedure for any reason or patients who died during the study.
The pressure ulcer sizes varied across the groups and were divided as follows: Group 1 (control group), which received hydrogel as therapy. In this group, 10 pressure ulcers will be assessed using the PUSH score, and at least 5 of the 10 ulcers will be evaluated histopathologically. Group 2 (treatment group) received PRP injection. Similarly, 10 pressure ulcers will be assessed using the PUSH score, and at least 5 of the 10 ulcers will undergo histopathological assessment. Group 3 (treatment group) received topical PRP. In this group, 10 pressure ulcers will also be assessed using the PUSH score, with at least 5 of the 10 ulcers undergoing histopathological examination. This sample size met the sample size calculation regarding the Federer formula for experimental study [23].
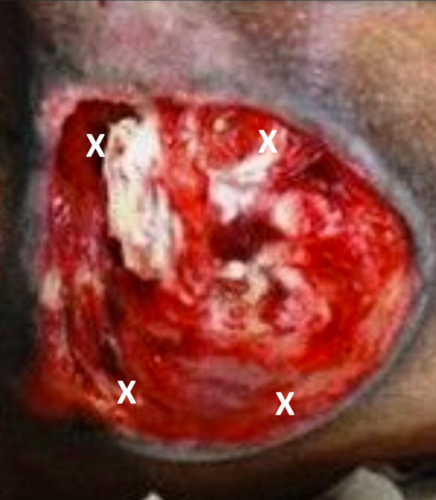
For topical hydrogel administration, 0.1 mL/cm2 of hydrogel was applied to cover the entire wound surface [24]. In this study, we used leucocyte-rich PRP. PRP injections were administered using a 1 cc syringe and a 26G needle, with 0.01 mL/cm2 injected into the tissue beneath the wound at four points: upper right, lower right, upper left and lower left, as shown in Figure 1. Topical PRP was applied at 0.01 mL/cm2, covering the entire wound surface (Figures 2 and 3).
- Wound size: Calculated from the largest length and width dimensions of the wound, which are multiplied and expressed in cm2. These measurements are then converted into scores according to the PUSH Tool 3.0 (Appendix 1).
- Exudate amount: Assessed macroscopically after the dressing is removed and before the application of topical agents. Exudate amount is categorised as none, light, moderate, or heavy, and is then converted into a score according to the PUSH Tool 3.0.
- Tissue type: Assessed macroscopically and categorised as closed, epithelial tissue, granulation tissue, slough, or necrotic tissue.
The higher the score, the more severe the pressure ulcer condition. In this study, the PUSH score will be evaluated at the initial assessment and when therapy begins (day 0), and subsequently after each therapy session (on day 6, 12 and 18), as well as on the 21st day after receiving therapy.
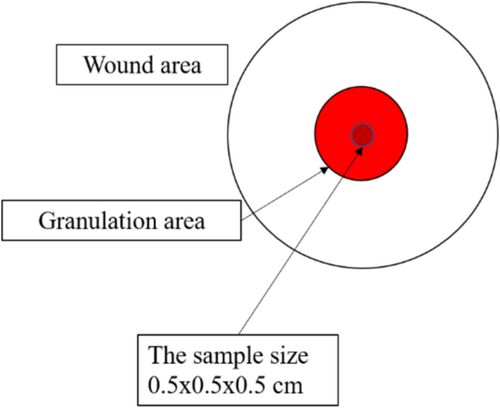
Collagen density was observed on day 0 and day 21 using granulation tissue samples obtained through the punch biopsy technique, followed by haematoxylin and eosin staining. Collagen density was measured using the ImageJ application. The percentage of collagen density for day 0 and day 21 was recorded, and the difference in collagen density (Δ) between these two time points was calculated. The assessment on day 21 is to observe the changes 7 days after the end of the proliferative phase of the ulcer, with the debridement as a starting point. The differences will be observed and compared within a consistent timeframe.
2.1 Platelet-Rich Plasma Manufacturing Process
PRP production was carried out in our clinical pathology laboratory using a two-stage centrifugation method. Autologous peripheral venous blood was collected from the patient using a 10 cc vacuum tube containing sodium citrate anticoagulant. Blood collection was performed by a nurse. The PRP production process was conducted by a clinical pathology officer or doctor. In the first stage of centrifugation, the blood was spun at 900 rpm at room temperature for 5 min. The plasma fraction was then aspirated using a pipette. In the second stage, the sample was centrifuged at 1500 rpm at room temperature for 15 min. The top layer, which is the PRP, was aspirated using a pipette. The number of cells in the PRP sample was then counted by flow cytometry. Finally, the PRP suspension was mixed with a calcium gluconate solution to reach a concentration of 1:10 per volume. The total volume of PRP produced was 2.5 mL.
2.2 Treatment of Research Subjects
Pressure ulcer patients who met the inclusion and exclusion criteria were selected using consecutive sampling. Each patient drew one of three lottery papers, each indicating the therapy they would receive. The next patient drew one of the two remaining papers, and the cycle continued until each treatment group had 10 patients. The caregivers were unaware of the therapy assigned to each patient based on the lottery. Five patients from each group underwent histopathological examination on day 21. Odd-numbered patients (1, 3, 5, 7 and 9) in each group were selected for biopsy.
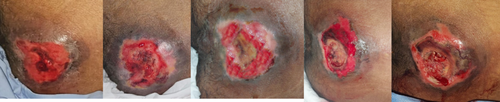
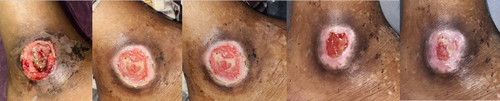
In this study, all pressure ulcers containing slough or necrosis were debrided to standardise wound conditions. (Figures 4-6) Debridement was done using tweezers, scissors, and/or a scalpel, followed by cleaning the wound with normal saline and chlorhexidine. Exudate was controlled using dry gauze. Clinical assessments of the wounds were performed based on the PUSH score by both the researcher and caregiver, with scores kept confidential until the study's conclusion.
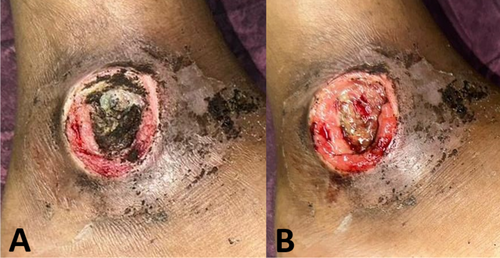
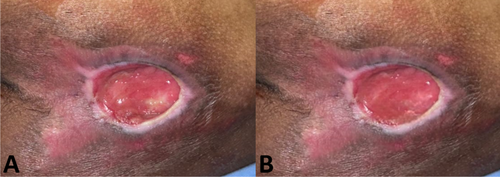
On day 0, the wound surface was treated according to the group assigned by the lottery draw. Three groups were included in this study: Group I received topical hydrogel, Group II received PRP injection, and Group III received topical PRP. Topical hydrogel was applied to the entire wound surface at a volume of 0.1 mL/cm2. PRP injection was administered using a 1 cc or 3 cc syringe, with 0.01 mL/cm2 injected at four points (upper right, lower right, upper left and lower left) on the tissue beneath the wound, spaced 0.5 cm from the wound edge (Figure 1). Topical PRP was applied to the entire wound surface at 0.01 mL/cm2. Treatment was administered by the researchers.
After treatment, all wounds were covered with gauze and a plaster or transparent dressing. On day 3, 9 and 15 (multiples of 6 days), or if contaminated with faeces or urine, only the dressing was changed by the caregiver, without assessing the PUSH score. On day 6, 12, 18 and 21 (multiples of 6 days and the final day of the study), the wounds were reassessed clinically by both the researcher and caregiver using the PUSH score. Wound documentation was completed on day 0, 6, 12, 18 and 21 to track the healing timeline.
At each assessment on day 6, 12 and 18, the respective treatments (topical hydrogel, PRP injection, or topical PRP) were reapplied as needed. All patients were mobilised every 2 h and the areas over bony prominences were kept moist by applying olive oil. For histopathological assessment, punch biopsies were taken under local anaesthesia (2% lidocaine, maximum dose 7 mg/kg body weight) from the granulation tissue area, measuring 0.5 × 0.5 × 0.5 cm, on day 0 and 21. (Figure 2) The biopsies were processed and evaluated by the Anatomical Pathology Department and the researcher.
2.3 Haematoxylin–Eosin Staining
After the tissue sampling, specimen preparation for haematoxylin–eosin (HE) staining was carried out. The excised tissue was fixed in 10% formalin solution at room temperature for 24–48 h, with a formalin-to-tissue ratio of 5–10 times. Following fixation, the tissue underwent a dehydration process, which involved sequential immersion in ethanol solutions: 70% ethanol for 2 h (changed 2 times), 80% ethanol for 1 h (changed 1 time), 95% ethanol for 1 h (changed 1 time), and 100% ethanol for 4.5 h (changed 3 times). After dehydration, the tissue was cleared using xylene for 4.5 h (changed three times) to remove the alcohol. The cleared tissue was then infiltrated with liquid paraffin in an oven set at 58°C–60°C for 2 h (with two changes of paraffin). The tissue was embedded in paraffin blocks until hardened, and then cut using a microtome into 3–10 μm thick sections, typically 5 μm.
The prepared sections were deparaffinised and rehydrated by immersing them in xylene solution for 2 min (with three changes), 100% ethanol for 2 min (with three changes), 95% ethanol for 2 min, 80% ethanol for 2 min, and deionised water for 2 min. The tissue was then stained with Mayer's haematoxylin solution for 3 min, followed by washing with running water for 5 min. After washing, the tissue was stained with eosin Y solution for 2 min, then dehydrated in ethanol (20 dips), followed by immersion in 95% ethanol for 2 min, 100% ethanol for 2 min (with two changes), and xylene for 2 min (with three changes). Finally, the slides were covered with a cover slip, dried, and examined under a microscope.
2.4 Clinical Evaluation of Wounds
Wounds were clinically assessed using the Pressure Ulcer Scale for Healing (PUSH) score, which evaluates three main parameters: wound size, exudate amount and tissue type. The wound size was measured using a plastic measuring tape to assess the largest length and width dimensions. The length and width were multiplied to obtain the area in cm2. These measurements were then converted into a score according to the PUSH scoring guidelines (Appendix 1). The amount of exudate was assessed macroscopically and categorised as none, light, moderate, or heavy. Each category was assigned a score based on the PUSH scoring system (Appendix 1). The tissue type was evaluated based on visual inspection and classified into one of the following categories: closed, epithelial tissue, granulation tissue, slough, or necrotic tissue. These categories were then converted into scores according to the PUSH system (Appendix 1).
The total PUSH score is the sum of these individual parameters, with scores ranging from 0 to 17. A score of 17 indicates the most severe pressure ulcer. Clinical wound assessments were performed on days 0, 6, 12, 18 and 21. Both the researcher and the patient caretaker conducted the wound assessment independently, with the note that both assessors did not know each other's assessment scores until the end of the study. The final score for each patient was determined by averaging the scores from both assessors.
2.5 Histopathological Evaluation of Wounds
The wound was assessed histopathologically using samples obtained via punch biopsy from the granulation tissue, as shown in Figure 2. The punch biopsy technique involved using a 5 mm biopsy punch to collect tissue samples measuring approximately 0.5 × 0.5 × 0.5 cm. These tissue samples were then stained with Haematoxylin and Eosin (HE) for evaluation. Collagen density was measured using the ImageJ application. The measurements were taken on day 0 (initial wound condition) and day 21 (end of the study). The collagen density was expressed as a percentage, and the difference in collagen density between day 0 and day 21 (Δ, delta) was calculated. The histopathological evaluation of the wound samples was performed by both the researchers and the anatomical pathologists. The final collagen density data were averaged from the results obtained by the researchers and the anatomical pathologists. The overall research flow for this study is illustrated in Figure 3.
2.6 Data Analysis
The data were processed using SPSS version 22.0 as the operational system. A p-value < 0.05 was considered statistically significant. Before performing any statistical tests on numerical data, the normality of the data was assessed using the Shapiro–Wilk test. For the comparison of the mean PUSH scores across more than two paired groups based on observation times (day 0, 6, 12, 18, 21), a parametric statistical test, repeated measures ANOVA (Analysis of Variance), was used, followed by a post hoc test. To compare the mean difference (Δ) in PUSH scores between day 0 and day 21 across the three groups, an unpaired t-test was employed. Whilst for the comparison of mean collagen thickness between day 0 and day 21 within each group, a paired t-test was used. To compare the mean difference (Δ) in collagen thickness between day 0 and day 21 across the three groups, an unpaired t-test was applied.
This study was conducted after obtaining approval and recommendations from the Ethics Committee of the Faculty of Medicine, Padjadjaran University, Bandung (LB.02.01/X.6.5/254/2023).
3 Results
The research was conducted over 6 months at the inpatient ward and Plastic Surgery polyclinic of a tertiary referral hospital. Initially, 24 subjects were enrolled in the study; however, 2 patients dropped out due to death, resulting in a final sample size of 22 patients with 30 pressure ulcers that met the inclusion criteria. These subjects were divided into three groups and observed over 21 days to analyse the effects of PRP injection and topical PRP on wound healing in pressure ulcer patients. The evaluation focused on changes in the PUSH score and increased collagen density as indicators of the healing process.
The characteristics of the research subjects were as follows: 11 (50%) men and 11 (50%) women. The most common location of the pressure ulcers was the sacrum, which accounted for 22 samples (73.3%) (Table 1).
| Groups | Variable | n (%) | |
|---|---|---|---|
| Gender | Male | 11 (50) | |
| Female | 11 (50) | ||
| Hydrogel | Wound location | Sacrum | 8 (26.67) |
| Right trochanter | 1 (3.33) | ||
| Left trochanter | 1 (3.33) | ||
| PRP injection | Wound location | Sacrum | 6 (20) |
| Right ischium | 2 (6.67) | ||
| Right trochanter | 1 (3.33) | ||
| Right malleolus | 1 (3.33) | ||
| Topical PRP | Wound location | Sacrum | 8 (26.67) |
| Left ischium | 1 (3.33) | ||
| Left malleolus | 1 (3.33) | ||
In this study, clinical wound assessments were based on the objective measurement scale, the PUSH score. The normality test showed that the data were mostly normally distributed (p > 0.05). Consequently, a repeated ANOVA test was conducted to perform a comparative analysis of the average PUSH score assessments across each wound care group. Several clinical images of wounds in each treatment group on day 0, 6, 12, 18 and 21 demonstrated various stages of wound development (Figures 7-9).
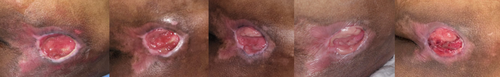
In the observation of the study, the average PUSH score in the control group on day 0 was 13.65 ± 1.64, on day 6 was 13.40 ± 1.63, on day 12 was 13.15 ± 1.89, on day 18 was 12.70 ± 2.66, and on day 21 was 13.20 ± 2.89. In the PRP injection group, the average PUSH score on day 0 was 14.15 ± 1.53, on day 6 was 13.75 ± 1.16, on day 12 was 13.00 ± 1.05, on day 18 was 12.75 ± 1.27, and on day 21 was 11.90 ± 2.08. In the topical PRP group, the average PUSH score on day 0 was 12.85 ± 3.00, on day 6 was 12.70 ± 1.96, on day 12 was 12.55 ± 2.36, on day 18 was 12.05 ± 2.11, and on day 21 was 11.75 ± 2.02. A significant difference in the average PUSH score was found between the PRP injection and topical PRP treatment groups on day 0, 6, 12, 18 and 21 (p < 0.05). (Table 2).
| Groups | Day | PUSH score (mean ± SD) | p |
|---|---|---|---|
| Control (hydrogel) | 0 | 13.65 ± 1.64 | 0.304 |
| 6 | 13.40 ± 1.63 | ||
| 12 | 13.15 ± 1.89 | ||
| 18 | 12.70 ± 2.66 | ||
| 21 | 13.20 ± 2.89 | ||
| PRP injection treatment | 0 | 14.15 ± 1.53 | 0.001 |
| 6 | 13.75 ± 1.16 | ||
| 12 | 13.00 ± 1.05 | ||
| 18 | 12.75 ± 1.27 | ||
| 21 | 11.90 ± 2.08 | ||
| Topical PRP treatment | 0 | 12.85 ± 3.00 | 0.046 |
| 6 | 12.70 ± 1.96 | ||
| 12 | 12.55 ± 2.36 | ||
| 18 | 12.05 ± 2.11 | ||
| 21 | 11.75 ± 2.02 |
- a Repeated ANOVA test based on observations of researchers and patient caretaker.
We conducted a post hoc test on each day based on the wound care group, to determine the range of days with the most significant mean PUSH score differences (Table 3). In the hydrogel group, all days had a p-value > 0.05, indicating no significant difference across the observation period. In the PRP injection group, a significant difference was observed starting on day 12 (p = 0.045). In the topical PRP group, a significant difference was observed starting on day 21 (p = 0.001) (Table 3).
| Groups | Day | vs | Day | p |
|---|---|---|---|---|
| Control (hydrogel) | 0 | 6 | 0.513 | |
| 12 | 0.204 | |||
| 18 | 0.097 | |||
| 21 | 0.422 | |||
| 6 | 12 | 0.397 | ||
| 18 | 0.234 | |||
| 21 | 0.735 | |||
| 12 | 18 | 0.295 | ||
| 21 | 0.906 | |||
| 18 | 21 | 0.148 | ||
| PRP injection | 0 | 6 | 0.196 | |
| 12 | 0.045 | |||
| 18 | 0.009 | |||
| 21 | 0.017 | |||
| 6 | 12 | 0.129 | ||
| 18 | 0.034 | |||
| 21 | 0.015 | |||
| 12 | 18 | 0.440 | ||
| 21 | 0.111 | |||
| 18 | 21 | 0.113 | ||
| Topical PRP | 0 | 6 | 0.726 | |
| 12 | 0.536 | |||
| 18 | 0.141 | |||
| 21 | 0.034 | |||
| 6 | 12 | 0.576 | ||
| 18 | 0.013 | |||
| 21 | 0.000 | |||
| 12 | 18 | 0.015 | ||
| 21 | 0.002 | |||
| 18 | 21 | 0.051 |
In this study, a comparative analysis of the average ∆ PUSH score between day 0 and day 21 across the wound care groups was conducted. In wounds observed by both researchers and patient caretakers, the average ∆ PUSH score in the control group was 0.45 + 1.69, whilst in the PRP injection group, it was 2.25 + 2.07, and in the topical PRP treatment group it was 1.10 + 1.39. The comparison revealed a significant difference between the control group and the PRP injection treatment group in the average ∆ PUSH score (p = 0.047) (Table 4).
| Groups | ΔPUSH score (mean ± SD) | p |
|---|---|---|
|
Control (hydrogel) PRP injection |
0.45 ± 1.69 2.25 ± 2.07 |
0.047 |
|
Control (hydrogel) Topical PRP |
0.45 ± 1.69 1.10 ± 1.39 |
0.360 |
|
PRP injection Topical PRP |
2.25 ± 2.07 1.10 ± 1.39 |
0.162 |
- a Unpaired t-test based on observations of researchers and patient caretakers.
Microscopic assessment was conducted in this study to evaluate the collagen density before (D0) and after treatment (D21). The examination was carried out by the anatomical pathology department using ImageJ software. The tissue preparations were converted into images, with colour separation applied: pink for collagen, purple for fibroblasts and inflammatory cells, and pale pink for the background (Figure 10). The collagen density results were expressed as a percentage of the area.
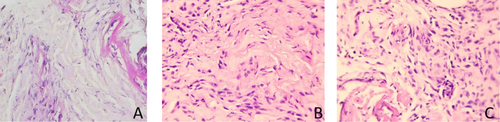
In the analysis of collagen density measurements, a paired t-test was conducted to compare the average collagen density on day 0 and day 21 within each group. For the comparison of the average change (Δ) in collagen density between day 0 and day 21 amongst the three wound care groups, an unpaired t-test was used.
In the comparison of the average collagen density on day 0 (before treatment) and day 21 (after treatment), an increase was observed amongst all groups. The average collagen density (expressed as a percentage of the area) in the control group on day 0 was 39.92% ± 3.64%, and on day 21 was 45.47% ± 6.78%. In the PRP injection group, the average collagen density on day 0 was 33.83% ± 10.63%, and on day 21 was 49.09% ± 8.30%. In the topical PRP group, the average collagen density on day 0 was 35.94% ± 12.44%, and on day 21 was 49.93% ± 10.33%. Table 5 shows that in the PRP injection group, the p-value was 0.001, indicating a significant increase in collagen density from day 0 to day 21 in wounds treated with PRP injection.
| Groups | Day | Collagen density (mean ± SD) | p |
|---|---|---|---|
| Control (hydrogel) | 0 | 39.92 ± 3.64 | 0.072 |
| 21 | 45.47 ± 6.78 | ||
| PRP injection | 0 | 33.83 ± 10.63 | 0.001 |
| 21 | 49.09 ± 8.30 | ||
| Topical PRP | 0 | 35.94 ± 12.44 | 0.108 |
| 21 | 49.93 ± 10.33 |
- a Paired t-test based on observations of researchers and anatomical pathologists.
Table 6 shows the difference in mean collagen density (Δ collagen density) between day 0 and day 21 amongst the wound care groups. In the control group, the Δ collagen density was 5.55% ± 5.12% area. In the PRP injection group, it was 15.26% ± 4.08% area, and in the topical PRP group, it was 14.00% ± 15.17% area. The comparison of Δ collagen density between the control group and the PRP injection group revealed a significant difference (p = 0.011).
| Groups | Δ collagen density (mean ± SD) | p |
|---|---|---|
|
Control (hydrogel) PRP injection |
5.55 ± 5.12 15.26 ± 4.08 |
0.011 |
|
Control (hydrogel) Topical PRP |
5.55 ± 5.12 14.00 ± 15.17 |
0.271 |
|
PRP injection Topical PRP |
15.26 ± 4.08 14.00 ± 15.17 |
0.862 |
- a Unpaired t-test based on observations of researchers and anatomical pathologists.
4 Discussion
In this study, the number of female and male patients was equal: 11 patients (50%) were female and 11 patients (50%) were male. This contrasts with the findings of Aboud et al., who reported a slightly higher incidence of pressure ulcers in women [25]. Additionally, a study by Kottner et al. suggested that men are more likely to experience pressure ulcers. However, gender is not considered an independent risk factor for pressure ulcers, as the difference between men and women in terms of incidence is minimal [26].
The most common location of pressure ulcers in this study was the sacrum, with 22 samples (73.3%). This finding aligns with the observations of Aboud et al. and Soedjana et al., who reported that the hip and sacrum areas account for about two-thirds of all pressure ulcers [25, 27]. These pressure ulcers are primarily caused by prolonged exposure to friction and shearing pressure over bony prominences. Several intrinsic factors also contribute to their development, including skin fragility, reduced blood flow, muscle atrophy, spinal cord injuries, nutritional deficiencies, and moisture due to urinary and/or faecal incontinence.
In the clinical observation of wounds using the PUSH score from day 0 to 21 by both researchers and patient caretakers, statistically significant results were obtained based on repeated ANOVA tests in the PRP injection and topical PRP groups, with a p-value < 0.05 (Table 2). This indicates that pressure ulcer treatment with PRP injection and topical PRP leads to significant clinical improvement. Post hoc testing revealed that the PRP injection treatment group showed a significant difference in mean PUSH score starting from day 12, whilst the topical PRP treatment group demonstrated a significant difference starting on day 21 (Table 3). Table 4 shows that the control group and the PRP injection group had a statistically significant difference in the Δ PUSH score (p = 0.047).
Overall, the findings of this study indicate clinical improvement in each wound care group, but statistically, only the control and PRP injection treatment groups showed a significant difference. When comparing the PRP injection and topical PRP groups, although the Δ PUSH score in the PRP injection treatment group was higher than that in the topical PRP group, this difference was not statistically significant. This suggests that by day 21, both the PRP injection and topical PRP groups showed similar clinical improvement in wound healing.
Studies by Elbarbary et al. and Rani et al. have suggested that PRP injection is more effective than topical PRP in the treatment of chronic wounds [15, 28]. Both studies found that PRP injection led to better wound healing rates compared to topical PRP, due to more effective delivery of growth factors to the wound with minimal side effects. PRP injections can provide a higher platelet concentration to the wound's edge and depth, ensuring a sustained release of growth factors for a longer period compared to topical PRP [15, 28]. Additionally, Rani et al. noted that topical PRP may be less practical, as the PRP fluid tends to spread beyond the wound edge or get absorbed into the secondary dressing, reducing its effectiveness [28].
Elbarbary et al. found that PRP injection led to a higher and faster rate of chronic wound healing, as evidenced by smaller wound sizes and shorter healing times compared to topical PRP [15]. The theoretical explanation for the superiority of PRP injection over topical PRP, as outlined by Rani et al., is that PRP injections release growth factors both above and around the wound, promoting cell repair and regeneration. The fibrin present in the injected PRP also provides a foundation for cell repair, stimulates tissue regeneration, and aids in wound contraction. Furthermore, the higher levels of white blood cells and monocytes in PRP injections help inhibit or eliminate pathogens, clear necrotic cells, and play an anti-infective role, ensuring a longer and more effective release of growth factors than topical PRP [28].
These findings explain why the PRP injection group showed a greater reduction in ∆ PUSH score and why its effects were observed more quickly compared to the topical PRP group.
Microscopically, Table 5 shows a significant difference in the mean collagen density on day 0 and day 21 in the PRP injection group, with a p-value of 0.001. Table 6 further reveals that the PRP injection group exhibited the highest difference in collagen density amongst all three groups. A significant difference in collagen density was observed between the control and PRP injection groups, with a p-value of 0.011. These findings align with a study by Xu et al., which demonstrated that collagen deposition significantly increased in the group treated with PRP injection compared to the control group. Their study concluded that PRP can provide a supportive environment for tissue remodelling [29].
According to the TIMERS (Tissue Management, Inflammation and Infection, Moisture Balance, Edge or Epithelial Advancement, Regeneration and Repair and Social Factors) wound healing model, a deficiency in growth factors can impair the advancement of the wound edge. PRP injection helps control local and systemic inflammation through various proteins, including IL-1 receptor antagonists, vascular endothelial growth factor, platelet-derived growth factor, and epidermal growth factor. These proteins play crucial roles in promoting tissue repair and wound healing [30, 31].
In this study, the protocol for administering topical PRP and PRP injections involved 4 sessions, with each administration consisting of 0.01 mL/cm2 (adjusted according to wound size) every week. This dose was based on the study by Görgü et al., which investigated PRP injection into target tissue with various doses per cm2 area: 0.01, 0.03 and 0.05 mL [32]. Another study by Güleç et al. used a dose of 0.01 mL per cm2 of wound area in Sprague Dawley rats [33]. The author used the smallest dose from various existing studies.
Additionally, in a study by Abdelhafez et al., PRP treatment was administered every 2 weeks until the wound appeared to heal, with a maximum of five sessions and a follow-up period of 10 weeks [34]. In their study, the PRP volume used was based on wound size (5 mL per 100 cm2), which was larger than the dose used in our research. Abdelhafez et al. injected a total of 0.2–0.4 mL of PRP around the wound, spaced 1 cm apart and administered subcutaneously. Their results showed that 96% of wounds in the PRP injection and topical treatment group achieved complete healing, compared to 88% in the topical PRP-only group [34].
Despite using a smaller dose of PRP, the approach in our study demonstrated significant effects on the clinical progression of wound healing, suggesting that even minimal doses of PRP can provide therapeutic benefits.
In this study, PRP was prepared using a two-stage centrifugation method. Peripheral venous blood was collected from the patient's arm into a 10 cc vacuum tube containing sodium citrate anticoagulant. The first stage of centrifugation was performed at 900 rpm at room temperature for 5 min. Afterward, the plasma fraction was carefully pipetted out. In the second stage, centrifugation was carried out at 1500 rpm at room temperature for 15 min. The upper layer, which consisted of PRP, was then extracted using a pipette. The PRP suspension was subsequently mixed with a calcium gluconate solution to achieve a 1:10 concentration. This process resulted in 2.5 mL of PRP.
This method differs from those used in other studies. For example, in a review by Dhurat and Sukesh, PRP was prepared from 30 cc of venous blood to yield 3–5 cc of PRP, with various protocols for preparation [35]. One such method by Amanda et al. involved processing 3.5 mL of blood at 100 × g for 10 min (first round), followed by 400 × g for 10 min (second round). In this method, 2/3 of the remaining plasma was withdrawn, resulting in platelet recovery of 70%–80% and a platelet concentration five times greater, which helped maintain platelet integrity and viability [35].
Karina et al. reported the use of PRP in the treatment of stage IV pressure ulcers in ICU patients with severe COVID-19. After debridement, the wound was treated with topical hyaluronic acid (2 mg), silver sulfadiazine (10 mg), and tulle framycetin sulphate. The first dose of PRP was administered both as an intralesional injection and an intravenous infusion on day 1. Subsequent PRP treatments and evaluations were conducted every 2 weeks. Clinical wound healing was observed on the 44th day. PRP was prepared using 24 mL of venous blood, which was divided into eight tubes containing sodium citrate. The blood was centrifuged at 1000 rpm for 10 min, and the separated plasma was then centrifuged at 3000 rpm for 10 min. The platelets were isolated by removing the excess platelet-poor plasma, leaving 2.5 mL of PRP. The final activation process involved adding 0.15 mL of calcium activator to each tube. After the clot was removed, 10 mL of normal saline was added to each tube [20].
A study by Liu et al. on the effects of topical PRP for the management of pressure ulcers used 10–20 mL of venous blood, which was centrifuged for 10 min at 1500 rpm to separate the red blood cell layer at the bottom. The plasma was then resuspended and subjected to a second-stage centrifugation for 10 min at 1500 rpm. Then, the supernatant layer was separated and mixed with calcium and thrombin. Topical PRP was applied every 7 days, with a total of 3 administrations. After each application, the wound was covered with a sterile dressing. The study found that the topical PRP group showed a significantly higher wound healing rate (92.16%) compared to the control group (p < 0.05) [14].
Numerous studies have been conducted on the administration of PRP for chronic wounds, and it is widely accepted as a safe, effective, and promising therapy compared to conventional treatments. However, before this study, there were no studies in the literature that compared the effects of PRP injection and topical PRP on pressure ulcer healing, particularly in patients in Indonesia. The dose of PRP used in this study, specifically 0.01 mL/cm2, has emerged as a novel approach, resulting in significant clinical improvement of the wound. Another key finding of this study is that the PRP injection group showed faster clinical improvement than the topical PRP group, with notable effects beginning on day 12. In contrast, the topical PRP group demonstrated significant clinical improvement starting on day 21.
The limitations of this study include the relatively small sample size of 30 pressure ulcer cases observed over 21 days. Additionally, the systemic effects of PRP on patients and the potential influence of various pressure ulcer locations on the study outcomes were not addressed. Limited funding also restricted the ability to conduct more comprehensive histopathological examinations. To improve and expand upon these findings, future research could involve randomised controlled trials and multicentre studies with larger sample sizes and extended observation periods. Further studies should also examine the comparative effects of PRP injection and topical PRP on a broader scale whilst standardising the PRP preparation method, dosage and administration schedule.
5 Conclusions
We concluded PRP injection significantly reduces PUSH scores and increases collagen density compared to the control group. By day 21, no significant differences were observed between PRP injection and topical PRP. However, PRP injection demonstrated faster clinical improvement, with significant changes in PUSH score evident by day 12, compared to day 21 for topical PRP.
Acknowledgements
The authors would like to express their gratitude to Hasan Sadikin General Hospital, Bandung, Indonesia, as a tertiary referral hospital for providing the authors with the opportunity to conduct research on pressure ulcer patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.





