RETRACTED: The impact of ulinastatin on wound infection and healing in patients with burn wounds: A meta-analysis
Abstract
Burn injuries result in localised tissue damage and precipitate systemic responses; routine clinical treatments, which typically include metabolic nutritional support and anti-infection therapies, do not yield optimal outcomes. Therefore, we aimed to systematically evaluate the effects of ulinastatin on wound infection and healing in patients with burns to provide reliable evidence-based recommendations for burn treatment. An electronic search of the Web of Science, PubMed, Cochrane Library, Embase, Wanfang, Chinese Biomedical Literature Database, and China National Knowledge Infrastructure databases, supplemented by manual searches, was conducted from database inception to October 2023 to collect randomised controlled trials (RCTs) assessing the efficacy of ulinastatin for the treatment of burns. Two researchers screened all retrieved articles according to the inclusion and exclusion criteria; the included studies were evaluated for quality, and the relevant data were extracted. Stata 17.0 software was employed for data analysis. Overall, 8 RCTs with 803 patients were included, with 404 and 399 in the ulinastatin and conventional treatment groups, respectively. The analysis revealed that wound infections (odds ratio [OR] = 0.08, 95% CI: 0.02–0.35, p = 0.001) and complications (OR = 0.21, 95% CI: 0.10–0.42, p < 0.001) were significantly lower, and wound healing time (standardised mean differences [SMD] = −1.31, 95% CI: −2.05 to −0.57, p = 0.001) was significantly shorter, in the ulinastatin groups than in the control group. This meta-analysis revealed that ulinastatin can effectively reduce the incidence of wound infections and complications and significantly shorten the duration of wound healing in patients with burns, thereby promoting early recovery in these patients.
1 INTRODUCTION
Burns are a serious public health concern worldwide, with approximately 265 000 individuals dying as a result of burns each year.1 The majority of these deaths are due to burn infections; disruption of the skin barrier, interruption of the vascular supply, systemic immunosuppression, and microbial invasion are all important causes of burn susceptibility.2 As a loss of the body's natural barrier, skin burns lead to dysregulation of the immune system, increasing the patient's risk of infection. Furthermore, the interaction between inflammatory mediators is an important factor leading to dysregulation of the patient's innate immune response, which in turn leads to further complications, such as systemic inflammatory response syndrome, severe sepsis, and multiple organ dysfunction syndrome (MODS). Moreover, during the treatment of burns, spatial and regional bacterial changes promote the development of multi-drug resistance. Multi-drug-resistant bacterial infections are becoming increasingly serious, especially in the intensive care unit, and the “waterfall” inflammatory response caused by extensive burns is becoming more difficult to control.3 Thus, controlling burn infections, inhibiting the early initiation of post-infectious inflammatory responses, and reducing the release of systemic inflammatory mediators are key aspects to improve the prognosis and survival of patients with extensive burns.4, 5
Ulinastatin belongs to a group of endogenous human anti-inflammatory factors which are protease inhibitors extracted from human urine that broadly inhibit inflammation and have therapeutic effects on a wide range of diseases with the same physiological and pharmacological basis. Currently, ulinastatin is widely used clinically in the treatment of acute pancreatitis and acute circulatory failure.6 It has anti-inflammatory cytokine properties and anti-oxidative stress activity. It is a trypsin inhibitor with two sugar chains at the N-terminal and O-terminal ends, allowing the protein to contain two functional regions that partially overlap; these generate multiple enzyme-binding target sites and inhibit a variety of serine proteases, such as trypsin, chymotrypsin, and fibrinolytic enzymes. In animal burn models, ulinastatin has been shown to significantly reduce the production of inflammatory mediators and oxygen-free radicals and protect numerous organ functions by inhibiting lipid peroxidation. Ulinastatin has also been reported to have a protective effect against sepsis-induced multi-organ damage and reduce the mortality rate of patients with extensive burns.5, 7 In recent years, studies on ulinastatin treatment of patients with burns have gradually increased, establishing that its effect is dose-related; however, its effectiveness and safety remain controversial. In this meta-analysis, we comprehensively evaluated the effects of ulinastatin on wound infection and healing in patients with burns to provide reliable evidence-based data regarding burn treatment.
2 MATERIALS AND METHODS
2.1 Literature search
An electronic search of the Web of Science, PubMed, Cochrane Library, Embase, Wanfang, Chinese Biomedical Literature Database, and China National Knowledge Infrastructure databases was conducted from database inception to October 2023 to collect randomised controlled trials (RCTs) on the administration of ulinastatin for the treatment of patients with burns. The literature search was performed by combining subject and free words using the following keywords: ulinastatin, burns, and burn wounds. This was supplemented by a manual search to examine the references of the included literature for relevant studies that were not initially retrieved.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
The inclusion criteria were as follows: (1) participants: patients with a total burn area >30% of the total body surface area (TBSA) or third degree burns covering ≥20% of the TBSA, regardless of sex; (2) intervention: ulinastatin treatment was administered to the experimental group, and conventional treatment was implemented in the control group; (3) outcomes: wound infection, complications, and wound healing time; (4) study design: RCT.
2.2.2 Exclusion criteria
The exclusion criteria comprised inconsistent interventions; studies with unavailable raw data, inaccurate data descriptions, or missing data; duplicate articles; and conferences, reviews, lectures, and case reports.
2.3 Data extraction and quality assessment
Two researchers independently screened the literature and extracted data from the included studies; disagreements were resolved through discussion and negotiation or adjudication by a third researcher. Basic characteristics of the studies (authors, year of publication, sample size, sex, and age) and outcome indicators (wound infection, complications, and wound healing time) were extracted. The risk of bias of the included studies was evaluated using the quality evaluation criteria recommended by the Cochrane Handbook 6.08: (1) random sequence generation, (2) allocation concealment, (3) blinding of subjects and researchers, (4) blinding of outcome assessors, (5) completeness of outcome data, (6) selective reporting of results, and (7) other biases. Each entry was classified as high, unclear, or low risk, according to the likelihood of the risk of bias. After assessing all the included literature according to the evaluation table, the results were cross-checked by two researchers and any inconsistencies were adjudicated by a third researcher.
2.4 Statistical analyses
Stata 17.0 software was employed for data analysis. Count data are expressed as odds ratios (OR) and 95% confidence intervals (95% CI) and measurement data as standardised mean differences (SMD) and 95% CI. I2 and χ2 tests were applied to determine the presence of heterogeneity.9 If there was no significant heterogeneity (I2 <50%, p > 0.1), a fixed-effects model was applied; otherwise, the random-effects model was chosen. The robustness of the results was determined using sensitivity analysis. If more than 10 papers were included in the analysis, a funnel plot was drawn to assess publication bias.
3 RESULTS
3.1 Study characteristics
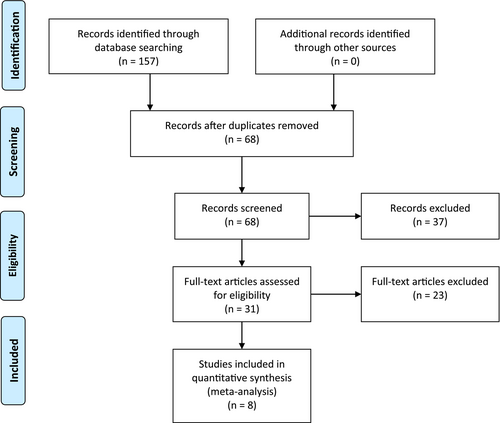
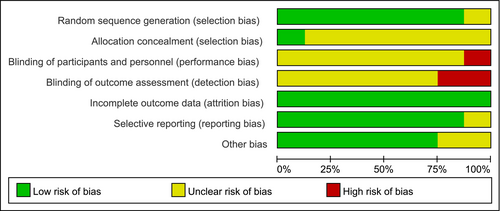
The literature screening process is illustrated in Figure 1. The database search identified a total of 157 relevant articles, which were imported into Endnote X9, a literature management system, for duplicate screening; consequently, 89 duplicates were removed. After reviewing the titles and abstracts, 37 studies that were not relevant were removed. Lastly, the full texts were read, and 8 RCTs were ultimately included.10-17 The risk of bias of the included studies is shown in Figure 2. The basic characteristics of these studies are listed in Table 1. A total of 803 patients were included, with 404 and 399 patients in the ulinastatin and conventional treatment groups, respectively.
| Author | Year | No. of patients | Age (years) | Sex (male/female) | |||
|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | ||
| Zhao | 2022 | 86 | 86 | 43.08 ± 1.72 | 42.79 ± 1.36 | 51/35 | 50/36 |
| Xie | 2020 | 50 | 50 | 40.22 ± 3.13 | 41.23 ± 3.09 | 27/23 | 28/22 |
| Wang | 2012a | 42 | 38 | 34.76 ± 9.73 | 65/15 | ||
| Wang | 2012b | 20 | 20 | 33.5 ± 11.2 | 31.03 ± 11.34 | 15/5 | 13/7 |
| Ma | 2016 | 13 | 12 | 36 ± 2 | 35 ± 2 | 9/4 | 7/5 |
| Li | 2021 | 43 | 43 | 42.26 ± 10.65 | 41.66 ± 10.43 | 22/21 | 25/18 |
| Zheng | 2018 | 110 | 110 | 15–45 | 186/34 | ||
| Jin | 2018 | 40 | 40 | 47.5 ± 9.2 | 47.4 ± 9.1 | 29/11 | 30/10 |
3.2 Wound infection
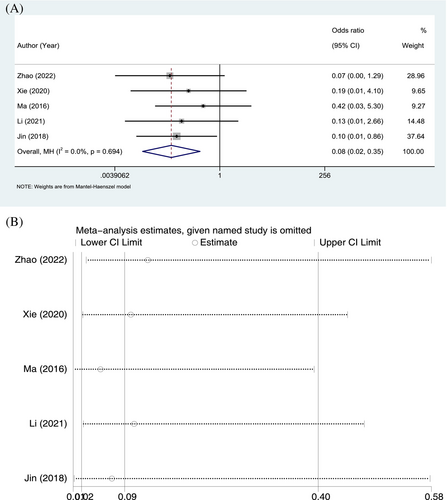
Wound infections were reported in 5 RCTs. Of the 232 patients in the ulinastatin group, 2 developed wound infections, with an incidence of 0.86%; in contrast, 21 of the 231 patients in the conventional treatment group developed wound infections, with an incidence of 9.09%. The homogeneity of the included studies was good (p = 0.694, I2 = 0.0%), and a fixed-effects model was applied. In terms of wound infection, the incidence was significantly lower in the ulinastatin group than in the conventional treatment group (OR = 0.08, 95% CI: 0.02–0.35, p = 0.001; Figure 3A). The sensitivity analysis was performed by individually excluding studies and revealed no significant changes in the combined OR, indicating stable results and reliable conclusions (Figure 3B).
3.3 Complications
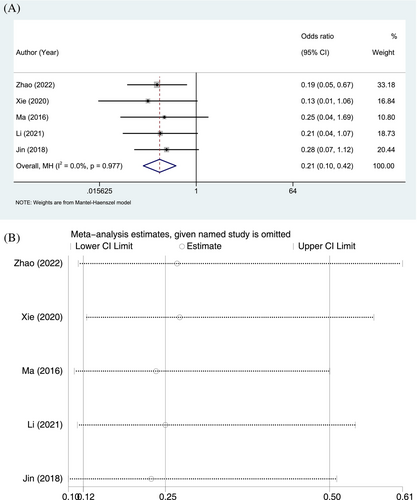
Complications were reported in 5 RCTs. In the ulinastatin group, 11 of 232 patients experienced complications, with an incidence rate of 4.74%, whereas 43 of 231 patients in the conventional treatment group, with an incidence rate of 18.61%. The homogeneity of the included studies was good (p = 0.977, I2 = 0.0%), and a fixed-effects model was applied. In terms of complications, the incidence was significantly lower in the ulinastatin group than in the conventional treatment group (OR = 0.21, 95% CI: 0.10–0.42, p < 0.001; Figure 4A). As the sensitivity analysis revealed no significant changes in the combined OR, the results and conclusions were considered to be stable and reliable (Figure 4B).
3.4 Wound healing time
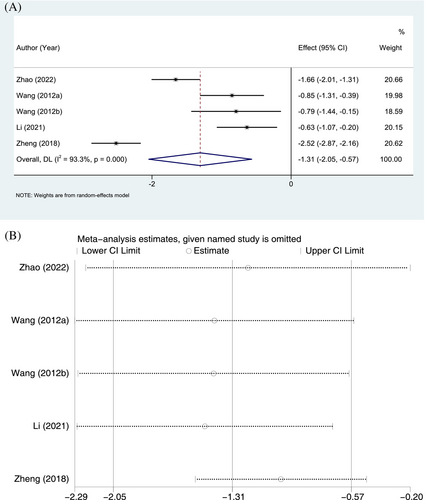
Five RCTs reported wound healing time, with 301 and 297 patients in the ulinastatin and conventional treatment groups, respectively. Significant heterogeneity was observed (p < 0.001, I2 = 93.3%), and a random-effects model was chosen. In terms of wound healing time, the ulinastatin group was significantly shorter than that in the conventional treatment group (SMD = −1.31, 95% CI: −2.05 to −0.57, p = 0.001; Figure 5A). Sensitivity analyses indicated no significant changes in the combined SMD, demonstrating stable results and reliable conclusions (Figure 5B).
4 DISCUSSION
Infection secondary to severe burns can cause systemic inflammatory response syndrome, MODS, and even multiple organ failure, thereby constituting one of the primary causes of death in patients with severe burns.18, 19 Thus, the key to improving the prognosis and survival of these patients is to control post-burn infections, inhibit the early initiation of post-infectious inflammatory responses, and reduce the release of systemic inflammatory mediators.3, 20
Ulinastatin was first used for the treatment of acute pancreatitis, and in recent years, it has also been widely used in treating patients with acute and critical illnesses such as acute circulatory failure. Ulinastatin has a protective effect against sepsis-induced multi-organ damage, inhibiting oxidative-induced hyperpermeability in human endothelial cells and decreasing the levels of the cytokines tumour necrosis factor-α and interleukin-6 in damaged organs.7, 21 Chen et al.22 found that, in healthy adults, high-dose ulinastatin was well tolerated up to a maximum dose of 8 million units. Basic science and clinical research studies have shown that ulinastatin can inhibit the activity of various proteases, downregulate the formation of inflammatory factors and excessive superoxide, and scavenge oxygen-free radicals, thereby protecting the heart, lungs, brain, liver, kidneys, and other organs, as well as improving immune function and coagulation.23, 24 In this meta-analysis, we collected RTCs on ulinastatin treatment for burns from Chinese and English databases and conducted a systematic analysis of their conclusions to provide evidence-based recommendations for the treatment of burns with ulinastatin. Overall, 8 RCTs were included, and our results showed that ulinastatin was more effective than conventional treatment in terms of reducing wound infections, complication rates, and wound healing times.
Studies have shown that ulinastatin can reduce the degree of liver injury by decreasing the production of pro-inflammatory cytokines in the peripheral blood, scavenging oxygen free radicals, and improving hepatic perfusion; thus, any impairments of hepatic function may be mitigated and prevented. Furthermore, the effect of high doses of ulinastatin is more significant than that of conventional doses.25, 26 High-dose ulinastatin can improve the circulatory status during shock by scavenging for superoxide and oxygen-free radicals; it can also increase tissue perfusion, reduce renal ischemia–reperfusion injury, and reduce the kidney damage caused by inflammatory factors, thus protecting renal function.4, 27 With early application of high-dose ulinastatin, respiratory function indicators and inflammatory factor levels are significantly improved in patients with severe burns; therefore, high-dose ulinastatin may protect the lung function of patients with severe burns and delay the progress of the inflammatory response. This effect is also more significant with high-dose ulinastatin than with conventional doses.28, 29 When wound infections of patients with burns were well controlled, these were negatively correlated with the incidence of complications and wound healing time, which is in line with our findings.
The relatively small number of articles included in this study is one of its limitations. Moreover, the optimal dosage and duration of ulinastatin treatment throughout the disease progression of burns is unknown, and the dose at which significant adverse effects occur is unclear. This needs to be further explored in large-sample, high-quality, multicentre, clinical RCTs.
5 CONCLUSION
In conclusion, our results demonstrated that ulinastatin treatment in patients with burns can effectively reduce the incidence of wound infections and complications, significantly shorten the wound healing time, and promote the early recovery of patients.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




