Recent advances on 3D-bioprinted gelatin methacrylate hydrogels for tissue engineering in wound healing: A review of current applications and future prospects
Jiaming Wan and Hongyu Wang contributed equally to this work.
Abstract
Advancements in 3D bioprinting, particularly the use of gelatin methacrylate (GelMA) hydrogels, are ushering in a transformative era in regenerative medicine and tissue engineering. This review highlights the pivotal role of GelMA hydrogels in wound healing and skin regeneration. Its biocompatibility, tunable mechanical properties and support for cellular proliferation make it a promising candidate for bioactive dressings and scaffolds. Challenges remain in optimizing GelMA hydrogels for clinical use, including scalability of 3D bioprinting techniques, durability under physiological conditions and the development of advanced bioinks. The review covers GelMA's applications from enhancing wound dressings, promoting angiogenesis and facilitating tissue regeneration to addressing microbial infections and diabetic wound healing. Preclinical studies underscore GelMA's potential in tissue healing and the need for further research for real-world applications. The future of GelMA hydrogels lies in overcoming these challenges through multidisciplinary collaboration, advancing manufacturing techniques and embracing personalized medicine paradigms.
1 INTRODUCTION
Advancements in tissue engineering, particularly the revolutionary technology of 3D bioprinting, have ushered in a new era of regenerative medicine.1, 2 Central to this innovation is gelatin methacrylate (GelMA), a bioink derived from the natural polymer gelatin, known for its optimal physicochemical and biological properties.2, 3 GelMA undergoes modification to allow photopolymerization, addressing the instability of gelatin at physiological temperatures.4, 5 With demonstrated biological functionality supporting cell adhesion, proliferation and differentiation, GelMA proves ideal for 3D cell culture and various tissue engineering applications, including skin regeneration and wound healing.6-8
Despite its benefits, GelMA-based hydrogels face challenges such as mechanical integrity and rapid degradation, necessitating careful optimization for structural robustness in dynamic physiological environments.9, 10 Researchers have tackled these challenges through composite bioinks, integrating GelMA with polymers or nanomaterials to enhance physical properties and functional outcomes.11 Additionally, the incorporation of bioactive molecules into GelMA hydrogels aims to replicate the natural extracellular matrix's microenvironment for successful tissue regeneration.12-14 In skin tissue engineering, GelMA's versatility shines through its formulation into various formats like 3D bioprinted scaffolds, injectable gels and electrospun fibrous membranes, addressing unique therapeutic needs.15, 16 Notably, GelMA's low viscosity allows for delivery to irregular wound sites, forming robust scaffolds through in situ photo cross-linking.17 Empirical studies underscore GelMA hydrogels' efficacy in promoting wound healing and skin tissue regeneration, facilitating essential processes like cell migration, neovascularization and collagen secretion.18 Furthermore, GelMA's capacity for localized drug delivery offers a strategic advantage in wound management.19-21 Our review comprehensively explores GelMA hydrogels' role in the broader context of 3D bioprinting, emphasizing their transformative impact on skin regeneration and wound healing. Integrating insights from molecular foundations to practical applications and challenges, our review provides an informed vision for future research trajectories and therapeutic innovations.
2 CURRENT APPLICATIONS OF 3D-BIOPRINTED GELATIN METHACRYLATE HYDROGELS IN WOUND HEALING
The fusion of 3D bioprinting with biomaterials like GelMA is revolutionizing regenerative medicine and wound healing applications.22, 23 GelMA's versatility and biocompatibility are vital to this progress, leading to the development of tailored wound dressings and scaffolds.4 Figure 1 demonstrates how GelMA hydrogels support wound healing through cellular interactions, underscoring the advancements in 3D-printed GelMA hydrogels for these purposes.
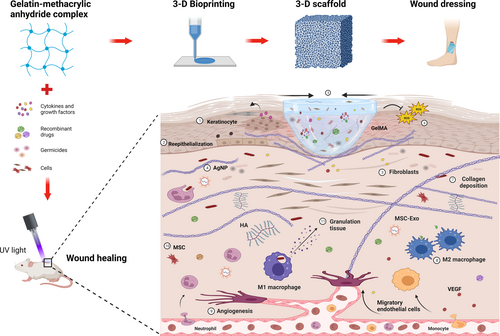
2.1 3D bioprinting and advanced structures
The goal of 3D bioprinting is to speed up the healing process and reduce difficulties for patients while repairing damaged tissues. GelMA hydrogel sheets containing gingival-derived stem cells were produced by Ansari et al., which speed up wound healing and may be used in both dentistry and plastic surgery.24 Another group led by Guan et al.25 developed a pro-angiogenic GelMA and hyaluronic acid methacryloyl (HAMA) peptide patch. Effective in pig skin models, a 3D-printed GelMA patch containing VEGF-mimicking peptides was also developed by Jang et al.26 Ibañez et al. studied GelMA scaffolds for scar reduction by suppressing myofibroblast activation, important for decreasing post-trauma scarring.27 Another group developed a novel in vivo printing technology employing a portable printer to directly apply VEGF-infused GelMA hydrogel to wounds, doing away with the need for sutures in the process and greatly enhancing wound care.28 To construct 3D scaffolds with superior structural integrity and biocompatibility,29 Xu et al. ingeniously combined cellulose nanofibrils with GelMA to produce a bioink. Additionally, Xia et al. coupled adipose-derived mesenchymal stem cells (ADSCs) with a 3D-printed GelMA hydrogel, which decreased oxidative stress and cell death and improved diabetic wound healing30 (Supplementary Table 1).
2.2 Anti-microbial and oxidative solutions
Treatments for microbial infections and wound healing have come a long way in recent years.31 Drug-resistant microorganisms are a major barrier to medical progress, but new methods are being developed to tackle them.32 Antibacterial activity and improved skin regeneration,33 this was shown by a cephalexin-loaded PCL/GelMA nanofibrous dressing developed by Bakhsheshi-Rad et al.
Hydrogel with quick curing and high adhesion, inspired by the mussel, with antibacterial qualities was produced by He and colleagues.34 Hang et al. have designed a near-infrared light-responsive nanovehicle in a gelatin-based hydrogel for increased antibacterial efficacy and skin healing.35 Silver nanoparticles destroy bacteria and accelerate wound healing, which is why they were studied at Jahan's lab.19
The pH and enzyme-responsive hydrogel for regulated polymyxin B release, developed by Dong et al., promotes healing throughout the inflammatory phase,36 making it a useful tool in the therapy of chronic wounds.37-39 The angiogenesis and immunological modulation facilitated by Yan et al.'s surfactin-enhanced hydrogel accelerated wound healing in diabetic rats.40 Antibacterial and anti-inflammatory activities were observed in a GelMA-based scaffold using Artemisia argyi extract developed by Xue and colleagues41 (Supplementary Table 2). To help diabetes patients recover faster from wounds, Zahid et al.42 created a GelMA hydrogel patch containing S-Nitroso-N-acetylpenicillamine (SNAP).
2.3 Angiogenesis and vascularization
Angiogenesis, necessary for tissue regeneration and wound healing, may be reduced in diseases like diabetes and fibrosis, leading to poor healing results.33, 43, 44 Improving wound healing and angiogenesis in diabetics, Chen et al. constructed a hydrogel scaffold and then improved it with desferrioxamine (DFO) to increase cell survival and activate hypoxia-inducible factor 1-alpha (HIF-1) production.45 Incorporating ADSC into a GelMA and HA hydrogel expanded hydrogel applications, as shown by Eke et al.,46 who demonstrated a considerable increase in vascularization and showed potential for skin regeneration. Using a customized hydrogel, Kim et al.47 evaluated therapeutic cells produced from human induced pluripotent stem cells (hiPSCs), finding only moderate wound reduction and substantial angiogenic and granulation tissue development.
The ADSC-conditioned medium in a GelMA hydrogel developed by Li's team improved blood vessel regeneration and perfusion in aged skin.48 To increase vascular development, collagen deposition and re-epithelialization during wound healing, they used this information to create a GelMA hydrogel containing endothelin-1 (ET-1).49 Sun et al.50 employed electrospinning to create a GelMA-based 3D fibrous scaffold, which they then used to enhance skin flap survival and microvascular growth in vivo (Supplementary Table 3).
2.4 Stem cell and tissue regeneration
Using cutting-edge scaffolding and bioactive chemicals, tissue engineering and stem cell research are at the heart of regenerative medicine.51 Rapid skin tissue development was shown by Hao et al.52 using 3D GelMA hydrogel scaffolds that were modulated with light. Epidermal development is stimulated by AKT signalling when human umbilical cord mesenchymal stem cells (hUC-MSCs) are cocultured with human umbilical vein endothelial cells (HUVECs) in GelMA hydrogels.53 Another work revealed that ADSCs paired with platelet-rich plasma (PRP) in oligomeric silsesquioxane-poly(ε-caprolactone) (POSS-PCL) nanocomposite scaffolds promote tissue integration and angiogenesis.54 Similar results were seen when GelMA hydrogels were combined with vesicles generated from hUC-MSCs,55 as shown by Tang et al.
Incorporating GelMA into UC-MSCs has been shown by Li et al. to improve fibroblast activity and wound healing.56 Last but not least, Yuan et al.57 (Supplementary Table 4) presented a GelMA/polyethylene glycol diacrylate (PEGDA) microneedle patch containing HUVEC-derived exosomes and tazarotene to improve angiogenesis in diabetic wounds.
2.5 Material innovations and applications
Materials science continues to unveil advancements that enhance understanding and improve quality of life, with hydrogel-based therapies like GelMA hydrogels playing a significant role in wound healing and tissue engineering. Huang et al. integrated antioxidative and immunoregulatory red jujube into GelMA hydrogels, suggesting its utility in wound dressings.58 Liu et al. introduced tannic acid and carbon nanotubes to GelMA hydrogels, improving their mechanical strength and adhesion, with potential applications in electronic skin.59
Addressing diabetic wound healing, Rehman et al. utilized graphene oxide (GO)-enhanced hydrogel dressings to speed up healing through enhanced cellular activities,60 while Shi et al. developed a bioink combining GelMA, collagen and tyrosinase for robust skin regeneration.61
In a similar vein, Zhao et al.62 manufactured light-controllable GelMA scaffolds for skin regeneration, while Annabi and colleagues63 developed a sprayable GelMA hydrogel with antibacterial characteristics for wound healing. Diabetic skin injury may be effectively treated with the glucose-responsive microneedle dressing developed by Guo et al.64 (Supplementary Table 5).
2.6 Miscellaneous innovations in healing
Especially for burn injuries, modern medical practices have revolutionized wound healing and tissue regeneration.65 By combining GelMA with anti-IL-6, which inhibits the cytokine IL-6,66 Uehara et al. (2019) improved skin transplant survival. Complete skin injuries may be treated using a GelMA and sulfhydrylated chitosan hydrogel that has been shown in studies to retain moisture, be strong and be antibacterial.67
Tao's group combined zinc nitrate, dopamine methacrylate and GelMA to make a hydrogel.68 Zhang's team developed oxygen-regulating microneedles out of black phosphorus and haemoglobin to speed up the healing of diabetic wounds.69 Another work by Zhang et al. enhanced wound healing using a GelMA composite hydrogel, boosting collagen production and angiogenesis70 (Supplementary Table 6).
3 PRECLINICAL TRIALS AND REAL-WORLD IMPLICATIONS
Preclinical research on GelMA's therapeutic applications, especially beyond rodent models, is scant, but three key studies using Hartley strain guinea pigs and micro-pigs offer critical insights. The first study demonstrated GelMA's potential over traditional suturing by showing reduced inflammation and enhanced protein expression, which might improve tissue healing in anterior vaginal wall repairs. GelMA was found to minimize the formation of profibrotic encapsulation around mesh fibres, suggesting a better alternative for epithelium and mesh anchoring.71, 72 The second study tackled complications from mesh-augmented prolapse repairs, like erosions and pain, by using GelMA hydrogels. These gels preserved collagen content and decreased immune response, which could lead to fewer postoperative issues and better reconstructive surgery outcomes for the vaginal wall.73 Finally, Jang et al. advanced preclinical research with a 3D-bioprinted GelMA patch containing VEGF mimetic peptides, which notably improved wound healing in pig skin by enhancing collagen deposition and angiogenesis, underscoring the efficacy of precise 3D-bioprinting in promoting cell growth and constructive remodelling.26 These studies underscore the potential of GelMA, suggesting further in-depth research is required for real-world applications and laying the foundation for human clinical trials.
4 CONCLUSION
Tissue engineering has just entered a revolutionary new phase, and recent developments in 3D bioprinted GelMA hydrogels promise to improve wound healing using bioactive dressings and scaffolding. GelMA hydrogels' biocompatibility, tunability of mechanical characteristics and facilitative function in cellular proliferation are all exploited by their incorporation into 3D bioprinting, heralding a new age of promise in regenerative medicine. However, there are several obstacles and restrictions to this novel technique, and optimizing its therapeutic value will need extensive study, technical development and interdisciplinary cooperation. The main problem with using GelMA hydrogels is that 3D bioprinting methods can only be used on a small scale at the moment because of the need for expensive and time-consuming specialist equipment. Getting this sorted out is crucial for taking GelMA hydrogels from the lab to the clinic. In addition, studies are still being conducted to determine the longevity of bioprinted constructions and their capacity to operate normally under physiological settings. Improving the mechanical strength, printability and incorporation of bioactive chemicals like growth factors into advanced bioinks is another area of focus with the goal of improving therapeutic effects. While GelMA's adaptability has its advantages, it may make it difficult to determine which formulations work best for certain therapeutic uses. Long-term use of GelMA in implanted devices or tissue scaffolds necessitates evaluation of its mechanical stability under the dynamic forces imposed by the human body. Regulatory hurdles increase the level of difficulty, necessitating thorough testing and validation to ensure the product is safe and effective enough for clinical use. Due to the high cost of developing and applying these materials on a large scale, this labour-intensive method may lengthen the time it takes before GelMA-based medicines become available to patients. When dealing with highly sensitive topics like organ or tissue bioprinting, ethical and social ramifications must also be considered.
Despite these obstacles, a wide range of bioprinting technologies from extrusion-based to hybrid approaches allow the creation of complex hydrogel structures that may be tuned to the demands of individual patients. Growth factor-, antimicrobial- and stem cell-enhanced bioactive dressings have been proven to hasten wound healing, promote tissue regeneration and prevent infection. Blood circulation and tissue healing may benefit greatly from the incorporation of bioactive substances inducing angiogenesis. Personalized care based on detailed imaging of each patient's wounds has the potential to improve outcomes. Production scaling, long-term stability, negotiating regulatory environments and cost reduction are all obstacles that must be overcome. To establish the safety and efficacy of these hydrogels in real-world circumstances, extensive clinical studies are required. In the future, we need to put more effort into perfecting bioink formulations, incorporating state-of-the-art manufacturing methods and adopting customized medical tenets.
In sum, the future of GelMA hydrogels in wound healing seems promising, but it will need a concerted effort to overcome the obstacles that now stand in the way. Intelligent bioactive dressings that react to the wound microenvironment are becoming a real possibility as new materials and methods are revealed via study. The incorporation of nanoparticles for prolonged therapeutic release has the potential to significantly improve these state-of-the-art dressings. A new era in wound treatment is on the horizon, and its realization requires the combined efforts of scientists, physicians and industry players.
ACKNOWLEDGEMENTS
The drawing platform was provided by Bio Render, for which we express our gratitude.
FUNDING INFORMATION
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no relevant financial or nonfinancial interests to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




