RETRACTED: Treatment of wound infections linked to neurosurgical implants
Abstract
As neurosurgery has advanced technologically, more and more neurosurgical implants are being employed on an aging patient population with several comorbidities. As a result, there is a steady increase in the frequency of infections linked to neurosurgical implants, which causes serious morbidity and mortality as well as abnormalities of the skull and inadequate brain protection. We discuss infections linked to internal and external ventricular and lumbar cerebrospinal fluid drainages, neurostimulators, craniotomies, and cranioplasty in this article. Biofilms, which are challenging to remove, are involved in all implant-associated illnesses. It takes a small quantity of microorganisms to create a biofilm on the implant surface. Skin flora bacteria are implicated in the majority of illnesses. Microorganisms that cause disruptions in wound healing make their way to the implant either during or right after surgery. In about two thirds of patients, implant-associated infections manifest early (within the first month after surgery), whereas the remaining infections present later as a result of low-grade infections or by direct extension from adjacent infections (per continuitatem) to the implants due to soft tissue damage. Except for ventriculo-atrial cerebrospinal fluid shunts, neurosurgical implants are rarely infected by the haematogenous route. This research examines established and clinically validated principles that are applicable to a range of surgical specialties using implants to treat biofilm-associated infections in orthopaedic and trauma cases. Nevertheless, there is little evidence and no evaluation in sizable patient populations to support the success of this extrapolation to neurosurgical patients. An optimal microbiological diagnostic, which includes sonicating removed implants and extending culture incubation times, is necessary for a positive result. Additionally, a strategy combining surgical and antibiotic therapy is needed. Surgical procedures involve a suitable debridement along with implant replacement or exchange, contingent on the biofilm's age and the state of the soft tissue. A protracted biofilm-active therapy is a component of antimicrobial treatment, usually lasting 4–12 weeks. This idea is appealing because it allows implants to be changed or kept in place for a single surgical procedure in a subset of patients. This not only enhances quality of life but also lowers morbidity because each additional neurosurgical procedure increases the risk of secondary complications like intracerebral bleeding or ischemia.
1 INTRODUCTION
Implant insertions are becoming more common in all surgical specialties, including neurosurgery. Age- and degenerative-related symptoms are among the indications, along with ischemic, neoplastic, and trauma-related illnesses. An estimated 450 000 neurosurgical implants were placed in US hospitals in 2004.1 About 3%–12% of craniotomies, 1%–24.4% of craniectomies, 1%–6% of neurostimulators, 4%–17% of cerebrospinal fluid (CSF) shunts, 2%–22% of external ventricular CSF drainages (EVD), and 5% of external lumbar CSF drainages (ELD) had implant-associated infections.1 These infections can have catastrophic effects on the patient and are linked to higher rates of morbidity and death because they typically require multiple revision surgeries, leaving the patient with unsightly skull deformities and no brain protection in between. Pain, mobility issues, and depressive symptoms can also worsen as a result of these infections.2 As such, it is critical to treat infections linked to neurosurgical implants. Neurosurgical implant-associated infections are difficult to diagnose.3 Clinical signs and symptoms in patients with neurological disabilities following intracerebral bleeding or trauma, as well as in patients with decreased consciousness as a result of sedative medication treatment, are frequently ambiguous, insensitive, and challenging to interpret. This also applies to laboratory tests that measure CSF parameters. A careful approach to the patient is required, one that takes into account the time elapsed since surgery as well as the existence of other nosocomial infection foci. This involves monitoring the dynamic of the clinical progression and laboratory tests.4 Implant surfaces are very prone to bacterial colonization, and a biofilm can build in a matter of minutes.5 Because the bacteria in biofilms are quiescent and not planktonic in tissue or CSF, biofilms make it more difficult to diagnose and treat implant-associated infections microbiologically.5 The majority of neurosurgical implants become infected exogenously when skin or mucosal flora germs are contaminated during surgery or during the early stages of wound healing disruption. As a result, during the first month following surgery, over two thirds of patients present.5, 6 Due to their intravascular location, hematogenous seeding from a distant infectious centre is extremely uncommon and almost solely observed in ventriculo-atrial (VA) shunts. The therapy of infections linked to neurosurgical implants lacks standardized procedures. Given that known techniques from trauma and orthopaedic surgery have been successfully clinically validated, it appears reasonable and appealing to apply them to the treatment of implant-associated infections.7 Sonication of extracted implants and extended incubation of cultures for up to 14 days can maximize microbiological diagnosis.8 Antimicrobial and surgical methods are combined in interdisciplinary treatment. Debridement and implant retention, exchange, or removal are always part of surgery. Furthermore, a protracted biofilm-active antimicrobial therapy is required.9 It is significant to remember that secondary injury to the central nervous system (CNS) makes thorough debridement and implant removal or exchange impractical in neurosurgery, and that antibiotic penetration across the blood–brain barrier is essential. In addition to discussing the difficulties in diagnosing and treating neurosurgical implant-associated infections, this study attempts to extend multidisciplinary management ideas from implant-associated infections in trauma and orthopaedic surgery, where the idea has been shown and empirically confirmed. Nevertheless, there is little evidence and no evaluation in sizable patient populations to support the success of this extrapolation to neurosurgical patients. This section will address infections related to craniotomy, cranioplasty, neurostimulators, internal CSF shunts (VA and ventriculo-peritoneal [VP] shunts), EVD, and ELD.
2 CATEGORIZATION OF INFECTIONS LINKED TO NEUROSURGICAL IMPLANTS
Implant-associated infections are categorized based on the infection's acuity and the time it first manifested after implantation, as these factors—along with the identified microorganism—define infection management.9 Both acute and chronic infections are included in the classification. Acute infections usually appear 6 weeks after implantation. In addition to a fever, patients typically exhibit pain, heat, edema, redness, and a foul-smelling discharge from the wound. Contrarily, chronic infections appear later—often months after implantation. Patients typically exhibit clear indications of localized inflammation, although they can also show up with an implant visible, a fistula, or persistent wound drainage. While a mature biofilm is present in chronic infections and cannot be removed without implant removal or exchange, an immature biofilm is present in acute infections and can be eradicated by implant debridement and retention combined with a 12-week biofilm-active treatment.7, 9 Since EVD and ELD are non-permanent implants, they are not covered by this classification. In the event of an infection, they can typically be eliminated, negating the need for biofilm-active medication.
3 CONCEPTS FOR DIAGNOSING INFECTIONS RELATED TO SURGICAL IMPLANTS
The standard of care changed to include sonication of the removed implants and extended incubation of cultures for up to 14 days, since most low-virulent infections require 7–14 days of cultivation.8 The risk of contamination does, however, grow with prolonged sample cultivation; therefore, the microbiological data must be interpreted accordingly. Because biofilms are separated from the implant surface and cultivated (sensitivity 80–90% vs. 60% in tissue cultures), sonication in orthopaedic surgery has a high specificity (up to 99%) and sensitivity that is much greater than in tissue cultures. Patients who have received antibiotic pretreatment in particular should be aware of this (sensitivity 75 vs. 45%).8 It is advised to take multiple (preferably three to five) independent tissue samples since biofilms are sporadically distributed throughout the periimplant tissue. Molecular diagnostic procedures, such as polymerase chain reaction assays or next-generation sequencing, may be used to identify the pathogen in illnesses that do not respond to culture. Sonication has been shown to be beneficial in neurosurgery for cranioplasties, CSF shunts, EVD tips, and osteosynthesis (as utilized for craniotomy fixation).10 In a study by Prinz et al., 22 patients with sonicated VP shunts were compared to 13 patients who received only standard culture.10 Whereas traditional culture only found the pathogen in 61% of cases (p = 0.018), sonication found it in all of them. When pathogen detection was analysed using a technique, only 22 out of 35 patients (60%, p < 0.001) had positive results from culture, while all patients who underwent sonication had positive results. Sonication was again positive in all 12 patients for those who had received antibiotic pretreatment (n = 18); however, traditional culture was only positive in 3 of 6 patients (50%, p = 0.005), highlighting the significant diagnostic impact of sonication. For infections linked to EVD, Jost et al.'s smaller investigation found the same thing.11 Sonication of the EVD tip was carried out in 14 patients, and the results showed a greater detection rate (64 vs. 14%; p < 0.05) than with traditional ventricular CSF culture. This is explained by the biofilm idea, which states that bacteria cling to the implant surface and are not planktonic in the CSF. However, contamination may still occur if the EVD tip is not collected appropriately (the distal 2–3 cm of the drainage must be collected, and the cutaneous exit site must be cleaned before the catheter is removed). Two of the five patients experienced a meningitis episode a few days following EVD removal, despite the fact that the five had a positive sonication culture and a negative CSF culture. The results were initially classified as contaminated.
4 THERAPIES FOR INFECTIONS RELATED TO NEUROSURGICAL IMPLANTS
The treatment approaches established in orthopaedic and trauma surgery can be applied to permanent neurosurgical implants (EVD and ELD excluded), albeit there is little data to support this extrapolation and it has not been verified in large neurosurgical patient groups.9 Including surgery and biofilm-active therapy, collaborative management is required to achieve high treatment effectiveness. In order to remove necrotic tissue and mechanically lower the pathogen burden in established biofilms, surgical debridement is always required. Implant management involves debridement and retention in acute infections and one- or two-stage exchange or implant removal in chronic infections, depending on the severity and timing of the infection following device insertion. In order to prevent subsequent implant colonization with skin infections, it is also crucial to ensure that the implant has sufficient postoperative soft tissue covering. Depending on the length of the implant-free interval and the intraoperative culture results, a biofilm-active therapy is then administered for a typical duration of 4–12 weeks, consisting of 1–2 weeks of intravenous treatment and 3–10 weeks of oral treatment. A drug holiday, or a period without antibiotics before reimplantation, is not advised because neurosurgical patients frequently require an implant immediately in order to prevent deformities of the skull that could be disfiguring and to protect the brain, as well as to lower the risk of sinking flap syndrome, which could cause progressive neurological deterioration. Furthermore, it was shown that continuous antibiotic therapy produced superior results for periprosthetic joint infections than did an antibiotic-free interval prior to reimplantation, based on extrapolation from the orthopaedic literature.12 Because no diagnostic test can reliably rule out persistent infection at the moment of reimplantation and because any viable organism can trigger an infection recurrence, constant antibiotic medication is necessary. In order to fully treat implant-associated infections, therapy should be continued upon reimplantation. Treatment failure in infections related to neurosurgical implants is a grave circumstance that needs to be completely prevented. If necessary, bactericidal antimicrobial medications that penetrate the CSF ought to be administered.9, 13 Bacteriocidal rifampin combinations are used in biofilm-active treatment against gram-positive pathogens (such as staphylococci and Cutibacterium spp.); fluoroquinolones are used in biofilm-active treatment against gram-negative bacilli. Since resistance to rifampin quickly develops, it should not be used as a stand-alone antibiotic (it reaches 56% of plasma levels in CSF). Instead, it must be combined with an antibiotic that has a similar good CSF penetration rate, such as levofloxacin (30%–50% of plasma levels in CSF), moxifloxacin (>50% of plasma levels in CSF), cotrimoxazole (40%–50% of plasma levels in CSF), doxycycline (26% of plasma levels in CSF), and levofloxacin (30%–50% of plasma levels in CSF).14 When coagulase-negative staphylococci are treated with intravenous vancomycin, which has poor CSF penetration and consequently leads in a rifampin monotherapy in CSF, leading to therapeutic failure, rifampin resistance has been shown to evolve in CSF shunt-associated infections.15 CSF levels of ciprofloxacin are approximately 26% of plasma levels.9, 14 Because of its effectiveness against the majority of relevant organisms, its ability to work in concert with other antibiotics, and its ability to sufficiently penetrate the blood–brain barrier in both non-inflamed (27%) and inflamed (50%–70%) meninges, intravenous fosfomycin may be a viable therapy option for CNS infections.16 A subgroup of patients receiving fosfomycin treatment was examined in a prospective, multicentre research involving patients with serious bacterial infections from 12 intensive care units in Germany and Austria.17 Severe CNS infections accounted for 22% of the cases, with pneumonia (15%), bone and joint infections (11%), stomach infections (11%) and bacteremia (11%), rounding out the top five. In 81.3% (148/182) of the cases and 84.8% (39/46) of the patients with ≥1 multidrug resistant pathogen, the overall clinical outcome was favourable. According to these findings, intravenous fosfomycin is a safe and efficient combination medication for the treatment of serious bacterial infections. Antimicrobial therapy administered intraventricularly is not usually advised.18 Nonetheless, it may be taken into consideration for individuals who do not respond well to systemic therapy or for infections that are tough to eradicate but are resistant to many drugs. The primary benefit is that large intraventricular concentrations can be achieved by avoiding the blood–brain barrier; nevertheless, there is little data to support this therapy approach, particularly in terms of dosage recommendations. Implant retention is feasible in certain patients. Acute infections with immature biofilms (≤6 weeks), therapies that are aggressive against biofilms, and sufficient soft tissue covering around the implant are necessary for implant retention.9 After 12 weeks of prolonged biofilm-active treatment, the biofilm is eliminated. Implant replacement or removal in one or two stages with a brief implant-free period of 14 days is advised in cases of chronic infections (>6 weeks) in order to eradicate the mature biofilm. After this surgical approach, biofilm-active therapy is often administered for 12 weeks. In the case of a two-stage implant exchange, a shorter treatment time of 4–6 weeks may be possible if the findings of the intraoperative culture test are negative. Treatment continuation is advised even in the event of negative intraoperative culture results after reimplantation, which is typically carried out under antibiotic therapy. This is because culture outcomes obtained under antibiotic management may be falsely negative. Recurrence of an infection can also lead to severe consequences for the patient, such as the need for additional surgery. If the pathogen causing the infection is not treatable with biofilmactive therapy (e.g. rifampin-resistant staphylococci, quinolone-resistant gram-negative bacilli, and Candida spp.), then an implant-associated infection that is difficult to treat is present. Depending on the pathogen and the infection site, as explained below, eradication of infection can only be achieved with implant removal and antimicrobial therapy without any implants in place for 1–6 weeks. Antibiotic therapy should ideally be continued until reimplantation in order to prevent the growth of any viable microorganisms. Long-term antimicrobial suppression therapy is an option if implant removal is not practical, although it is uncomfortable for the patient and may have unfavourable side effects. The susceptibility pattern indicates that clindamycin should be added to doxycycline and cotrimoxazole as substitutes for intradural suppression management, provided that blood–brain barrier penetration is not required in the event of extradural infections. Since it has been demonstrated that streptococcal implant-associated infections on orthopaedic implants are difficult to cure, a suppression treatment for at least a year—and possibly longer if well tolerated—is required.19
5 CERTAIN INFECTIONS
5.1 Infections related to cranioplasty and craniotomies
A bone flap is taken out and replaced with titanium plates or clamps in order to access cerebral tissues for procedures such as brain biopsies, abscess or haemorrhage drainage, vascular malformation trimming, or surgery for a skull base tumour. We refer to this technique as a craniotomy. The term “craniectomy” refers to a decompression surgery operation when a larger bone flap is removed and not immediately replaced in the event of trauma, malignant cerebral infarction, intracerebral haemorrhage, or an infected bone flap. The process known as autologous cranioplasty allows the extracted bone flap to be cryopreserved and then reinserted at a later time. When there is aseptic bone necrosis, many fragments, mature biofilm, or chronic bone flap infection or resorption, the bone flap is not reusable and a synthetic cranioplasty (made of titanium, polyether ether ketone [PEEK], or polymethyl methacrylate [PMMA]) is employed.20 Diabetes, the existence of an EVD, CSF leaking, intracerebral haemorrhage, and tumour surgery are risk factors for infection.21 Infections related to craniotomies and cranioplasty typically appear within the first month following surgery and are most frequently brought on by staphylococci, a type of bacteria found in the skin flora.21 In addition to purulent wound drainage and difficulties with wound healing, other clinical presentations include fever, headaches, seizures, or localized neurological abnormalities.22 Compared to cranioplasties (1%–24.4%), craniotomies have a lower infection rate (0.3–12%).4 The infection rate for cranioplasties is not influenced by the type of material used (autologous versus synthetic).23 There is no additional evidence linking delayed cranioplasty to a lower risk of infection, despite the association between extremely early cranioplasty (within 14 days of craniectomy) and infection in a recent cohort research.24 However, the majority of cranioplasties take an average of 7.3 months (with a range of 1–40 months) to complete, leaving the patient with a deformity of the skull that is disfiguring and does not protect the brain, as well as disturbed circulation in the CSF and the potential for sinking flap syndrome, which could lead to progressive neurological deterioration.2, 24 An earlier cranioplasty should be taken into consideration because of this. Since there is a thin layer of soft tissue covering craniotomies and cranioplasties and because the superficial and deep compartments are not physically separated early after surgery, all infections should be regarded as deep wound infections and hence connected with craniotomies or cranioplasty. After the infection is completely cured, current clinical treatment typically entails delaying cranioplasty and removing the contaminated implant or bone flap. A novel idea derived from orthopaedic surgery implant-associated infections permits either a one- or two-stage implant exchange with a brief implant-free interval of 2 weeks, or surgical debridement and implant retention in acute infections in selected patients where biofilm-active therapy is available and soft tissue coverage is adequate.9 After that, the patient receives biofilm-active therapy for a duration of 12 weeks.9 With this notion, the patient's quality of life is improved because they remain with a disfiguring skull deformity without brain protection for a relatively short period of time, if at all. It has recently been demonstrated that this idea works well for patient populations undergoing neurosurgery.25 Of the 12 patients who had an infection related to autologous craniotomy and resection craniectomy, 10 (83%) underwent an immediate titanium cranioplasty after receiving intravenous (range: 3–8 weeks) and oral (range: 0–16 weeks) antibiotic treatment.26 Another study found that, when combined with intravenous (range: 2–6 weeks) and oral (range: 0–6 weeks) antibiotic treatment, debridement and bone flap retention were successful in 10 of 11 patients (91%).25 These short trials confirm that immediate implant exchange or retention are viable options instead of removal. A favourable outcome was noted despite the fact that most patients received poor antibiotic treatment (no biofilm-active medication). Given the small number of patients, it is only reasonable to infer that, when combined with biofilm-active treatment, surgical debridement is unquestionably a key component of the therapeutic concept “debridement and implant retention” and leads to a high rate of treatment success. By using the idea put forth here, together with optimized biofilm-active therapy, a 12-month follow-up showed an 87% infection-free survival rate in a larger cohort.27 medication failure was linked in univariate analysis to insufficient antimicrobial therapy, which is defined as not receiving biofilm-active medication during the designated treatment time.
5.2 Infections linked to deep brain and spinal cord stimulators, or neurostimulators
While spinal cord stimulators are mostly used to treat chronic back pain, deep brain stimulators are being employed more and more in the treatment of movement disorders like Parkinson's disease and dystonia. Leads and electrodes are parts of a stimulator system that are inserted into the spinal epidural space (for spinal cord stimulators) and the brain (for deep brain stimulators) as shown in Figure 1-3.28-30
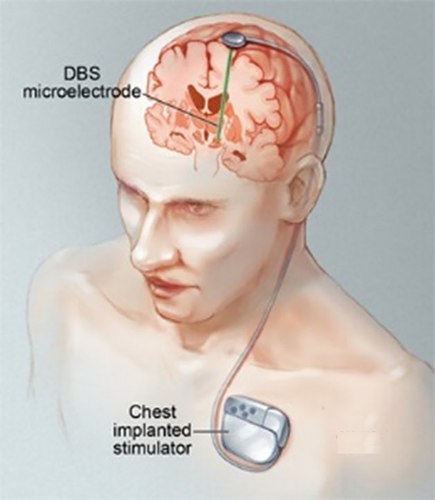
The generator, which is positioned in the abdomen for spinal cord stimulators or the subcutaneous tissue of the chest for deep brain stimulators, is connected to the leads and electrodes by wires. The term “pocket infection” refers to the 31–85% of cases where infections happen at the generator pocket; nevertheless, wires (15%–69%) may also be affected. Leads and electrodes that have brain or epidural abscesses are rarely affected; the most common pathogens are staphylococci and other skin flora.31 Deep brain stimulators have an infection rate of 4.5% to 6.5%, the bulk of which manifests 3 months after implantation.32 A 5% infection rate for spinal cord stimulators is reported, with 38% of cases presenting as early implant-associated illnesses.31 Several publications advise against device removal in cases of infection or advocate a lead/electrodesparing surgery in which the device is partially explanted and then reimplanted weeks after the infection is cleared. The patient will have considerable morbidity and annoyance from this treatment, though, since repeated brain surgeries are required, and symptoms return throughout the implant-free period. High medical expenses as a result of the lost stimulator system are also to be anticipated.32 Consequently, our recommendation entails extending the orthopaedic surgery treatment algorithm once further, allowing the implant to be kept or only partially exchanged in certain individuals. Acute infections, the availability of biofilm-active treatment, and sufficient soft tissue covering of the implant are prerequisites for this approach. A 12-week biofilm-active treatment is then administered.9 We recommend the following procedures based on the portion of the neurostimulator that is infected. If biofilm-active therapy is available, generators in acute pocket infections can be debrided and kept. The generator's insertion location should ideally be moved due to the often poor soft tissue state associated with infection. Wires, electrodes, and leads may be kept. Wires should be replaced as soon as feasible in cases of extracranial or spinal wire infections; however, generators, leads, and electrodes can be kept. The infected portion of the stimulator and the surgical technique selected determine the course of the antimicrobial treatment and how long it will last. Long-term antimicrobial suppression management is an option for treating difficult-to-treat infections in the absence of biofilm-active therapy, particularly if device removal is thought to be impractical. In the event of meningitis or other potentially fatal infections, such as lead and electrode-associated brain or epidural abscesses, the device must be removed.
5.3 Infections related to ventriculoperitoneal and ventriculoatrial cerebral fluid shunts
Patients with persistent hydrocephalus can have their CSF drained from the cerebral ventricles to either the right cardiac atrium or the peritoneal cavity by VP or VA shunt devices, respectively. The infection prevalence is 4%–16% in both CSF shunt systems, although VA shunts have more frequent and typically more severe consequences.4 In cases of distal shunt disconnection, intracardiac thrombus formation, and shunt migration—which can lead to myocardial perforation—the latter require more surgical revisions and have a high morbidity rate.33 A number of factors, such as prior CSF shunt infection, postoperative CSF leakage, revision surgery, concurrent EVD, and neuroendoscope use, increase the risk of CSF shunt-associated infections.34 Recent research has demonstrated that CSF shunt systems covered with antibiotics (such as rifampin and clindamycin) can lower the infection rate.35 However, utilizing local antibiotics carries a risk of antimicrobial resistance emergence, especially for rifampin, where resistance can arise quickly due to point mutation. In vitro vascular grafts soaked in rifampin have shown this danger.36 The sole antibiotic that is biofilm-active against staphylococci is rifampin, and once resistance develops, it is impossible to completely eradicate biofilm infection. Given that many staphylococci are clindamycin-resistant, the combination of rifampin and clindamycin typically does not stop the development of resistance. Patients who have infections linked to CSF shunts typically show symptoms early in the first month following the shunt's implantation, and staphylococci and Cutibacterium species are the predominant pathogens.37 Patients frequently show up with few or no clinical symptoms.37 VP and VA shunt-associated infections appear differently clinically, particularly if the distal shunt portion is infected.37 Patients may present with symptoms of peritonitis, intraabdominal pseudocysts, or intestinal shunt perforation if the distal VP shunt portion is infected. Similarly, if the distal VA shunt portion is infected, patients may experience stigmata of right-sided endocarditis, including persistent bacteremia and septic lung emboli. Shunt nephritis, an immune complex-mediated glomerulonephritis, is an uncommon consequence of long-term VA shunt-associated low-grade infection.15 The only indication of an infection in the event of a low-grade infection of the proximal shunt portion may be shunt dysfunction accompanied with worsening hydrocephalus symptoms, such as headache, vomiting, and seizures. On the other hand, patients with high-grade infections may exhibit symptoms like severe meningitis or brain abscesses, which are urgent signs that the CSF shunt needs to be removed. Even though it is the most frequent clinical feature, only 78% of cases have a fever.15 Because clinical indications and symptoms are sometimes non-specific, CSF examination is crucial. The microbiological yield from shunt valve puncture was 68%–91%, compared with 8%–45% in lumbar and 20%–70% in ventricular CSF, as per the most recent literature. This indicates that CSF from shunt valve puncture had a greater diagnostic yield than CSF from lumbar puncture or ventricular CSF.15 Similarly, the CSF cell count is only raised in 80% of cases overall, but it is unquestionably larger in lumbar puncture and shunt valve CSF (median 484 cells/μL and 573 cells/μL, respectively) than in ventricular CSF (median 8 cells/μL).15 The biofilm concept explains these results once more: leukocytes and microorganisms are closely surrounding biofilms on the shunt valve, as evidenced by the high positivity rate of 49–78% for shunt tip cultures (undoubtedly an underestimate, since sonication of the implants was not available in both studies). Additional reasons for these CSF results can include variations in CSF circulation in hydrocephalus patients, as well as the retrospective study methodology, which makes it impossible to rule out bias related to treatment and sample.15 Because the distal shunt portion is intravascular, blood cultures are a valuable diagnostic tool in VA shunt-associated infections, with positive rates exceeding 80%.15 The available research on various therapy techniques is highly inconsistent. An earlier randomized research by James et al. evaluated shunt removal, one-stage shunt exchange, and shunt retention in 50 patients with infections related to CSF shunts. The course of antibiotic treatment included 3 weeks of intravenous and 2 weeks of intraventricular administration of antibiotics that showed no efficacy against biofilms.38 Surgery is necessary to treat implant-associated infections because high failure rates (64%) for shunt retention contrasted with high cure rates (95%) for shunt removal and 88% for one-stage shunt exchange. 81% of adult patients with CSF shunt-associated infections in a retrospective research underwent combination surgical and antibiotic treatment, which included shunt removal (47%) and one- or two-stage shunt exchanges (10%) or (23%), with or without an intercurrent EVD.15 Among those patients, the total cure rate was a high 98%. It's interesting to note that, in contrast to James' trial, the 19% of patients who did not have surgery had an 87% cure rate. This could be explained by the more current study's optimized antimicrobial therapy combined with biofilm activity.15 Another retrospective investigation revealed that the surgical treatment approach involved the removal of the CSF shunt (28%) and the exchange of the shunt in one or two stages (22%–43%), whereas the use of antibiotics alone has gradually been abandoned over time (only 7%).37 A number of patients with staphylococcal infections may not have received biofilm-active treatment with rifampin, and five patients' distal shunt externalization strategy may have contributed to the overall lower cure rate of 70% (17% with antibiotics alone, 31% with one-stage and 89% with two-stage exchange, and 83% with shunt removal). However, in this investigation, CSF shunt retention was the only risk factor for treatment failure (odds ratio 46.04, 95% confidence interval 5.30–399.88).37 The following treatment regimen is recommended based on these studies, which demonstrate that CSF shunt retention in acute infections or one-stage shunt exchange in chronic infections where biofilm-active medication is available are beneficial treatment options with decreased patient morbidity. A one-stage operation that involves either retention or exchange of the CSF shunt is followed by a 12-week course of biofilm-active treatment. It is important to note that in cases of acute meningitis or ventriculitis, brain abscess, shunt dysfunction, skin erosion over the shunt system, or gut perforation, CSF shunt retention is not feasible. The pathogen and the intraoperative culture results determine the length of treatment and implant-free period if the CSF shunt is removed or exchanged in a two-stage procedure. After removing the CSF shunt, the CSF infection should be completely eradicated in 5–7 days for coagulase-negative staphylococci and Cutibacterium spp., 14 days for Staphylococcus aureus, Streptococcus spp., Enterococcus spp., and culture-negative infections, and 21 days for gram-negative bacilli.18 A 12-week biofilm-active treatment is administered if intraoperative cultures obtained upon CSF shunt replacement are still positive; in the event that intraoperative culture findings are negative, 4–6 weeks of treatment are advised to get rid of any leftover germs. If shunt removal is not a possibility, a long-term antibiotic suppression medication (cotrimoxazole or doxycycline) is recommended when a difficult-to-treat infection is detected while a new CSF shunt is in place. Though extra subtherapeutic vancomycin blood levels cannot be ruled out because of the study's retrospective design, it is possible that inadequate vancomycin CSF penetration causes rifampin monotherapy to result in resistance development. The susceptibility testing revealed that patients receiving intravenous vancomycin and oral rifampin for CSF shunt-associated infections caused by coagulase-negative staphylococci experienced treatment failures.15 For intradural infections, therefore, optimal therapy combinations with CSF penetration are necessary. For endocarditis linked to VA shunts, extended IV therapy may be sufficient.39
5.4 Infections connected to the drainage of lumbar and external ventricles' cerebrospinal fluid
Temporary implants called EVD and ELD are primarily used to treat acute hydrocephalus resulting from severe craniocerebral injuries or intracranial bleeding. Management of EVD- and ELD-associated infections is different from that of CSF shunt-associated infections because of the temporary installation; biofilm-active therapy is not required. 36However, managing infections linked to EVDs and ELDs can be difficult, particularly if the patient has a permanent CSF shunt and is dependent on external CSF drainage. This shunt must frequently be implanted promptly, ideally at the same time as the infected EVD or ELD is removed. A CSF shunt is required in up to 44% of patients with an EVD or ELD.40 With an infection rate of 8%, EVD and ELD-related illnesses affect up to 22% of people.4 Although polymicrobial infections can also occur, staphylococci and other skin flora members like Cutibacterium spp. are the most frequent pathogens.40 A cranial fracture accompanied by CSF leaking, a CSF catheterization duration of more than 8 days, and repeated CSF sample from EVD or EVD irrigation are risk factors for infection.41 The use of an ELD or silver-coated EVD, or whether it was situated at the patient's bedside in an emergency room or critical care unit, had no effect on the infection rate; on the other hand, a tunnelled EVD was linked to a decreased infection rate.41 Using rifampin-coated EVDs and ELDs is strongly discouraged as it exposes microorganisms in the CSF to rifampin monotherapy and promotes the growth of rifampin-resistant bacteria, primarily coagulase-negative staphylococci.42 Since there is not a biofilm-active treatment for the resulting CSF shunt-associated infection, it cannot be cured. It can be difficult to diagnose infections linked to ELD or EVD. Clinical manifestations, CSF haemorrhage resulting in a sterile inflammation, and CSF analysis results coincide with the underlying condition.3 Furthermore, sedative medication used in conjunction with the underlying condition's low state of consciousness can disguise indicators of a central nervous system infection.43, 44 As a result, considerable clinical suspicion is required. Surprisingly, meningitis only manifested in 23% of individuals within 10 days following the removal of EVD.11 Clinical and CSF analyses were compared at three distinct time points in a retrospective analysis involving 39 patients: the time of EVD insertion, 48 h prior to the emergence of an EVD-associated infection, and the time of CSF culture-positive infection.45 The CSF values were not different, and the only noteworthy signs of infection were a higher frequency of fever, an elevated respiratory rate, and a lower mental state.46-49 Clinical and CSF parameters were compared between the time-points of EVD insertion and EVD-associated infection in another study involving 48 patients. The results showed that fever, headache, vomiting, and stiff neck were more common in the latter (79% vs. 15%), and that the CSF cell count was higher (177 vs. 46 cells/μL) in the former.40 The majority of EVD-associated infections are identified based on CSF pleocytosis and fever since more sensitive and specific criteria are lacking. This leads to a much greater rate of hypothesized infections than microbiologically proved infections, which are the gold standard.50 According to certain research, CSF lactate (at a cut-off of 4 mmol/L) and an elevated cell index are indicative of infections linked to EVD.51 The ratios of the white blood cell and red blood cell counts in the CSF and blood are used to compute the cell index. According to Lunardi's research, an infection was indicated by a cut-off point of 2.9 and a 4.33-fold increase in the cell index over time.52 In light of the above described facts, we recommend the following course of action. For each febrile patient with an EVD or ELD, the exclusion of an alternate nosocomial infection focus is required.53 If there is a high suspicion of an EVD or ELD-associated infection and the CSF analysis is consistent with infection (CSF leucocyte count >300 cells/μL or increasing cell index, lactate >2.1 mmol/L, decreased glucose CSF/blood ratio <0.5), a CSF sample should be obtained for microbiological culture and empirical antibiotic treatment should be initiated, such as intravenous vancomycin plus either ceftriaxone, cefepime, or ceftazidime based on local surveillance data. If the patient responds to intravenous treatment, there is no evidence that intraventricular antimicrobial therapy is beneficial.18 It is strongly advised against using rifampin as a biofilm-active therapy for EVD and ELD as they are solely intercurrent implants. If a permanent CSF shunt is implanted and gets infected, rifampin may be required later.54 Treatment periods vary depending on the causative pathogen: 7 days for low-virulent pathogens like Cutibacterium spp. and coagulase-negative staphylococci, 14 days for S. aureus, Streptococcus spp., Enterococcus spp., and culture-negative infections, and 21 days if gram-negative bacilli are isolated.18 Since EVD and ELD manipulations are linked to an elevated risk of infection, it is best to limit their use. As a result, prophylactic EVD or ELD exchange and daily CSF sample are not advised.18 However, in the event of a high-grade infection (S. aureus, gram-negative bacilli, or Candida spp.) or an inadequate response to therapy, the EVD or ELD should be adjusted if needed.
6 CONCLUSIONS
The use of more and more neurosurgical implants as a result of technological advancements in neurosurgery has made device-associated infections in this field increasingly important. The majority of recommendations are derived from other implant-associated infections; however, there is a lack of established diagnosis and treatment methods for neurosurgical patients. Additionally, the efficacy of this extrapolation has not been thoroughly tested in sizable patient populations. Management is difficult because biofilms are involved; well-known, clinically verified multidisciplinary principles from orthopaedic or trauma surgery, where high cure rates of >90% are attained, should be followed. Multidisciplinarity is essential for achieving success. Microbiological diagnosis is much enhanced by prolonged culture incubation and sonication of the removed implants. Surgery is part of the treatment approach. A comprehensive debridement and stage-specific implant management are included. For acute infections, this means debridement and retention; for persistent infections, it means one- or two-stage implant exchange. Furthermore, a biofilmactive treatment is typically required for 4–12 weeks. Therefore, novel treatment options that reduce morbidity include curing without removing the implant or employing a one-stage exchange. This is especially important because neurosurgical implant removal is sometimes challenging or impossible due to the possibility of injuring brain tissue or producing intracerebral haemorrhage. This approach can lower the number of surgeries performed while improving the quality of life for patients. Biofilmactive treatments must be used to optimize antimicrobial treatment in conjunction with less invasive surgical techniques. Particular consideration must be given to antibiotic penetration across the blood–brain barrier if the intradural compartment is infected.
FUNDING INFORMATION
Anhui Provincial Key Research and Development Plan (2022e07020069); Scientific Research Foundation of Education Department of Anhui Province (2022AH050769, KJ2021ZD0036); Fundamental Research Funds for the Central Universities (WK9110000069); Health Scientific Research Foundation of Chuzhou (CZWJ2022A001).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
On request, the corresponding author is required to provide access to the meta-analysis database.




