Mechanical and contact characteristics of foam materials within wound dressings: Theoretical and practical considerations in treatment
Abstract
In the treatment of acute and chronic wounds, the clinical performance of a given foam-based dressing, and, ultimately, the wound healing and cost of care outcomes are strongly influenced by the mechanical performance of the foam material/s within that dressing. Most aspects of the mechanical performance of foam materials, for example, their stiffness, frictional properties, conformability, swelling characteristics and durability, and the overall mechanical protection provided by a foam-based dressing to a wound strongly depend on the microstructure of the foam components, particularly on their microtopography, density and porosity. This article, therefore, provides, for the first time, a comprehensive, self-inclusive compilation of clinically relevant theoretical and practical considerations, based on published analytical and experimental research as well as clinical experience related to the mechanical performance of foams in foam-based wound dressings. The current bioengineering information is useful for establishing understanding of the importance of mechanical properties of foams in foam-based dressings among clinicians and researchers in industry and academia, and other potential stakeholders in the wound care field, for example, regulators and buyers. This information is also particularly important for the development of standardised test methods for the evaluation of foam-based wound dressings and resulting standard mechanical performance metrics for these dressings.
Abbreviations
-
- COF
-
- coefficient of friction
-
- DFU
-
- diabetic foot ulcer
-
- ECMO
-
- extracorporeal membrane oxygenation
-
- MARSI
-
- medical adhesive-related skin injuries
-
- PU/PI
-
- pressure ulcer/injury
-
- SEM
-
- scanning electron microscopy
-
- SF
-
- shape factor
-
- VLU
-
- venous leg ulcer
1 INTRODUCTION: STRUCTURE-FUNCTION PRINCIPLES APPLIED TO DRESSINGS IN A MECHANICAL CONTEXT
Various materials are used innovatively in the making of modern wound dressings and each type has a unique microarchitecture and function that is determined by how they are composed, combined and constructed. How the dressings are affixed to the skin and wound, handled and removed, are the foundations for their clinical performance, contributing to the experience of the patients using the product and the cost-effectiveness of the treatment course.1-8 Foam materials within dressings appear to be a particularly good case for demonstrating the above concept because of their popularity in clinical practice. The clinical outcomes of wound care are, of course, not only derived from the properties of the foam/s in the dressing per se, but the complexities of dressing designs also have a profound effect on dressing performance. Importantly, it is uncommon for a dressing alone to heal a chronic wound, and even the best dressings require a holistic approach that targets the underlying cause of the wound to achieve wound healing. However, the application of a dressing that is mismatched with the demands of the wound may make complete healing difficult, if not impossible. For example, the compressive and tensile stiffness and strength of a foam material within a dressing, affecting the ability of the foam-based dressing to protect the wound mechanically, and the bending stiffness of foams, which influences the conformability performance of foam-based dressings, all depend on the apparent density* and porosity of that foam material, which in turn, influence the absorbency performance of the foam while managing exudates, as demonstrated in the clinical case described in Figure 1. Likewise, the coefficient of friction (COF) of the wound-facing surfaces of foam dressings is influenced by the microtopography of the surface. Hence foams used within wound dressings deserve specific attention in the literature. The interaction of these bioengineering factors and how they eventually shape the clinical outcome is currently unknown and was not thoroughly studied before. For example, in their Cochrane review work, Walker et al9 stated, specifically for foam dressings applied to treat a pressure ulcer/injury (PU/PI), that ‘Clinicians need to carefully consider the lack of robust evidence in relation to the clinical and cost-effectiveness of foam dressings for treating PUs/PIs when making treatment decisions, particularly when considering the wound management properties that may be offered by each dressing type and the care context’ (p. 2). The clinical authors of this article share this view and observe that many wound care specialists do not understand the differences between commercially available foam dressings, which likely leads to suboptimal care outcomes. The current article takes the first step in addressing these gaps, by identifying key characteristics of foams in wound dressings that should be studied and reported for standardisation, as suggested in clinical work.10
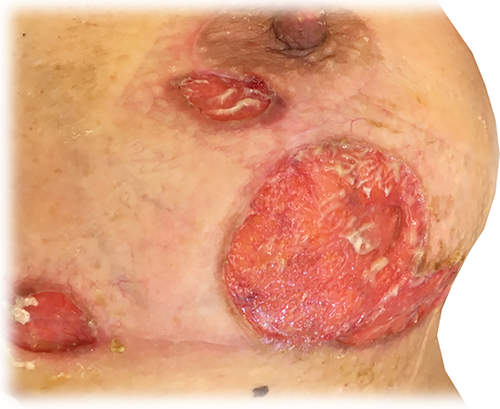
The well-established structure-function principle in material science provides insights into the properties of the microstructure (also often referred to as the microarchitecture) of the studied material that explain the resulting mechanical, thermal, fluid transport and retention metrics related to the function of each dressing.5, 6, 11-16 The characteristics of each of these material properties and their collective quality, together with the durability of the materials and sustained integrity of the dressing structure while in use under different real-world conditions and activities shape the clinical performance of wound dressings. Understanding material behaviours and how to quantitatively describe the material microarchitecture is therefore vital to achieve desirable clinical performance through appropriate design, processing and manufacturing of wound dressings.2, 5 The purpose of this article is to review the contemporary knowledge and formulate the bioengineering theory that applies to porous materials in wound dressings, and primarily, to foams that are in extensive use in contemporary dressings.17 Foams in foam-based dressings are most commonly made of soft polyurethane, which is often treated in various ways to create a hydrophilic non-adherent membrane that allows the passage of exudate through to the insulating foam body of the dressing. Foams have many important advantages as a wound dressing material over traditional textile dressings (eg, cotton bleached gauze) or hydrocolloid dressings, in light of their superior fluid absorbency and lower risk to adhere to fragile wound bed tissues. Foams are also preferred for their mechanical cushioning properties, thermal insulation for the wound, flexibility to conform relatively well to curved body contours (and further mimic skin tones to minimise the negative effect of wounds on the body image, and therefore, improve compliance to the treatment plan), and maintenance of a moist wound healing environment. However, according to the aforementioned structure-function principles, the quality of the clinical performance of a foam-based dressing in any of the above aspects stems from the microstructure and properties of the specific foam types used in the dressing.
It is noteworthy that most wound dressings contain different material types in addition to polymeric foams alone. Many foam-based dressings incorporate layers combining polymeric with cellulose or superabsorbent materials in multi-layered dressings.16, 18-20 Since their invention in the 1950s, polymeric foams have received extensive attention from material scientists in numerous fields of engineering and technology (such as in the packing industry, seat and mattress industry, for sound management components and in building construction to mention a few), and analytical (mathematical) formulations describing their mechanical behaviour were developed in these diverse fields. Nevertheless, foams have not been carefully scrutinised in terms of their performance as a wound dressing material9 and there is no mathematical-analytical theory for foams in foam-based wound dressings in the literature yet. Moreover, the relatively simple structural and mechanical behaviours of foams with respect to other (such as hydrocolloid and superabsorbent) dressing materials allow closed-form mathematical-analytical formulations to be developed and utilised specifically for analyses of foams in wound dressings. Other wound dressing materials may require sophisticated computer codes and simulation tools for complex numerical computer analyses to achieve similar aims. Foams are, therefore, a natural, solid starting point in the formulation of a rigorous scientific theory for the mechanical behaviour of wound dressings.
The specific aims of this work are therefore: (a) to review, for the first time in the literature, the mechanics and contact characteristics of foam materials within foam-based or foam-made dressings; and (b) provide key theoretical and practical considerations, which are relevant to the design, efficacy research and development of new testing standards for foam-containing or foam-made wound dressings. As a first step towards the development of better, clinically relevant testing standards for foam-containing dressings, there is a need to understand and establish a scientific (bioengineering) theoretical framework around the importance of the mechanical properties of foams in dressings. This framework should ultimately take into consideration the perspectives of wound care researchers, clinicians, patients, manufacturers, regulators, payers and likely also other relevant stakeholders in the wound care arena such as professional societies, health economists and health policy makers. The current work is a cornerstone of this initiative.
2 THE STIFFNESS OF FOAMS AND HOW THEY RELATE TO FOAM DENSITY AND POROSITY
Foams in wound dressings are composed of a solid polymer phase and a dispersed air phase. In addition to offering storage space for excess exudate in the voids of the foam, from a mechanical perspective, foams are lightweight and have the ability to absorb mechanical energy through deformation, which promotes mechanical protection and cushioning capabilities. Foams also facilitate good thermal insulation of the wound bed against heat loss to the ambient environment in the absence of native skin. Foam dressings in the wound care industry can theoretically be designed to optimise these mechanical properties, for example, balancing between stiffness and conformability determined by the foam density. Ideally, a foam dressing should have a stiffness level that is similar to that of native skin, to avoid sharp stiffness gradients between the dressing and peri-wound skin which may imprint the skin, that is, create localised, sustained soft-tissue distortions under the dressing and particularly at the perimeter or borders of the applied dressing, which may compromise cell and tissue viability over time (Figure 2A).5, 22, 23 Concurrently with this requirement, the borders of a foam dressing should have the capability to prevent any leakage of exudate to the peri-wound skin, to prevent inflammation or maceration and subsequent skin breakdown.24, 25 McKee et al26 reviewed the range of stiffnesses reported for human skin evaluated by means of indentation testing, which is the relevant loading mode to the interaction with dressings. They noted a wide range of reported skin stiffness data and decided to conduct a statistical outlier analysis of the reported empirical data. They concluded that the elastic moduli of human skin are within the 6 to 222 kPa range, averaging at approximately 85 kPa. Overly stiff dressings that substantially exceed the aforementioned skin stiffness can be an outcome indicator of an inadequate design and/or material selection (ie, excessively low porosity). More commonly, however, a foam dressing with a suitable stiffness under no mechanical strain or moderate strain can become exceedingly stiff if it is compressed to produce strains that exceed the strain domain for the intended use. For example, excessive dressing strain may develop under compression bandaging or medical devices, or when subjected to the bodyweight as in a non-offloaded diabetes-related foot ulcer (DFU).5, 22, 23, 27-29 Orlov and Gefen23, 30 recently reported that the compressive stiffness of certain foam dressings compressed to 50% strain can reach approximately 300 kPa, that is, ~3.5 times the mean compressive skin stiffness reported by McKee et al,26 which indicates that currently, the biomechanical compatibility of foam dressings with skin is not always optimal.23
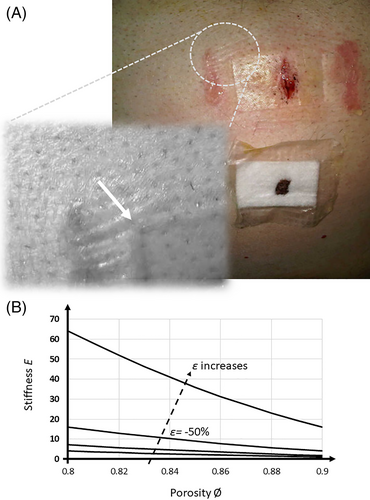
The chemistry of the raw foam material and the details of the manufacturing process of foaming eventually determine the strength and stiffness of the final foam material. In particular, these factors influence both the properties of the solid polymer phase and the microarchitectural characteristics of the porosity of the foam, that is, the percentage and shape of the microscopic void spaces in the microstructure designed to collect and store the wound exudate when the foam is included in a wound dressing (please see Figure 1 for a clinical case description demonstrating the importance of this porosity property). The denser a foam is, the less air that it contains in its pores, and so the work of compressive forces will mostly be invested in deforming the solid polymer, that is, the foam stiffness increases when density increases. Of course, the density and porosity of foams are interchangeably affected by external mechanical forces that act to compress the foam and narrow or collapse the spaces in the microarchitecture (which further reduces the absorbency capacity of the dressing subjected to the loads31). The foam densities reported by Lee et al32 for polyurethane foams used in commercially available dressings were between 0.06 and 0.26 g/cm3, hence there is relatively large variability in this property among existing products. The detailed derivation of how the strength and stiffness of foam materials depend on their porosity and density is provided in Appendix A.1 for readers interested in the technical aspects of foam material science. The theoretical influence of the porosity and compressive strain level on the stiffness of a foam dressing is further illustrated in Figure 2B. Generally, for foams made of the same solid polymer phase, there is a non-linear increase in the stiffness with the decrease in the porosity and increase in the level of the applied external loading (or the non-dimensional deformation, ie, strain level), as depicted in Figure 2B.
2.1 Foams in wound dressings under large mechanical deformations and repetitive loading
Despite best effort and intention to ensure proper offloading, many wounds are subjected to mechanical loads, unintentionally or deliberately, and such loads may be sustained (static) or dynamic/cyclic in nature.30, 33 For example, acute care patients requiring cardiopulmonary support through extracorporeal membrane oxygenation (ECMO) but develop a sacral PU/PI during the supine ECMO support period, which is then treated by applying a dressing to their non-offloaded sacral wound are difficult to reposition as the postural changes may dislodge the device. Likewise, patients with spasticity, those with a severe gastroesophageal reflux disease, patients with dementia or delirium, and those with psychiatric disorders are challenging to reposition and often apply their bodyweight on their wounds, and on their dressings. Another example is the gold standard treatment of a venous leg ulcer (VLU) because of venous insufficiency, which typically includes the application of a dressing on the VLU, and graduated compression therapy over the applied dressing, so that the dressing is deformed in compression and must function under the sustained compression.34-36 Patients with a DFU will preferably have their wounds partially or completely offloaded by means of an offloading (plantar pressure redistribution) device, for example, a total contact cast, but ambulatory patients are sometimes non-adherent with either offloading or immobilisation, and apply their bodyweight on the DFU.29, 37-40 Moreover, even when offloading devices are used by patients with DFUs as indicated, and in combination with foam dressings (which is a common clinical practice41, 42), the dressing may move under the device, for example, because of minor misalignments of the device causing shear forces on the dressing that lead to migration of the dressing during walking. Prior studies suggest that individuals with a DFU walk over 4000 steps per day on average, in particular those who use removable offloading devices for whom the estimated average of daily steps is approximately 4500 steps.43-45 Moreover, the duration of standing, as another important measure of weight-bearing activities, is estimated to be three-folds longer than the duration of walking for these persons.46 Thus, extra attention should be paid to the resilience of wound dressings applied to DFUs against repetitive pressure and shear loading. This phenomenon, observed by the clinical authors of this work, not only challenges the absorbency of the applied dressing but also its capacity to stay-on, and while a dressing migrates, it shears the peri-wound and may deteriorate it. For repetitive or impact loads such as those occurring in a wound dressing attached to the plantar surface of the foot to treat a DFU, Equation (A8) in Appendix A.1 may not be a sufficient description and specific experimental characterisation will be required.
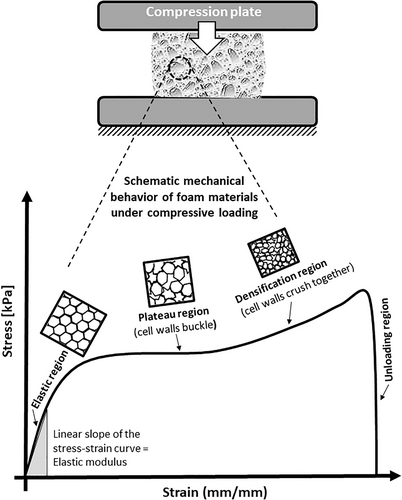
All the above clinical scenarios necessitate the understanding of how foams within foam-based wound dressings behave under compressive mechanical loading. The stress-strain curve of a soft polyurethane foam undergoing repetitive or impact compressive loading can be divided into four typical regions: elastic, plateau, densification and unloading regions (Figure 3).27, 49-51 These regions correspond to the variation in the apparent mechanical properties, because of the changes in the microstructure of the foam. Specifically, in the elastic (also called the linear) region, the stress linearly increases with the strain and reaches an initial peak stress. This region is generally observed at a strain of 5% to 10%, and when the compressive load is removed, the foam specimen returns to its original shape. Of note, foams in foam dressings, particularly when subjected to bodyweight loads or under compression therapy, may be deformed to substantially larger strains. While the elastic region corresponds to a relatively intact cell structure that begins to deform with the bending and stretching of the cell walls, in the plateau (also called the buckling) region, the cells are already substantially deformed. The apparent foam stresses in the plateau region may fluctuate slightly with the strain, but overall, the stress maintains the average stress level despite an increase in the strain, corresponding to the altered cell structure and collapse of the cells in an elastic buckling mode. The densification region forms when a substantial load is applied to the foam. This region corresponds to a structure in which most of the cells are distorted to such an extent that the cell walls are already in contact with each other (Figure 3). In the unloading region, the stress rapidly decreases to zero.50 The strain that decreases because of the rebounding of the specimen during unloading is the elastic strain (Figure 3).
The above theoretical and experimental formulations clearly demonstrate that the stiffness properties of foam dressings may vary considerably for different clinical usage scenarios, primarily depending on the magnitude of the mechanical loads to which the dressing is subjected, from zero for a completely offloaded wound to very large strains as occurring under compression bandaging. Of note is that the compressive strain ε of a foam dressing may not necessarily remain constant over time. While in the context of a non-offloaded PU/PI, or a VLU treated by dressings under compression bandaging, the strain level in the dressing materials may be approximately steady for long time periods, dressings applied to non-offloaded plantar DFUs may be subjected to repetitive and rapid deformations. For dressings applied to such plantar DFUs, the compressive strain can, therefore, be theoretically simplified as cyclic (sinusoidal), that is, where ε0 is the strain amplitude, t indicates time and ω indicates the step frequency for the relevant activity; the frequency of human walking, which is relevant in this regard, is within the range of 1.7 to 2.1 Hz.52, 53
2.2 Additional micro-morphological foam characteristics related to foam function in wound dressings
Of note, the SF of a perfectly circular pore is 1, and elongated pores have lower SFs. The work of Yunus et al54 demonstrated that the SF of open-cell polyurethane foams (often used in wound dressings) is typically substantially below unity, and may approach 0.5,55 which is indeed visualised by the elliptical shapes of pores in the SEM micrographs obtained by Lee et al.32
Another important morphological parameter is the thickness of the walls that make the pores, also known as the struts, which was investigated by Heit et al,56 also using SEM. They found that in foams used in wound healing products, the wall thickness increases with the size of the pores, and is approximately 70 μm for small pore sizes (720 μm), 130 μm for medium pore sizes (1300 μm) and 300 μm for large pore sizes (3100 μm) in the foams examined in their study. Both the pore size and the wall thickness affect the stiffness and strength of the foam, as both influence the porosity value and, thereby, the level of material resistance to mechanical forces through deformation (Equation A8 in Appendix A.1). Indeed, the work of Lee et al32 demonstrated that different foam materials used in wound dressings have strength properties that span over orders of magnitudes, from 0.011 to 0.248 kg/mm2 (108 kPa to 2432 MPa), and likewise, the elongation at failure of the tested foams ranged between 180% and 1101%.
2.3 Mechanical durability of foams in wound dressings
The mechanically vulnerable element in foams is the struts, and hence, fatigue-related mechanical microdamage in foams is associated with bending, elastic buckling and ultimately, plastic (irreversible) collapse of the struts.59 Accordingly, the durability of foams also originates at the microstructure and is linked with the sizes and shapes of the pores and the thickness of the walls that separate them, as the micro-failure (microdamage) accumulation is influenced by the extent of wall bending and stretching.47 In terms of the apparent (bulk) properties, the mechanical stresses leading to microdamage in foams, therefore, strongly depend on the foam density or porosity, as well as on the strength of the solid phase of the polymer material (Equations A2 and A5 in Appendix A.1).60 In addition, any existing defects such as edge cracks or internal holes in the foam can compromise its mechanical durability, with edge defects being more likely to compromise the strength of foams with respect to internal defects.61 The latter point has practical implications on the function of foams in foam-based wound dressings. A dressing with a visible physical defect caused by mechanical forces, such as because of intense rubbing, sustained indentation or scratching, is more prone to further damage from (subsequent) mechanical forces, which may lead to disintegration of the dressing and, thereby, a compromised healing of the wound.14, 15, 62
3 THE BENDING STIFFNESS OF FOAM DRESSINGS AND THE ASSOCIATED WOUND BED PROTECTION
The bending stiffness of foams used in wound dressings is highly important in the context of the mechanical protection provided by the dressing to the wound bed and the peri-wound tissues, as well as in terms of the conformability of the applied dressing to curved body surfaces, and its ease of use (ie, how much a dressing would mechanically ‘resist’ to applying it on convex body surfaces). Park et al63 measured the bending stiffness of polyurethane foams in the context of flexion of shoe insoles, and as expected, their results demonstrated that the bending stiffness of foams depends on the thickness and elastic modulus† of the specific foam material. This aligns with the fundamental theory of flexural bending, which determines that the bending stiffness (also known as the flexural rigidity) of an elastic material is the product of the elastic modulus (Figure 3) and the moment of inertia of the cross-section. For a rectangular cross-section (which is characteristic to the vast majority of wound dressings), the moment of inertia of the cross section is wt3/12 where w is the width and t is the thickness of the dressing, hence the thickness of the dressing plays a major role in the bending stiffness (because of the third power in the latter formula). For example, to illustrate the effect of the thickness on the bending stiffness, and hence on the conformability of foams in foam-based dressings, in order to double the bending stiffness of a single-layer foam dressing having a unit thickness, it is possible to increase its elastic modulus two-fold through lowering the porosity (Equation A3 in Appendix A.1) without changing its thickness, or alternatively, to use the same material but increase its thickness by just 25%. A thicker dressing not only increases the bending stiffness but also increases the absorbency reservoir of the foam for wound fluid uptake. On the other hand, a greater bending stiffness may cause discomfort to patients if the dressing is placed on a body part that is relatively mobile such as over a joint (eg, in flexion/extension). A higher bending stiffness of the dressing can also make it more difficult to apply the dressing on curved body surfaces (because the dressing will resist in shape change to achieve conformability, and it will be more challenging for the adhesive mechanism of the dressing to maintain the curved shape due to an elastic recoil). As the body is not a flat plane and most of it has convex surfaces that often move substantially (such as the feet, elbows, knees, hips, or neck), some manufacturers have developed specialised flexible dressings with a deliberately low bending stiffness achieved, for example, using patterned perforations in the material, to match anatomy-specific or patient-specific needs of conformability and/or body movements with the dressing.64, 65 The added flexibility of the dressing by means of such treatments of the foam materials contributes to decreasing dressing wastage through this enhanced capacity to remain in place for as long as needed.64
4 ADHESION OF THE DRESSING BORDERS TO THE PERI-WOUND AND IMPLICATIONS OF NON-ADHERENCE
Optimising the adhesiveness of dressings has been a persistent challenge to the wound care industry. In modern dressings, an adhesive mechanism is integrated in at least a border area around the wound pad so that no further fixation (with secondary adhesive tape or polyurethane film layer) is needed (though for some highly convex or moving anatomical regions, such as the female breast and the knees or axilla, respectively, an adhesive border of a standard-shape dressing is often not suitable or needs to be cut to shape66). Repeatedly changing these adhesive wound dressings frequently causes pain and stripping damage, which is removal of a large amount of stratum corneum from the newly formed epithelium and/or the peri-wound skin with the adhesive.67, 68 With the introduction of silicone adhesives, the extent of this problem decreased‡,69, 71 yet peri-wound skin trauma caused by the repetitive removals of adhesive dressings still occurs and is known to increase the size of wounds and the risk of infections and delays healing, and thereby, adversely affect the quality of life of patients and have cost implications for health care providers.70 In the more recent literature, this is referred to as medical adhesive-related skin injury (MARSI), which is associated with a history of contact dermatitis or prolonged bed rest periods and is considered preventable, including through the selection of appropriate dressing products, in addition to suitable procedures for application, monitoring, reapplication and removal of the dressings.72-75
Despite that foam materials are generally considered non-adhesive, there are many foam-based dressings with an adhesive skin contact layer that enable the dressings to be secured in place without the need for secondary support or fixation. An overly aggressive adhesiveness to the wound or to the fragile peri-wound skin may result in trauma and pain upon removal, and may also cause stripping of the stratum corneum, leading to an inflammatory response and compromised barrier function.76-78 However, it is important to balance this consideration with the need to adequately secure the dressing in place for the intended period of use.66 An insufficiently strong (or durable) adhesiveness leads to frequent dressing detachments, therefore requiring more dressing changes, which may also cause skin irritation or MARSI, leading to discomfort and pain, in addition to increased costs of wound care. Importantly, the frequency of dressing changes, which is strongly affected by the quality of the adhesiveness of the applied dressing over time, directly impacts the quality of life of patients and the overall cost of care§.4, 70, 82 Dykes et al77 indicated that foam dressings with an acrylic adhesive require the greatest separation forces to detach from the forearm skin (2.2 N), followed by polyurethane adhesives (1.7 N), and the least force to peel the dressing occurred when the dressing had a soft silicone adhesive (1.1 N) or a hydrocolloid adhesive (1 N). Their findings regarding a soft silicone adhesive agree with visual analog scale-based and McGill questionnaire-based pain evaluations conducted by Woo et al83 who compared a soft silicone foam dressing (Mepilex® Border; Mölnlycke Health Care AB, Gothenburg, Sweden) with an adhesive hydrocellular polyurethane foam dressing (Allevyn Adhesive; Smith & Nephew, Hull) and reported that the foam dressings with the soft silicone adhesive were associated with less pain, both before and during dressing changes. Surprisingly, the Dykes77 work highlighted that the level of force needed to separate the dressing from the skin does not always correlate to the damage to the stratum corneum, which was greatest for the acrylic adhesive (95%) and negligible for the soft silicone adhesive. However, the hydrocolloid adhesive associated with relatively low peeling forces caused stratum corneum damage which was equivalent to that of a polyurethane adhesive (85%-90%). An important observation described by Waring et al78 is an overall wide inter-subject variation in the levels of adhesion to human skin (ie, variability in inter-subject peeling forces), and likewise, product-related differences in attachment to dry versus moist or oily skin.
Differences in peel force levels are reported in the literature, for example, by Dykes et al77 (approximately 1 to 2 N) and Waring et al78 (approximately 0.5 N), which may be associated with anatomical site differences and test method settings, such as the dressing removal technique, angle and the speed of removal, which plays a crucial role. As the skin is viscoelastic, the state of contact stresses between the dressing and the skin strongly depends on the speed of removal: the faster the removal, the greater the skin (and wound) stresses are.5 The latter point has important clinical implications, in the sense that the dressing removal technique, as taught, trained and implemented in practice, is of critical importance in order to minimise the risk of MARSI. Indeed, current clinical MARSI prevention guidelines recommend a ‘low and slow’ removal technique, that is, to slowly pull back the adherent dressing at a low horizontal angle (approximately parallel to the skin), away from the corner or edge so that it is gradually separated from the skin, which not only provides greater control of the removal action, but also reduces the localised wound and peri-wound skin stresses as indicated above.73, 84
Standard peel test methods utilising standardised substrates, in particular steel and glass, are commonly used by the wound dressing industry, as reviewed by Bernatchez and Bichel.85 A few experimental synthetic viscoelastic substrata, which mimic adhesion on human skin, were developed in an academic research context to measure adherence properties,86, 87 or for surgical simulations.88 However, from a standardisation point of view, repeatable and robust methods are required, hence complex substrates run the risk of increasing variability substantially. In addition, information is needed regarding how the adhesiveness performance may be affected by the wound fluid over time for different exudate types, compositions and viscosities.
A great promise for research in this area of wound dressing science is in dynamic computer models to simulate the behaviour of both the viscoelastic human skin during dressing detachment and the adhesive technology under investigation. Computer modelling and simulation work conducted at the group of the lead author (AG) indicate that the adhesion forces, angle of removal and speed of removal of the dressing all affect the state of mechanical stresses of the peri-wound skin, and thereby the risk for developing a MARSI. The pattern by which peri-wound stress concentrations change in location and magnitude throughout the removal process for a dressing peeled from a planar wound is demonstrated in such a computer simulation in Figure 4, and further bioengineering analysis is provided in Appendix A.2. Specifically, the formulation in Appendix A.2 indicates that the most sensitive region of the peri-wound skin to stripping damage is where the wound width is maximal (assuming that there is no adhesion between the wound pad and the wound bed). However, the equations in Appendix A.2 also demonstrate that the level of the applied force and the angle of application are important factors affecting the skin stress concentration magnitudes, and can be controlled by a trained clinician. As mentioned above, under real-world conditions, this interaction becomes more complicated as it also depends on the speed of the removal process, which cannot be kept constant as this is a manual manoeuvre. Another factor affecting the intensity of the tissue stress concentrations during the peeling is the convexity of the wound and peri-wound surfaces, which is not taken into account in the analyses detailed in Figure 4B-D and Appendix A.2; stress concentrations are typically amplified on curved surfaces.89 Importantly, the current theory indicates that faster dressing removals create larger skin reaction forces because of the viscoelasticity of the skin. Accordingly, the dressing removal rate is a critical factor to consider for avoiding the occurrence of MARSI, as recent relevant guidelines and clinical practice indeed highlight.73, 75
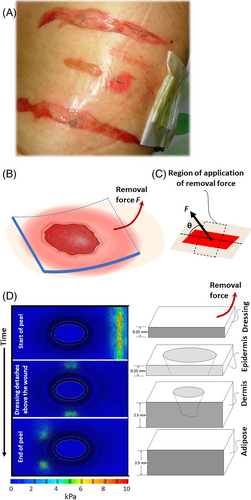
Lastly, it is important to note that the laboratory tests of peeling forces reviewed above, or the current computer modelling of the peel process of dressings (Figure 4D), do not account for the patient-specific and wound-specific, time-dependent biological interactions of the wound and dressing.2, 28 For example, an important mechanism that increases the adhesion forces in real-world clinical practice (though it is not very common for foam dressings), is potential ingress of new granulation tissue into the wound-facing aspect of the dressing (5, 6, 90, 91). Another biological influence that may appear in real-world scenarios is if the exudate within the dressing dries out and fibrin-containing scab forms, which binds the dressing to the wound and result in greater peel forces when the dressing needs to be removed.5
5 THE COF OF THE OUTER AND INNER DRESSING SURFACES
A wound dressing can be subjected to frictional forces causing shearing in numerous clinical scenarios, such as when the region of the wound is rubbing against the bedsheets, clothing or any other object (eg, the safety rails of a hospital bed), or during head-of-bed elevation, repositioning, turning, bathing, or transferring patients, or while a patient spontaneously moves in their bed or chair. During such events, the wound and peri-wound tissues are exposed to single-instance or repetitive shear deformations. Repeated frictional distortions may possibly cause breaks in the wound surface that allow pathogens to penetrate to the vasculature, and eventually generate secondary infections.92 These friction-related tissue distortions can be minimised if the COF of the dressing with the contacting elements is optimal.93 Specifically, a high COF at the outer dressing surface with, for example, the bedsheets or clothing, will cause delivery of greater frictional forces to the dressing (the frictional force is the normal force times the COF), resulting in more shear within the dressing structure, which may further distort the wound bed or peri-wound in shear if the dressing is not effective in absorbing the shear internally.33, 94 Likewise, excess friction at the dressing-skin interface may compromise the peri-wound tissues, by inducing continuous or repetitive shear loads on them. Hence, typically, low COFs are desired for dressing materials in contact with either an external object (ie, at the outer dressing surface) or at the wound-facing aspect of the dressing. In addition, adequate attachment of the dressing to the skin is required to minimise frictional sliding movements of the dressing over the skin/wound.95
The COF of polyurethane foams for potential use as a skin-contacting material in wound dressings varies from approximately 0.15 for the harder foams up to 1.5 for the softest grades.93, 96-98 Specifically, Vilhena and Ramalho93 measured the COF of different materials used in a hospital setting, including a polyurethane foam, against the ventral forearm for natural dry adult skin, and obtained a range of COFs between 0.25 and 0.47 with a midrange of 0.36, which is similar to the COFs of polyester-fabric or cotton-made bed protectors (being around 0.499), but greater than for specialised hospital fabrics (0.27) or diapers (0.28). The above foam-skin COF values are at the lower end of those that can be expected in a moist wound environment, which is constantly exposed to exudate fluids, and/or where there is substantial sweating from the peri-wound region. Typically, COFs of synthetic materials with skin largely increase in the presence of moisture97 because of the hydrosensitive mechanical behaviour of the stratum corneum that softens in the presence of water (which is known as the plasticizing effect). Exposure to moisture and wetness may further smoothen the skin roughness asperities profile, and consequently, increase the de-facto contact area of materials with skin, thereby inducing more frictional adhesion, that is, a correlated adhesion and friction behaviour.93, 100 Indeed, Schwartz et al97 measured the COFs of porcine skin, dry and wet, with saline, sweat or urine, when rubbing against a single-layer polyurethane foam dressing using a tilting-table tribometer, and reported that the wet contact had a 1.3-fold greater COF with biological fluids (sweat and urine), overall increasing the COF more than saline (pointing to the importance of testing dressing materials with realistic biological fluids, not just saline). Of note, some body regions such as the axilla are more densely populated with sweat glands,101 hence the above effect of perspiration increasing the COF of polyurethane foams with skin is affected by the anatomical site. To reduce the COFs of wound-contacting foams, surface polishing or treatment with lubricants can be applied93; however, such surface modifications will likely be more needed if the dressing is indicated for highly perspiring peri-wound or largely exuding wound conditions where greater dressing-skin COFs are expected because of the continuous fluid exposure.97
The COF of the external surface of the dressing with potentially contacting materials such as the clothing and bed linen is also highly important, as high COFs will lead to high external frictional forces, shearing of the dressing and potential transmissibility of the shear stresses forming in the dressing to the wound bed and peri-wound region. Related to this, Ohura et al102 measured the COF between the outer layers of wet dressings and clothing, and found, as may be expected, that these COFs were sensitive to the level of wetness in the dressing. They also found that a foam-based dressing exhibited a lower COF with the clothing with respect to a hydrocolloid for simulated highly exuding conditions.102
6 SHAPE CONFORMABILITY OF DRESSINGS TO THE WOUND BED AND TO BODY SURFACE CONTOURS
The curved and asymmetrical surfaces of the human body are challenging for application of foam dressings, specifically bordered ones.66, 103 In particular, the clinical demand for placing dressings on irregular body surfaces at various anatomical regions to treat different wound aetiologies is often contradictory to manufacturers' interests in standardisation of the shapes (including symmetry) and sizes of dressings to optimise the processes and costs of production. Conformability of a foam material to the wound shape is warranted but needs to not overstress the wound bed because of swelling and dilatation as the dressing absorbs exudate fluids. The shape conformability of dressing materials and structures results from the interaction of the foam material characteristics such as the elastic modulus, the bending/flexural modulus and the Poisson's ratio, and the swelling behaviour of the foam. Theoretically, a dressing material with a high-volume capacity storage (which may intuitively be perceived as beneficial for fluid handling) could have the potential negative effect of dilatation in a confined wound bed space to an extent causing localised pressure increases on the delicate wound bed tissues. Excessively high pressures may lead to tissue stress concentrations in the wound bed and possible wound deterioration, by mechanically damaging an ongoing epithelialization and/or by inducing pain and psychological stress to the patient. With that said, low-level mechano-modulation has been shown to be linked to the stimulation of inflammatory, fibroblast migratory and fibrosis-related tissue remodelling responses, and hence theoretically, gentle, moderate pressures on the wound bed may, in fact, contribute positively to the wound healing process.28, 104, 105
The theory and derivation of the swelling pressure of foams in foam-based dressings are detailed in Appendix A.3. The formulation in this Appendix demonstrates that the swelling pressure of a foam-based dressing in the wound bed depends on the elapsed time post-application of the dressing and the effective diffusion coefficient of the foam, which in turn depends on the porosity and tortuosity of the foam. Specifically, the derivation in Appendix A.3 indicates that the swelling pressure will increase faster for a greater porosity ∅ and lower tortuosity in the microarchitecture of the foam-based dressing. In other words, to control the compression and the rate of compression applied by a foam material onto the wound bed when the foam-based dressing continuously absorbs exudate fluids, the swelling pressure can be regulated through appropriate selection of the microstructural features of the foam, specifically, by limiting the porosity Ø, increasing the tortuosity or doing both (Equation A13 in Appendix A.3). Similarly, to the other dressing characteristics reviewed here, the extent of swelling differs notably across manufactures and products, and this can be associated, at least partially, to the above theory, as the foam microstructural features also differ considerably between commercially available foam-based dressings.16
7 SUMMARY AND CONCLUSIONS
All the key aspects of the mechanical performance of foams within foam-based wound dressings strongly depend on the microstructure of the specific foam. For example, the compressive and tensile stiffness and strength of foams, affecting the ability of the dressing to protect the wound mechanically, and the bending stiffness of foams, which influences the conformability performance of foam-based dressings, all depend on the relative density and porosity of the foam materials. Likewise, the COFs of foam dressings with the wound bed and peri-wound skin depend on the microtopography of the surfaces. Although there are not many published works focusing on the microarchitecture of foams in foam-based wound dressings, the variability in microstructural properties reflected in the published studies reviewed here, with even the most fundamental property of the pore size ranging over an order of magnitude across manufacturers and products (ie, 100-1000 μm), reflects that an optimal porosity range of values for foam dressings is unknown at this time. As evident from the theoretical formulations in this article, the variability in pore sizes then reflects on high variabilities in foam stiffness, strength, conformability performance and durability of the foam—all of which are critical for delivering adequate mechanical protection to the wound bed and peri-wound, consistently over the intended period of use. In addition, multiple balances and optimizations are needed in the chosen mechanical properties of foam materials used in foam dressings, and the structure of foam dressings as a construct, for achieving an adequate and clinically efficacious dressing design; relevant examples are summarised in Table 1.
| Mechanical property | If excessively low, will result: | If excessively high, will result: |
|---|---|---|
Compressive material stiffness (elastic modulus)a |
|
|
Structural (bending) stiffness |
|
|
Adhesive strength |
|
|
- a Please see Figure 3.
Currently, no test standards exist that characterise the microstructure of foam-based dressings as part of their performance evaluation, and of course, no target values exist to guide manufacturers regarding the foam porosity, shape of pores and the variations in pore size and shape that would provide superior performance in the mechanical aspect of wound protection. Likewise, COFs and peeling forces are not standardised in the wound dressing industry, despite the critical impact of both on potential wound bed and peri-wound mechanical damage related to movements of the dressing against the skin or against elements in the outer environment, dressing removals and repetitive changes. Testing methods such as the ASTM 903,106 which is a standard test method for peel or stripping strength of adhesive bonds, are used in the absence of specific peeling tests for wound dressings. A particular challenge in this regard is to standardise a material substrate to represent the skin in such testing, although a range of options potentially exists, such as elastomers (silicones), ethylene/methyl acrylate films, epoxy resins, textiles and metals, or alternatively, excised porcine skin.86, 107, 108 Preliminary research towards the standardisation of testing of adhesive bandage tapes, which are, of course, much less complex than advanced foam-based wound dressings, already indicated that the peeling forces depend on the width of the tape and storage conditions, two example properties that are typically not considered in the evaluation of adhesiveness of wound dressings.109 Importantly, theoretical and computational analyses such as those reported here can be used to identify influential factors, for example, as in Equations (A9 and A10) (Appendix A.2), which point to the role of the dressing width in the skin loading state during removals, but details of theoretical-computational frameworks for wound dressings are also lacking in the literature. This article, therefore, provides, for the first time, a scientific compilation of theoretical considerations and experimental results related to the mechanical performance of foam materials in foam-based wound dressings, in the light of their frequent usage and clinical relevance, en route to the development of standard, clinically relevant mechanical performance metrics for wound dressings.
ACKNOWLEDGEMENTS
The current research was conducted by members of the International Wound Dressing Technology Expert Panel (IWDTEP). The IWDTEP consists of consultants paid by Mölnlycke Health Care AB (Gothenburg, Sweden). Molnlycke has not controlled (or regulated) the research carried out by the members of the IWDTEP. Mr Paulo Ramos (Council Member, EWMA and Board Member, APTFeridas, Portugal) of the Northern Regional Health Administration, ARS Norte Vila do Conde, Portugal, is thanked for providing the medical adhesive-related skin injury (MARSI) photograph shown in Figure 4A and Ms. Yael Shlomo, Ms. Lotem Dalal, Ms. Mai Dabas, Ms. Belle Kriger, Ms. Natali Gafni and Ms. Efrat Gerstman, students supervised by author AG are thanked for the simulation work in Figure 4D and related computational feasibility analyses of dressing removals.
Endnotes
APPENDIX A: FORMULATION AND DERIVATION OF THE THEORY OF FOAM MECHANICAL PERFORMANCE
A.1 The relationship between the porosity of foams and their strength and stiffness
There are several factors that shape the microarchitecture, including the porosity of foams, and thereby the mechanical properties of foam materials such as the chemistry of the solid and the foaming process defined by the manufacturing protocol.110 Specifically, in the production of polyurethane foams, the foaming and gelling reactions are critical for cell nucleation, growth, distribution and collapse and will therefore eventually determine the porosity Ø of the finished foam product.110 Indeed, porosity values of foam dressings differ remarkably, as demonstrated by Lee et al32 who investigated the microstructure of 11 commercial foam-based dressing types. For example, Lee et al32 demonstrated that dressings have non-homogeneous pore sizes and morphologies, and that the pore sizes in a cross-section range from approximately 100 to 1000 μm, that is, by an order of magnitude across products, which is a surprisingly immense variation.
A.2 Factors affecting the state of peri-wound stresses during removal of dressings
It is natural to first pull the edge of the dressing upwards to detach it (ie, so that θ➔90°), which maximises the normal stress on the peri-wound as the edge of the dressing detaches (Figure 4D; top frame of computational simulation). At that region of the edge of the dressing, W = 0, and therefore, the normal stress concentration is simply F/Dd. However, to progress the peeling process further, the force must take a lower angle, and hence, over the wound, the shear stress τ increases and maximises when (D-W) minimises, that is, at the widest region of the wound.
A.3 Swelling of foam in a confined space
The latter Equation (A13) demonstrates that the swelling pressure of a foam-based dressing in the wound bed depends on the elapsed time post-application of the dressing and the effective diffusion coefficient of the foam, which in turn depends on the porosity and tortuosity of the foam.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




