Evaluation of wound healing effects of micronized acellular dermal matrix in combination with negative pressure wound therapy: In vivo study
Funding information: Daewoong Foundation
Abstract
Acellular dermal matrix (ADM) grafts can provide coverage for full-thickness skin defects and substitute for dermal defects. We tested the effectiveness of micronized ADM (mADM) as a dressing material, combined with negative pressure wound therapy (NPWT), for managing superficial wounds. We compared the wound healing effect of mADM in combination with NPWT with those of gelatin and mADM applied with a foam dressing. These therapeutic materials were applied to 36 cm2 excisional wounds in a porcine full-thickness skin defect model. Wound healing kinetics and new tissue formation were assessed 10 days after the initial treatment by measuring the wound area. Collagen deposition and neovascularization were histologically evaluated. Compared with the other two groups, mADM plus NPWT combination group had a significantly larger wound area at the baseline (P = .0040), but the smallest on the 7th day (P = .0093). In addition, collagen formation and neovascularization were more histologically promoted than in the other two groups. mADM showed better results than the gelatin group but less collagen and revascularization than the combination group, and there was no significant difference in wound area. Our results show that the combination of mADM and NPWT has a synergistic wound healing effect.
1 INTRODUCTION
Regeneration of healthy and functional skin remains a considerable challenge because of the multilayer structure of this tissue and the different cell types within its extracellular matrix (ECM). Over the years, substantial efforts have been made to accelerate the wound healing process using traditional therapies, comprising natural compounds, such as plant extracts and honey, as well as modern techniques involving hyperbaric oxygen, negative pressure therapy, skin substitutes, growth factors and stem cells.1, 2
Skin substitutes are biomaterials designed to accelerate wound healing by replacing the ECM. These materials are increasingly being used for the management of both acute and chronic wounds.3 Autologous tissue transplants, such as flaps or skin grafts, may be the best option for soft tissue reconstruction. However, they have many limitations, including widespread skin loss, immune rejection, pain, scarring, slow healing, availability of the donor site and aesthetically undesirable donor sites.4, 5
The human acellular dermal matrix (ADM) is currently one of the most widely used skin substitutes. It contains collagen and elastin, which contribute to its tensile strength and elasticity; proteoglycans, required for angiogenesis; laminin, which maintains binding with connective tissues; and a basement membrane consisting of collagen type IV. Thus, this material can act as a biological scaffold for neovascularization, fibroblast infiltration and enhanced reepithelization during the wound healing process.6 Over the past 20 years, ADM has been applied to various acute and chronic wounds, including burn wounds and diabetic foot ulcers (DFUs).5
ADM is a leading type of skin substitute that was first developed as a thin sheet. Sheet types have been widely used in the past because of their size, thickness and easy adaptation to wounds or skin envelope pockets. However, sheet-type ADMs cannot easily be placed in narrow or deep spaces, and intact attachments must be sutured in aseptic circumstances that require specialised skills. Therefore, to solve this problem, micronized-type ADM products have been developed to be easily applied to various shapes of wounds. In a clinical case of chronic pressure sores and sinus wounds with micronized ADM (mADM), the size and depth of the wound were improved.7-9 The mADM (CG Bio Co., Ltd., Seoul, South Korea) used in this study is a paste-type mADM that is currently being used safely in clinical settings.9-14 This paste-type ADM consists of mADM powders that have undergone aseptic tissue processing. In addition, it can be applied easily to the wound site because the materials are provided in syringes that are ready to be used without rehydration. Thus, it is less likely to dislocate or be a source of infection and can facilitate wound healing by creating a dynamic microenvironment within the wound.15
Another cost-effective method that is relatively easy to apply to a wound is negative pressure wound therapy (NPWT).16 NPWT is a widely accepted treatment modality used to promote any kinds of chronic wound. It provided moistened and aseptic environment to wound with a vacuum pump and facilitate the intrinsic mechanotransductive wound healing process. This approach has become an integral part of wound treatment protocols. NPWT can increase tissue granulation in wounds by stimulating cellular pathways required for healing and is a barrier against bacterial accumulation. Clinical studies have shown the healing effect of a combined treatment using mADM plus NPWT, but previous studies did not provide histological evidence to support widespread use or change in the gross sectional area of the wound during the healing process.10-14 The present study investigated the synergistic effect of mADM plus NPWT combined treatment using a porcine full-thickness skin defect model.
2 METHODS
2.1 Materials
mADM (CG Bio Co., Ltd.) is a mixed paste-type micronized allogenic ADM product comprising human tissue. It has a remarkable degree of attachment to wounds and is ready to be used without any need for hydrolyzation. This paste also has a uniform particle size (664.2 ± 389.9 μm), which facilitates faster graft inosculation. Gelatin solution (Sammi Industrial Co., Ltd., Ansan, Republic of Korea), a partially hydrolysed form of collagen, was used in the control group. CURAVAC (CG Bio Co., Ltd.) was used as the dressing material for NPWT and adjunctive dressing methods using mADM. In addition, Easyfoam (CG Bio Co., Ltd.) was used as a control dressing material.
2.2 Porcine full-thickness skin defect model
The experimental protocol used in this study was approved by the Animal Institutional Review Board of Asan Life Science Research Center and the animals were cared for according to the guidelines for care and use of animal research (document number: 2015-04-131). Yorkshire pigs were used in the experiments to generate a porcine model of wound healing. Briefly, eight pigs were sedated via an intramuscular injection of ketamine, xylazine and alfaxan (60:12:0.6). Tracheal intubation was then performed, and anaesthesia was maintained through inhalation of 1% halothane. All animals were intubated orally with cuffed endotracheal tubes, and mechanical ventilation was established using a Drager-Fabius ventilator (Dräger Medical AG & Co. KG; Lübeck, Germany) in the volume-controlled mode (70% nitrous oxide, 30% oxygen). Ventilatory settings were identical for all animals (respiratory rate, 15 breaths/min; minute ventilation, 12 L/min). The vital signs were continuously monitored throughout the procedure using an electrocardiogram and a pulse oximeter. After the procedure, 500 mg of acepromazine was mixed into the animal feed to sedate the pigs and stabilise the dressing material. An intramuscular injection of gentamycin was administered as prophylactic antibiotic. The animals were shaved and scrubbed for surgery and each dressing change procedure.
Full-thickness skin defects were created with the pigs in the prone position. Six wounds per pig were designed on the backs of the animals from the posterior superior iliac spine (PSIS) to the inferior margin of the scapula. Symmetrical wounds measuring 6 × 6 cm were created, each separated by 5 cm to avoid interference between groups (n = 16 per group). The wound at each end was 3 cm from the PSIS and the inferior border of the scapula landmark. After the final experiment, the animals were euthanised with a lethal dose (60 mM) of intravenously injected potassium chloride.
2.3 Wound treatments
According to the planned treatment methods, the experimental animals were divided into three groups: Group A, mADM with foam dressing; group B, mADM with NPWT; and group C, gelatin with a foam dressing (Figure 1). The wound dressings (foam dressing and NPWT dressing) were changed every 2 days until complete epithelialization was achieved. During the experiment, mADM was applied only once. CURAVAC was used to apply NPWT in accordance with standard treatment protocols, with a negative pressure of 125 mmHg maintained in a continuous mode. In the treatment intervals, all animals were housed in separate cages and given oral analgesics.
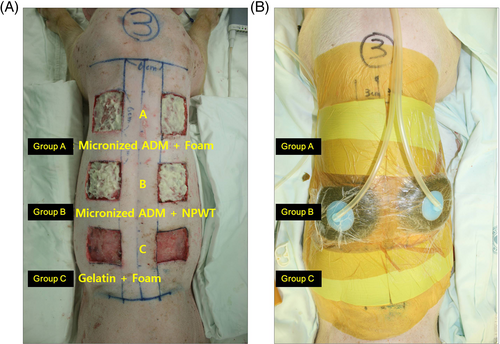
2.4 Measurement of wound area
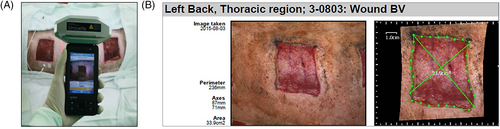
The sizes of the wounds were measured with a Silhouette hand-held device using infrared scanning technology (Aranz Medical, Christchurch, New Zealand) (Figure 2). Scanning with this device creates a 3D model of the wound and measures its size. In addition, changes in the wound area were continuously recorded using the device's built-in software.
2.5 Histological evaluation
Samples were taken from each group at postoperative day (POD) 10 to compare granulation formation histology. Each tissue sample was preserved in a paraffin block for sectioning and staining on a slide. Haematoxylin and eosin (H&E), and Masson trichrome (MT) staining, which are universal markers of wound healing, were used to evaluate angiogenesis and collagen formation.
2.6 Statistical analysis
Changes in the wound area were recorded and compared between the treatment groups. Data were entered into an Excel spreadsheet (Microsoft Corp, Redmond, Washington), and calculations and statistical analyses were conducted using SAS version 9.1 software (SAS Institute, Cary, North Carolina). Analysis of variance and Tukey's HSD tests were performed for multiple comparisons. A linear mixed model procedure was used to investigate significant interactions between factors affecting the wound area (POD timepoints and groups). Statistical significance was set at P < .05.
3 RESULTS
All eight pigs used in our wound healing model survived until the end of the experiments, and none were excluded from the analysis. In addition, no abnormalities, such as infections, ulcerations or weight loss were observed in any animal.
3.1 Wound size measurements
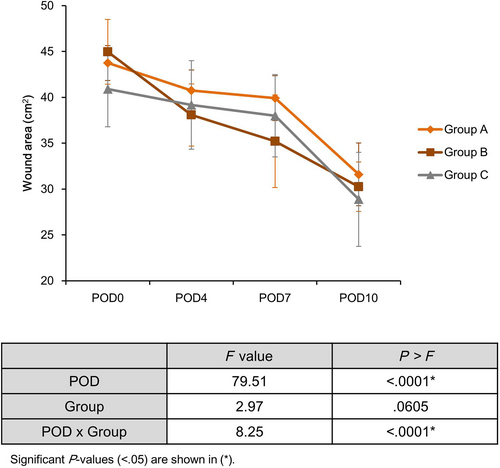
The mean wound area was measured on POD 0, 4, 7 and 10 (Table 1). Group B had the largest wound area at POD 0 baseline (Group A, 43.74 ± 3.53 cm2; Group B, 44.95 ± 1.90 cm2, Group C, 40.91 ± 4.11 cm2). The wound area on POD 0 was significantly different between the three groups (B > A > C, P = .0040). Post hoc analysis showed that this was because of the average difference between groups B and C (P = .0035). The wound area decreased over time in all the groups. The wound area at POD 4 was the smallest in group B, but there was no significant difference among the three groups (A > C > B, P = .1277). The wound area at POD 7 was the smallest in Group B (Group A, 39.91 ± 5.04 cm2; Group B, 35.21 ± 2.44 cm2; Group C, 37.98 ± 4.49 cm2). There was a significant difference in wound area on POD 7 between the three groups (A > C > B, P = .0093), which was because of the average difference between Group A and Group B, as shown through post hoc analysis (P = .0068). There was no significant difference in wound area between the three groups on POD 10 (A > B > C, P = .6279). In conclusion, although group B had the largest wound area at baseline, it had the smallest wound area at POD 7.
| Wound size, cm2 (mean ± SD) | ANOVA | Tukey HSD post hoc (P) | ||||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | F-test | P-value | A vs B | A vs C | B vs C | |
| POD 0 (n = 16) | 43.74 (±3.53) | 44.95 (±1.90) | 40.91 (±4.11) | 6.2603 | .0040* | .5605 | .0510 | .0035* |
| POD 4 (n = 16) | 40.74 (±3.39) | 38.08 (±2.23) | 39.18 (±4.83) | 2.1549 | .1277 | .1086 | .4527 | .6716 |
| POD 7 (n = 16) | 39.91 (±5.04) | 35.21 (±2.44) | 37.98 (±4.49) | 5.2004 | .0093* | .0068* | .3931 | .1531 |
| POD 10 (n = 4) | 31.60 (±2.68) | 30.25 (±3.43) | 28.88 (±5.13) | 0.4904 | .6279 | .8771 | .6008 | .8737 |
- Abbreviations: ANOVA, analysis of variance; POD, postoperative day.
- * Statistically significant difference: P < .05.
A linear mixed model was used to investigate the effect of time and group two factors on changes in wound area (Figure 3). The effect of time on the change in wound area was statistically significant (P < .0001), but the effect of the group alone on the change in wound area was not significant (P = .0605). The wound area decreased over time in all groups. The graphs of Groups A and C were parallel without intersecting each other, but in the case of Group B, after the baseline, the graphs of the other two groups crossed several times. This result indicated a significant interaction between time and the intervention applied to each group in the change in wound area (P < .0001).
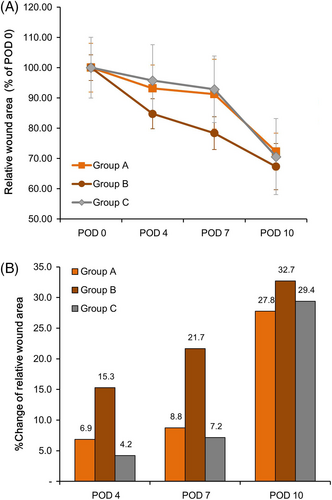
Because there was a significant difference in the baseline wound area between the three groups, it was converted to a normalised value when the baseline was considered to be 100% for adjustment (Figure 4). The absolute value of the X-intercept of the linear prediction trend line (Group A 8.562, Group C 9.1151, Group B 10.449) showed that the wound area decreased more rapidly in Group B than in Groups A and C (Figure 4A). In addition, the % reduction in wound area compared with baseline at all time points was greater in Group B than in Groups A and C (POD 4; Group B 15.3% > Group A 6.9%, >Group C 4.2%/POD 7; Group B 21.7% > Group A 8.8%, >Group C 7.2%/POD 10; Group B 32.7% > Group C 29.4%, >Group A 27.8%). This trend was more evident in the early stages of POD 4 and POD 7 (Figure 4B).
3.2 Histologic findings
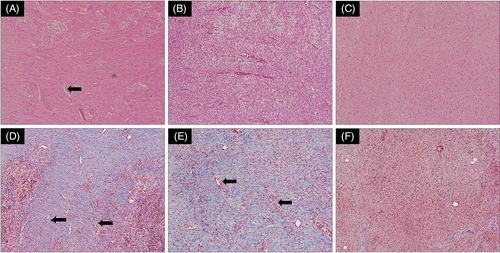
Neovascularization and collagen deposition were compared using H&E and MT staining. In Groups A and B, in which mADM was applied, neovascularization was observed in the thin endothelium lining with hollow lumen, and collagen was densely packed in the subcutaneous layer (Figure 5A,B). In particular, collagen deposition was significantly observed in Group B, wherein application was combined with NPWT, rather than in Group A (Figure 5D,E). In contrast, in Group C, wherein gelatin was applied, neither collagen deposition nor angiogenesis was observed (Figure 5C,F).
4 DISCUSSION
Here, we demonstrated the superior healing effect of a combined treatment of mADM plus NPWT compared with the effects of mADM and gelatin in a pig model with an acute open wound. Clinical studies have shown the healing effect of combined treatment using mADM plus NPWT, but previous studies did not evaluate changes in the gross sectional area size of the wound during the healing process or provide histological evidence to support its widespread use.
Many types of wound dressing materials, ranging from gauze to NPWT, are currently in clinical use. NPWT is known to promote wound healing by removing exudates containing inflammatory substances, improving wound perfusion, promoting tissue granulation, preventing wound infection, strengthening cellular signalling pathways involved in wound healing and mechanical shrinkage of the wound area.16-19 In this study, foam dressing was applied to the two groups (Group A, mADM; Group C, gelatin) where we did not apply NPWT. Foam dressing, such as Easyfoam, is one of the most widely used wound dressing materials, absorbing excess exudate from wounds to maintain an adequately moist environment.
The mADM used in this study was prepared in a paste formulation after grinding human-derived dermis into mADM powder. Compared with animal-derived ADM, which may induce allergic reactions because of foreign antigens (eg, alpha-gal) in the human body, even after decellularization, there is no concern regarding the immunogenicity of human-derived ADM. ADM induces nutritional diffusion and structurally provides a framework for cellular activities, such as cell adhesion, differentiation and migration,20 thus promoting revascularization and cellular repopulation.2 Unlike the sheet type, mADM is fractionated; therefore, it has a much larger contact area than general dressing materials and can be easily and quickly applied to deep and narrow spaces, such as vertical wound planes and cavities. When mADM was applied to severe patients with bone or tendon exposure, it formed a proper granulation tissue bed for skin grafting. According to a retrospective study by Jeon and Kim, when mADM was applied to seven patients with narrow and deep chronic wounds, healing was achieved in five patients (71.42%) within an average of 2.4 weeks.9
The clinical evidence for the effectiveness of mADM plus NPWT combined treatment has been presented through several case reports and clinical trials. In a case report of a patient with Buerger's disease, who had an ischemic entire dorsal foot ulcer, revascularization and healthy granulation tissue were observed on the wound surface after percutaneous transluminal angioplasty followed by a combined treatment three times a week for a total of 2 weeks. Epithelization began at the edge of the wound. After 6 months, the wound was completely healed, and there was no recurrence or complication of the ulcer.10 In a case report of a patient with skin necrosis in the lower leg because of a massive silicone injection, when the combined treatment was applied twice a week for 2 weeks, healthy granulation tissue and gross wound margins were observed after 3 months. The wound healed completely, and there were no treatment-related complications from treatment.11 In a retrospective clinical study of 20 patients with hard-to-heal wounds, when the combined treatment was applied twice a week. After 2 weeks, the wound depth started to decrease significantly compared with the previous week, and the wound depth at 4 weeks was significantly reduced compared with the baseline. The post-wound area was significantly reduced by 59.10% compared with the baseline, indicating an excellent wound healing effect.12 In a recent prospective randomised trial, the wound healing effect of the combined treatment was compared 1:1 with NPWT alone in a total of 30 patients with DFUs. After 42 days, the wound closure rate of the combined treatment group was 57.1%, which was significantly higher than that of the NPWT alone group (28.6%). After 120 days, the wound closure rates of the combined treatment group and the NPWT alone group were 93.3% and 85.7%, respectively, indicating that the combined treatment showed a superior wound healing effect than NPWT alone.14 Based on this clinical evidence, simultaneous application of mADM and NPWT is expected to augment the wound healing process.
In this study, changes in the gross sectional area of the wound were tracked for up to 10 days after treatment. In general, the wound healing phase can be divided into a haemostatic phase (~15 minutes), an inflammatory phase (~7 days), a proliferative phase (~3 weeks), and a remodelling phase (~2 years). The observation period of this study was 10 days; this extended up to when the inflammatory phase ended, and the proliferation phase began, with active granulation through collagen deposition. Although the wound area of the combined treatment group (Group B) was significantly larger at baseline (POD 0), it was significantly smaller at POD 7 (Table 1), and the highest % change in wound area with time was seen in Group B (Figure 3). The interaction effect of the intervention applied to Group B on the reduction of wound area over time was also confirmed through a linear mixed model (Figure 4). Therefore, in this study, we found that the wound area reduction effect was excellent when combined with NPWT, rather than mADM application only, and this was more pronounced in the early stages (POD 4 and 7).
Histological analysis (H&E and MT staining) was performed on the biopsy tissue to further evaluate the effects of the different interventions in each group (Figure 5). Because collagen fibres function as the framework of the dermis, the generation of new collagen fibres is considered a major factor in the wound healing process.21, 22 Higher levels of collagen deposition and neovascularization were observed in Groups A and B, in which mADM was applied, compared with Group C (gelatin). Furthermore, collagen deposition was more prominent in group B than in group A, confirming that the granulation-promoting effect of the combined treatment, compared with mADM alone, was histologically superior. Excluding the effect of NPWT, group A showed more collagen deposition than group C. This result is similar to that of Jang et al, who compared the wound healing effect of paste-type mADM with gelatin alone and collagen sponge using a rat skin defect model. After 48 days of MT staining, thick collagen bundles were generated in the mADM group and arranged in dendritic arrays close to normal skin, whereas only thin collagen fibres were observed in the gelatin alone and no treatment groups.15 However, the results of this study differed at the time of analysis (4 weeks vs 1 day), so the amount of collagen production or arrangement was judged to be relatively larger and more clearly observable than in this study. In conclusion, it was found that mADM containing various ECM protein components, such as elastin and fibronectin, in addition to collagen, showed a better collagen deposition effect than gelatin alone, containing only partially hydrolysed collagen.
To the best of our knowledge, this study is the first to compare the wound healing effect of a combined treatment of mADM plus NPWT with mADM and gelatin in pigs. NPWT would contribute to this kind of result by reducing the wound area and additional discharge. The mechanical feature of NPWT makes the wound more compact, and mADM penetrates better through this gap, reducing the dead space in the wound, which can be considered to further promote wound healing. It was demonstrated that the application of mADM has a superior wound healing effect compared with gelatin alone, and that when NPWT is used in parallel, a synergistic effect appears with regards both wound area reduction and collagen deposition.
This study has several limitations. First, there was a significant difference in the wound area between the three groups at baseline. Nevertheless, the NPWT group showed the most significant reduction in wound area, and showed the same trend in the normalised graph. Second, the observation period was as short as 10 days. The proliferation phase of the wound healing phases begins after about 10 days; hence, a more extended observation period will be required to observe more reliable collagen deposition and arrange patterns. In addition, it will be necessary to analyse how the combined treatment affects the shrinkage of the wound margin and scar formation through long-term observation. Third, histological analysis was performed using H&E and MT staining, but a quantitative analysis was not performed. In the future, if the distribution pattern of fibroblasts and myofibroblasts or the pattern of distribution of collagen type (type I vs III) is quantitatively analysed using various immunological markers, the wound healing effect of mADM plus NPWT combined treatment can be fully understood. Finally, this experiment was conducted on the porcine model. As it is not a product that has been clinical tested in humans, it can be acted differently in the actual model. It would be better to discuss the exact effectiveness if further clinical trials were conducted.
5 CONCLUSION
This study analysed the wound healing effect of paste formulation mADM combined with the NPWT technique using in vivo models. Compared with gelatin, mADM showed dense collagen deposition and neovascularization. Furthermore, when NPWT was combined with mADM, a synergistic effect appeared, and this resulted in significant reduction in wound area and improved quality of wound healing.
ACKNOWLEDGEMENTS
This study was supported by the Daewoong Foundation, and statistical analysis and English proofreading were supported by CG Bio Co., Ltd.
FUNDING INFORMATION
This work was financially supported by Research Support Project for Young Medical Scientists grant funded by the Daewoong Foundation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




