Development of ZnO/selenium nanoparticles embedded chitosan-based anti-bacterial wound dressing for potential healing ability and nursing care after paediatric fracture surgery
Abstract
The current study was aimed at the assessment of the effect of chitosan-ZnO/Selenium nanoparticles scaffold on infected wound healing and care of paediatric surgery treatment. The nanoparticle scaffolds were developed from sources such as chitosan (CS), different concentrations of zinc oxide (ZnO), and Selenium (SeNPs) nanoparticles by freeze-drying method. The structural and chemical properties of nanoparticles were investigated by UV–Vis, fourier transform infrared spectroscopy (FTIR), and phase identification by x-ray diffraction analysis. The surface morphology of CS, chitosan-ZnO (CS-ZnO) and chitosan-ZnO/SeNPs was analysed using a scanning electron microscope. The incorporation of ZnO and SeNPs along with CS polymer induces antioxidant and antimicrobial functions. The bacterial susceptibility to nanoparticle scaffolds against Escherichia coli and Staphylococcus aureus showed the excellent antibacterial effects of ZnO and SeNPs. In-vitro studies of fibroblast of NIH 3 T3 and HaCaT cell lines revealed the biocompatibility, cell adhesion, cell viability, and proliferation of scaffold in the wound site. Also, results of in-vivo studies strongly enhanced collagen synthesis, re-epithelialization, and rapid wound closure. Thus, the synthesised chitosan-ZnO/SeNPs nanoparticle scaffold resulted in significant improvement in histopathological indices in the full thickness of wound healing after nursing care of paediatric fracture surgery.
1 INTRODUCTION
Very limited data and reports have existed related to post-operative wound infection in paediatric patients, meanwhile, a large number of reports and literatures are available on this subject for adults. As previously reported studies, neonates were a more susceptible group and have a high possibility of wound infection that ranged from 21% to 38%.1, 2 Moreover, the incidence of skin wound infection has increased with the development of today's society.3 The regeneration of skin tissue at the site of injury is still really a challenging task in the field of biomedical science.4 Generally, wound repair is a complex process involving a series of overlapping phases, including matrix deposition, inflammation and tissue remodelling. The duration of these individual phases varies depending on the intensity and depth of the wound.5 Currently, most wound dressing treatments aim to facilitate these stages of wound healing by providing a moist environment, controlling excessive exudate buildup and protecting against infection that would perturb normal healing.6
However, the wound-healing effect has not yet met the clinical requirement. Moreover, there may be infections caused by various bacteria.7 Therefore, it is quite necessary to develop an alternative substitute in order to better repair the skin injury. In recent years, various biomaterials scaffolds have been developed for wound healing and skin regeneration.8, 9 However, functional recovery is still far from normal skin, and thus much better scaffolds for wound healing and skin regeneration are needed. In recent years, porous composite biomaterials, which have many advantages, have been widely applied in the field of tissue engineering and regeneration medicine.10, 11 A porous scaffold consisting of collagen and hydroxyapatite was developed for drug delivery devices.12 The study showed that the endocortical woven and lamellar bone formation could be enhanced with neurotrophic factor loaded by the scaffolds. Thus, the porous scaffolds were believed to be very suitable for filling the irregular defects in cosmetic surgery. In another study, the porous chitosan-siloxane scaffolds for nerve regeneration were fabricated, and the inflammatory response during wound-healing processes was evaluated. It was found that the treatment with porous chitosan-siloxane scaffolds showed low inflammation and better posttraumatic axonal regrowth, indicating a good functional recovery.13
In addition, both the chitosan-modified poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) porous scaffolds loaded with cord blood (CB)-derived unrestricted somatic stem cells (USSCs) and the collagen/chitosan porous scaffolds crosslinked by glutaraldehyde (GA) were found to have effects to promote skin regeneration.14 As mentioned above, the porous biomaterials scaffolds could act as a protective barrier, absorb excess wound exudate, and maintain a moist environment, which in turn helps in pain reduction. It is well known that a moist wound environment could accelerate wound healing.15 Therefore, the porous biomaterial scaffolds showed a promising future in clinical application for wound healing and skin regeneration. Keratin could be extracted from silk, feathers, nail, wool, and human hair. It contains cell adhesion sequence, RGD (Arg-Gly-Asp) and LDV (Leu-Asp-Val), which are found in the extracellular matrix (ECM) proteins such as fibronectin.16 The cellular-binding motifs on keratin could mimic the sites of cellular attachment found in the native ECM. Therefore, keratin could be utilised for the construction of tissue engineering implants. It has been reported that keratin biomaterials in the form of sponge scaffolds have been developed for different biomedical applications such as wound dressings and neural tissue engineering applications.17
The mouse fibroblast cells were found to proliferate well on the keratin scaffold,18 suggesting the good biocompatibility of keratin. Thus, keratin is expected to be applicable for biomedical use. However, the degradation property and unsatisfied mechanical properties are still the limitations for its sole application as a tissue scaffold.19 Chitosan (CS) used in scaffold material with excellent biocompatibility, good mechanical properties, non-toxicity and good antibacterial activity has been intensively studied and widely used in different tissue engineering fields.20 The collagen-chitosan (CCH) scaffold immobilised with RGD sequences was found to have an effect to promote rapid regeneration of injured sciatic nerves in rats.21 Chitosan combined with organic or inorganic biomaterials could form composite scaffolds, which had been used for bone tissue engineering.22
The chitosan/collagen scaffold, chitosan/poly (caprolactone) (CS/PCL) nanofibrous scaffold and chitosan/silk fibroin (CS/SF) blend scaffolds were developed by different research groups, and they were found to have effects to effectively promote skin tissue regeneration. In addition, the antibacterial activity of chitosan is also very important for most tissue regeneration. Zhao et al.,23 fabricated chitosan/sericin composite nanofibers by electrospinning, and they found that the composite nanofibers showed good bactericidal activity against both gram-positive and gram-negative bacteria, which are promising for wound dressing applications. The chitosan/phosvitin antibacterial scaffolds were fabricated via layer-by-layer deposition by Zhou et al.,24 the result of the microbial inhibition assay indicated that the composite nanofibrous mats had excellent antibacterial activity against Escherichia coli and Staphylococcus aureus, which could be used for antimicrobial packaging, tissue engineering, wound dressing, and so forth. Thus, according to the analysis mentioned above, the combination of keratin and chitosan is anticipated to improve both the mechanical properties and antibacterial activity of the composite scaffolds, which is beneficial for wound healing and skin regeneration, though the keratin-chitosan composite scaffolds were investigated.25
Studies on metal nanoparticles and drug delivery systems have shown that some of these materials can reduce minimum inhibitory concentration (MIC), thus increasing the effectiveness of antibiotics.25, 26 Some reports are available on the antimicrobial activity and wound healing properties of selenium nanoparticles (SeNPs),27-30 chitosan-based hydrogel31-33 and chitosan-ZnO (CS-ZnO) nanocomposites,34-36 but their efficacy such as MIC reduction along with mupirocin has not been studied so far. Selenium is a microelement that plays an important role in the health of the human body.37 Chitosan with known antimicrobial effect is a biocompatible and biodegradable polysaccharide that is produced by deacetylation of chitin,38 showing promising applicability in future drug delivery systems. It is postulated that based on the antibacterial activity and wound healing properties of chitosan and SeNPs, a combination of these two components may induce a synergistic effect, and their use along with mupirocin may help to increase its efficacy by reducing MIC. Moreover, it seems that the chemical modification of chitosan with cetyltrimethylammonium bromide (CTAB) in the nanohybrid system can improve the antimicrobial activity of chitosan.39-42 Zinc oxide (ZnO) was widely related to exhibit antimicrobial activity and higher stability than organic molecules.43-45 Also, it was used to accelerate the healing of both chronic and acute wounds,46 because of its epithelialization and bacteriostatic properties. ZnO represents today one of the most reliable choices in obtaining composites with potential applications in wound care.47, 48
In this work, we designed and developed a ZnO/SeNPs loaded CS hydrogel scaffold as a wound dressing material for potential healing and care of paediatric surgery. Antibacterial studies were then performed for CS-ZnO/SeNPs against S. aureus and E. coli Pathogens. Interestingly, we discovered that the chitosan-ZnO/SeNPs scaffold shows a significant improvement in planimetric and histopathological indices in the full thickness of wound healing. To the best of our knowledge, there are no reports published on wound healing applications for this scaffold made from the combination of CS-ZnO/SeNPs hydrogel dressing.
2 MATERIALS AND METHOD
2.1 Preparation of CS and CS-ZnO nanostructures
At the start, the extracted chitin (0.5 g) was dissolved in (2% v/v) of 10 mL acetic acid solution and then subjected to constant stirring in a magnetic stirrer at 1200 RPM for 2 h at 80–100°C, due to the bath temperature to obtain a pale yellow chitin solution. Thereafter, a freshly prepared 30 mL of 45% (W/V) of sodium hydroxide solution was added in a micro addition, until the formation of white-coloured CS precipitated in the bottom of the flask.42 The process settle down for 24 h, finally, the precipitate was filtered using a suction pump and dried in a hot air oven at 200°C. For the CS-ZnO nanocomposites, 30 mL of 25% (W/V) zinc chloride solution was added slowly dropwise into the pale yellow coloured chitin solution and then micro addition of 30 mL of 45% (W/V) NaOH solution.
2.2 Synthesis of SeNPs
A stock solution of 15 mM (4.306 mg m−1) sodium selenite pentahydrate (Na2SeO3. 5H2O) and 75 mM (13.678 mg m−1) ascorbic acid (Sigma-Aldrich) was prepared and then 24 mL of distilled water was added to 1 mL of sodium selenite stock solution.49 Then prepared stock solution of ascorbic acid 1 mL was slowly added to sodium selenite solution under magnetic stirring for 2 h and temperature of the bath was slowly raised to 70°C.50, 51 After changing the colour to light orange at 35–40 min, the mixture was diluted to 25–50 mL with distilled water.
2.3 Synthesis of chitosan-ZnO/SeNPs scaffold
A prepared chitosan-ZnO (CS-ZnO) was dissolved into (1% v/v) dilute acetic acid solution to a concentration of (2% w/v) while stirring on a magnetic hot plate. The solution was stirred with moderated heat of 100°C for 3 h. the resultant CS-ZnO solution was filtered through the Whatman filter paper after vacuum filtration to remove any undissolved particles. For the preparation of chitosan-ZnO/SeNPs, an appropriate amount of chitosan-ZnO 1.5 mL 0.227 mol/L Vc and 1 mL of 2.40 mol/L acetum, respectively. The appropriate amount of 5.36 mmol/L Se (IV) solution was added to the mixture, then the mixed solution was diluted to 10 mL. The above sample is designated as CS-ZnO/SeNPs.
2.4 Physicochemical analysis
The prepared scaffold as wound healing materials were characterised using the following instrumental analysis. The phase analysis of the prepared scaffold as wound healing material was identified using an x-ray diffractometer (X'Pert PRO PANalytical diffractometer) with Cu Kα radiation (λ = 0.15406 nm) and a scanning rate of 0.01°/step in the 2θ range of 10–80°. Functional groups were analysed by FTIR spectra and recorded using a Thermo-Nicolet-380 model (Thermo Fisher, Madison, USA) spectrum in the range of 4000–400 cm−1 at room temperature. UV–vis absorption spectra were recorded using a 2401 PC model spectrophotometer (Shimadzu, Kyoto, Japan) with a wavelength range of 200–800 nm. Morphological properties were analysed using a scanning electron microscope (Hitachi-S3000 H, Hitachi, and Tokyo, Japan).
2.5 Antibacterial activity
The antibacterial activity of synthesised CS-ZnO/SeNPs was examined using both gram-positive (S. aureus) and gram-negative bacteria (E. coli) by disk diffusion method. These two bacteria were grown in nutrient broth at 37°C for 24 h, approximately, 20 mL of molten nutrient agar was poured into the petri dishes and cooled. The two tested organisms were swapped over the nutrient agar medium, and the disks containing CS-ZnO/SeNPs were kept over the medium using sterile forceps. The CS-ZnO/SeNPs -loaded disks were prepared at different concentrations of 25, 50, 75 and 100 mg mL−1 disc. For antibacterial activity, gentamycin (25 μg/mL) was used as a standard drug. The plates were kept in a refrigerator at 2–8°C for a period of effective diffusion of test compounds and standards. Later, they were incubated for 24 h at 37°C, and the inhibition zone diameters were measured. The experiment was carried out three times.
2.6 In-vitro studies
The wound healing performance of fibroblast on NIH 3 T3 fibroblast and Human keratinocytes (HaCaT) cell lines was evaluated using MTT assay. The cultured cells with DMEM medium and cultivated over CS-ZnO/SeNPs in 24 wells plate for 24 h. The fetal calf serum supplemented with 100 Units mL−1 Penicillin, 150 mg mL−1 Gentamycin, 60 mg mL−1 Streptomycin and 2 mg mL−1 Amphotericin B was maintained at 37°C. Further 100 μL of solution was taken and transferred to 96 well plates. The cells without scaffold are considered as control. The absorbance of the solution was measured using a Universal Microplate reader at 570 nm. To evaluate the cell attachment, cell adhesion and morphology over the scaffold, the medium was removed and washed with Phosphate Buffer Saline (PBS). The in vitro cell proliferation was visualised using a fluorescence staining technique performed over the cells of the scaffold as wound healing materials at 24 h. Then, the stained cells were counterstained using DAPI (2 mg mL−1) and Calcein AM (2 μM) and incubated at 37°C for 30 min and visualised the nuclei cells and live cells using Phase Contrast Microscopy.
2.7 In-vivo studies
Male albino Wistar rats with a weight of 200–220 g at 5 weeks of age were purchased and used in this study. The rats were individually housed and monitored under light/dark phases at 25°C and were provided with standard rodent feed. In this study, rats were divided into three groups, each group containing three animals (control, CS-ZnO, CS-ZnO/SeNPs scaffold and standard drug). All the rats were acclimatised 1 week prior to the commencement of the study. The scaffold-treated groups showed significant wound healing from 4th day onwards which was comparable to that of the standard drug, that is, the povidone-iodine ointment-treated group in all three wound models.
2.7.1 Surgical and wound dressing procedure
In rats' excision wounds, CS-ZnO/SeNPs scaffold was evaluated to determine the potential wound healing efficiency. Also, the control and CS-ZnO scaffold were also examined with same excisional wound size. During this study, the rats were anaesthetised below the cervical region using standard anaesthesia thiopentone sodium. Before full-thickness excision wound creation (2.5 × 2.5 cm2), the dorsal surface of the skin was disinfected and shaved. Control (Group I) was treated with sterilised povidone-iodine gauze at the wound site. The test animal group CS-ZnO (Group II) and CS-ZnO/SeNPs (Group III) were applied with fabricated scaffolds. The wound dressings were sterilised before dressings and the dressings were changed periodically. Also, the three rat groups were sacrificed at each time interval of 4 and 16 days. The wounds were cleaned and granulation tissues were collected from each group and processed for histology studies and remaining stored at −80°C until analysis. Finally, the wound healing mechanism of all groups was evaluated periodically on the wound surface for wound contraction, tensile strength, biochemical, and histological analyses.
2.7.2 Rate of contraction and period of re-epithelialization
2.7.3 Biochemical analysis
During biochemical estimation, the amount of total protein, hydroxyproline and hexosamine of granulated tissues was analysed using accepted wound healing makers, the total amount of collagen (hydroxyl proline) on granulated tissues by the Woessner method.52 The protein content was analysed by the Lowery method53 and hexosamine was determined by using Elson and Morgan method42 for both treated and untreated wounds.
2.7.4 Histopathological analysis
The granulated tissues from various time intervals of post-wound healing were used for histopathological study. Further by using a tissue embedding procedure, 5 μm thick sections were sliced using a microtome and fixed on a glass slide.54 Later the staining was performed with haematoxylin-eosin stains to evaluate the dermal connectivity of granulated tissues. Finally, the stained sections were micro-graphed using a microscope.
2.8 Statistical analysis
All quantitative data were statistically analysed and presented by means of standard deviation (SD). Factual investigation was assessed using Graph Pad Prism (latest version). Also, a student's t-test (expecting equal variances) was performed to estimate the statistical significance between each experimental group. A value of P < .05 was considered to be statistically significant.
3 RESULTS AND DISCUSSION
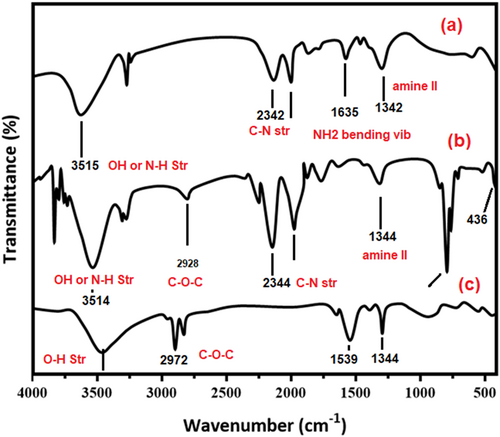
3.1 FTIR spectroscopy
The intermolecular interaction of CS, chitosan-ZnO (CS-ZnO) and chitosan-ZnO/Se nanoparticles (CS-ZnO/SeNPs) scaffold as wound healing materials was characterised by Fourier transform infrared spectroscopy (FTIR) in Figure 1A–C. Figure 1A shows the chitosan characterisation peaks observed at 3445,1645, 1568, 1376, and 1029 cm−1 were regarded as OH or NH stretching vibration, CO stretching from amide I, NH bending and CN stretching from amide-II and skeletal vibration of CO stretching, respectively. The spectrum of the SeNPs scaffold was different, with highlighted wavenumber ranging from 1000 to 3700 cm−1. The peak of OH or NH stretching vibration became wider and flatter; indicating that hydrogen bonding was enhanced.55 The newborn peak that appeared in Figure 1B at 550–400 cm−1 was attributed to the ZnO stretching vibration of chitosan-ZnO nanocomposites. The peaks of amide I and amide II in the blank chitosan/ chitosan-ZnO complex (free of SeNPs) overlapped and became an intensive absorption peak at 1569 cm−1, due to the electrostatic interaction between chitosan and chitosan-ZnO/SeNPs characteristic peak. Here we have included the comparison table for spectral data CS, chitosan-ZnO (CS-ZnO), and chitosan-ZnO/SeNPs (Table 1).
| Sample name | Wavenumber (cm−1) | Functional group |
|---|---|---|
| CS (chitosan) | 3445 | O-H or N-H stretching vib |
| 1645, 1568 | C=O stretching from amide I, N-H bending | |
| 1376 | C-N stretching from amide-II | |
| 1029 | skeletal vibration of C-O stretching | |
| CS-ZnO (Chitosan-ZnO) | 3495 | O-H or N-H stretching vib |
| 1630, 1443 | N-H bending primary amine | |
| 1023 | Chitosan Polysaccharide | |
| 550–400 cm−1 | Zn-O stretching vibration | |
| CS-ZnO/SeNPs | 3498 | O-H stretching vib |
| 2972 | C-O-C stretching vib | |
| 1569 cm−1 | Chitosan & chitosan-ZnO/SeNPs characteristic peak. |
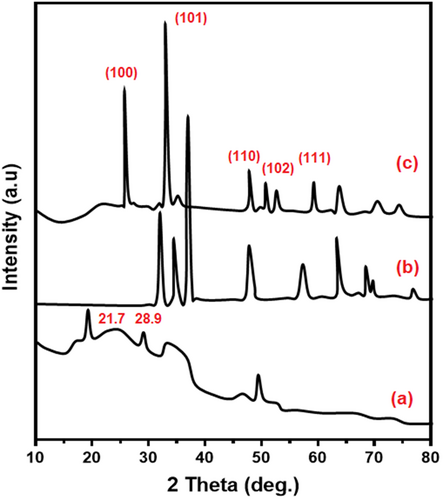
3.2 X-ray diffraction analysis
X-ray diffraction analysis (XRD) patterns of chitosan, chitosan-ZnO, and chitosan-ZnO/SeNPs scaffold wound healing materials are shown in Figure 2. Semi-crystalline nature of chitosan bio-polymer was confirmed by the appearance of peaks at 2θ = 21.7, 28.9° (Figure 2A). The crystalline nature of chitosan-ZnO nanocomposites shows the functional peaks at 2θ = 31.7, 34.6, 47.9, 56.7, 62.8, 66.7° of complexes are shown in Figure 2B corresponding to the (100), (110), (103), (112), (102), (103), and (200) lattice plane of ZnO (JCPDS No: 36–1451). When (0.5%) M/V of Zn and Se sources were incorporated in the composite with chitosan-ZnO and the selenium nanoparticle masked the chitosan-ZnO composite due to its biotransformation the SeNPs dominated. Whereas novel (111) SeNPs lattice planes are observed (JCPDS: 06–0362).56
3.3 UV–vis spectroscopy
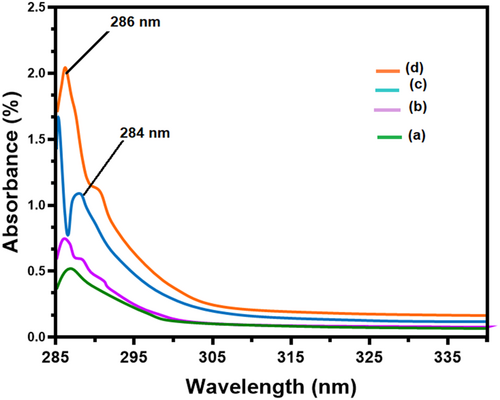
UV–vis spectra of CS, chitosan-ZnO (CS-ZnO) and chitosan-ZnO/SeNPs (CS-ZnO/SeNPs) scaffold as wound healing materials in the region of 200–800 nm are shown in Figure 3A–D. The pure CS and CS-ZnO nanocomposites showed absorption peaks at 287 and 286 nm, which is due to the electron promotion from the valence band to the conduction band by the absorption of light in the UV region. The corresponding UV absorption at 286 nm is due to the n → σ* transition resulting from the nonbonding electrons of the amide group from the chitosan.57 In Figure 3, peak-3c shows the absorption peak of selenium nanoparticles at 288 nm. The intensity of absorption band is linearly increased and increases of metal matrix ratio are up to 2% of Zn and Se sources to prepare the scaffold, and then merged eventually. Finally, the chitosan-ZnO/SeNPs scaffold has been observed in the absorption at 286 nm represented as peak-3d, as the incorporation of selenium blue shifted the wavenumber with a reduced band gap. This observation clearly enabled the strong formation of chitosan-ZnO/SeNPs.
3.4 Surface morphology
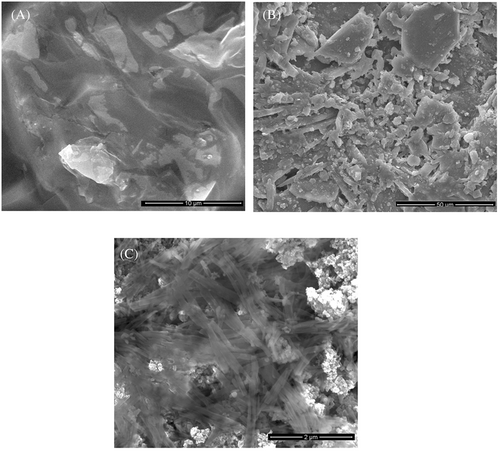
Surface morphology of chitosan, chitosan-ZnO, and chitosan-ZnO/SeNPs scaffold as wound healing materials are shown in Figure 4A–C. Figure 4A, B shows the semi-crystalline nature of the smoothed surface in that's particles are agglomerated (chitosan) and some of the microcrystalline particles again started to appear due to the presence of ZnO particles (chitosan-ZnO). Chitosan-ZnO/SeNPs images indicated the collection of various microparticles with the fibrous surfaces of structures was noticed. The size of these microparticles was 1–50 μm as shown in Figure 4C, determined by size distribution analysis based on Mie scattering theory.58
3.5 Antibacterial activity
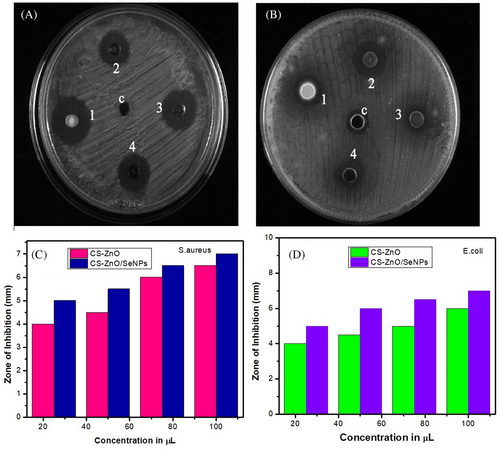
The antibacterial activity has been studied against gram-positive and gram-negative bacterial pathogens using four different concentrations of CS-ZnO/SeNPs samples (25, 50, 75, and 100 μL). Sharma and coworkers evaluated the ZnO nanoparticles against bacterial microbes and exhibit excellent antibacterial properties.59 Similarly, Srivastava and coworkers biosynthesized SeNPs and bacterial test results show excellent antibacterial and antifungal properties.60 Figure 5 shows the size of the zone of inhibition and antibacterial activity formed around each CS-ZnO/SeNPs loaded with test samples. The sample containing 100 μL of CS-ZnO/SeNPs shows the most significant effect on a zone of inhibition of 6.5 mm of S. aureus. The E. coli exhibit a modulated effect on the inhibition zone of 6 mm. Synthesised CS-ZnO/SeNPs showed better antibacterial activity against E. coli by disc diffusion method. In addition, green synthesised CS-ZnO/SeNPs were tested in antibacterial activity and showed a significant effect on gram-positive and gram-negative bacteria by disc diffusion method which is compared with standard drug gentamycin the scaffold showed excellent antibacterial activity.
3.6 In-vitro studies
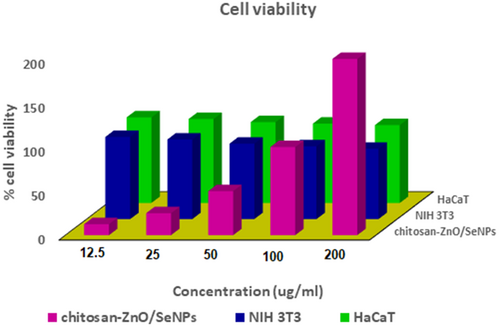
Smooth wound healing scaffold with enriched cell adhesion and cell proliferation are the important key factors in the tissue regeneration process. In-vitro biocompatibility of chitosan-ZnO/SeNPs scaffold as wound healing materials was evaluated on NIH 3 T3 and HaCaT cell lines after 24 h by MTT assay are shown in Figure 6. The cell seed over the chitosan-ZnO/SeNPs scaffold exhibits increased cell viability in both cells. The presence of Selenium nanoparticles in the scaffold enhances the cell viability in chitosan-ZnO/SeNPs. The in-vitro fluorescent staining activity of both scaffolds with Calcein Am and DAPI was determined using NIH 3 T3 fibroblast and HaCaT cell lines after 24 h. This top layer contains CS fibrous layer and the bottom layer contains ZnO/SeNPs fibrous layer completely adhered and undergoes cell proliferation on the cell line surface. In addition, the scaffold morphology supports flexibility and proliferates cells which reflect on the cell structure. The presence of chitosan attracts the cell migration of fibroblast, which induces cell adhesion in an increased manner. Also in keratinocytes, CS nanofibrous helps cell proliferation and induces cell migration at a later stage which is a good result for this wound dressing material. Moreover, the Selenium nanoparticles aid to stop the burning and irritation sensation at the wound site. Finally, the study result indicated the cytocompatible nature of the CS-ZnO/SeNPs.
3.7 Rate of wound contraction
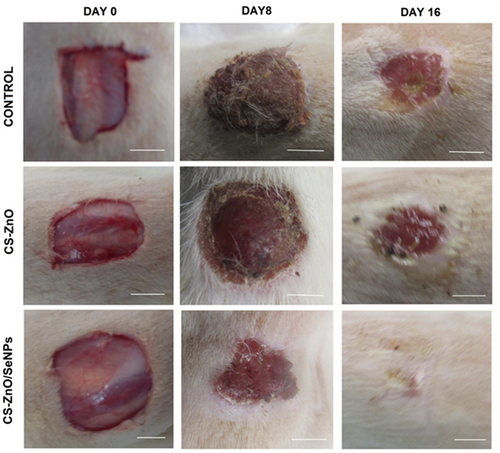
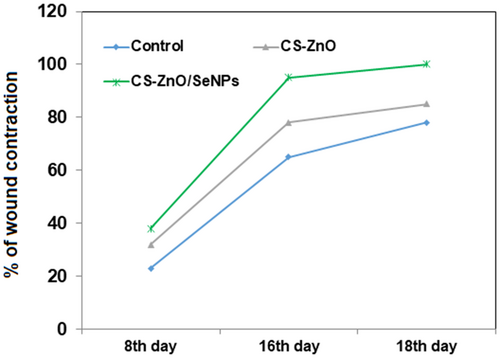
The wound healing of excisional wounds was calculated using planimetric sheet photographs. Figure 7 shows the phases of wound contraction at different time intervals. Also, the percentage of wound contraction was depicted in Figure 8. Here the graph clearly states that wound closure of the CS-ZnO/SeNPs scaffold exhibits the greatest healing rate from day 4 onwards when compared to the control group and CS-ZnO scaffold groups. However, the control group exhibits the least wound closure when compared to CS-ZnO and CS-ZnO/SeNPs on day 16. The presence of chitosan regenerates tissue growth but ZnO and SeNPs support faster healing from pathogenic inhibition.
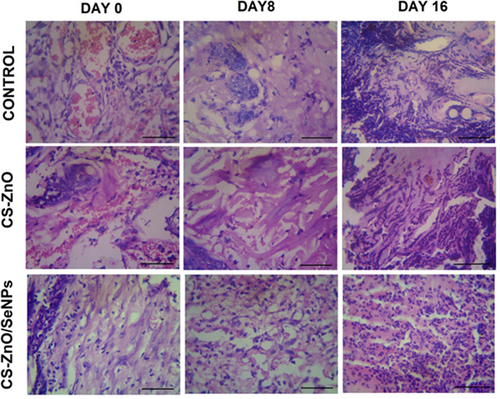
3.8 Histopathology study
From the histopathology evaluation, the phases of wound contraction in both groups were monitored. The inflammatory phase, re-epithelialization, and maturation phase can be measured through a cross-section of wounds. In our study also, the regeneration of tissues from the treated group performance was more prominent as granulation of tissues when compared to both the control and CS-ZnO groups. From day 0 to 8, H & S staining results clearly indicate that CS-ZnO/SeNPs treated group helps collagen deposition and induces more tensile strength with neovascularization shown in Figure 9. Also, fibroblast proliferation of the treated group exhibits the formation of more connective tissue and eliminates pathogenic infections on day 16, when compared to the control and CS-ZnO group. However, high proliferation index and blood vessel formation eventually create the formation of an extracellular matrix with a better healing rate. Thus, the reports of H & E clearly state that CS-ZnO/SeNPs treated group can be used as an alternate wound dressing substitute.
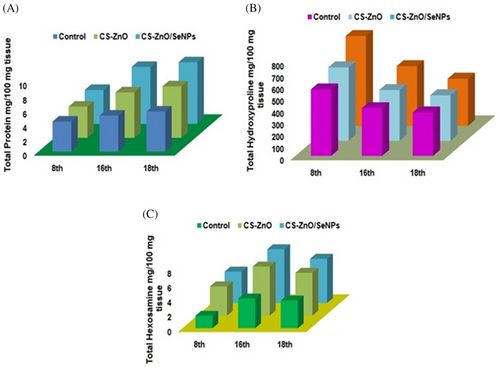
3.9 Biochemical study of excisional wounds
The granulation of tissues at the wound sites of both the control and treated group were biochemically analysed through total protein, hydroxyproline and hexosamine for a specific period of time after scarification as depicted in Figure 10. During granulation process, more amount of protein gets attached towards extracellular matrix, which formulates migration of fibroblast cells, increased haemostasis, and induces re-epithelialization for rapid wound healing. The chitosan and SeNPs significantly promote tissue granulation which was estimated by the presence of protein and given in Figure 10A. The protein content was increased from day 8 with a significant difference between the control group and CS-Zno/SeNPs treated group. However, on day 18, the protein content was slightly decreased due to the stabilised level of SeNPs. Thus, the level of protein and collagen synthesis of CS-ZnO/SeNPs treated group was carried throughout wound healing phase. The experimental groups exhibit significant increases in hydroxyproline and hexosamine content during day 8 of healing shown in Figure 10B, C. The hydroxyproline content of the treated group has more connective tissues and provides structural integrity to the tissues. From day 8 to 18, clearly states that collagen biosynthesis during granulation was prominently affected in CS-ZnO/SeNPs treated group compared to the control group. Thus, the hydroxyproline measurement indicates that an effective collagen deposition leads to complete wound closure during remodelling phase. Figure 10C shows an increased content of hexamine maintains collagen deposition and acceleration. However, ZnO and SeNPs increase the ability of collagen environment with infection free continuously. The wound healing process increases cell division, in CS-ZnO/SeNPs treated group on day 16, which indicates cumulative release and degradation of CS deliberates scaffold strength and integrates nearby cells. Thus CS-ZnO/SeNPs treated scaffold group effectively re-epithelialized and tissue adhesion on wounds without scar formation.
4 CONCLUSION
In conclusion, CS-ZnO/SeNPs scaffolds were developed from CS and ZnO and SeNPs using the freeze-drying method. The structural and chemical properties of the scaffold as wound healing materials were characterised by FTIR, XRD, and UV–Vis analysis. The surface morphology of the scaffold was analysed using SEM. Antibacterial activity of CS-ZnO/SeNPs against S. aureus and E. coli pathogens exhibits stronger activity. The chitosan-ZnO/SeNPs resulted in significant improvement in planimetric and histopathological indices in full thickness of wound healing. Thus, from this study, it could be concluded that chitosan-ZnO/SeNPs scaffold has a reproducible wound healing potential and hereby justifies its use in practice.
CONFLICT OF INTEREST
The authors declare that this manuscript has no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable for this article.




