Functional morphology of the reproductive system and sperm transfer in Stenopus hispidus (Crustacea: Decapoda: Stenopodidea), and their relation to the mating system
Abstract
The morphology of the reproductive system of a stenopodidean decapod is described here for the first time, with an interpretation of the sperm transfer process. Pairs of adults of Stenopus hispidus were maintained under laboratory conditions to observe reproductive cycles. Mating behavior and sperm transfer were video-recorded for analysis. After copulation, the shrimps were anesthetized and dissected to record the shape and location of the gonads, and pleopod morphology was described and illustrated. The reproductive systems (RS), thoracic sterna, and male and female genitalia were observed by scanning electron microscopy. The male reproductive system was restricted to the cephalothorax and was highly reduced compared with that of other decapods. Only the first pair of pleopods may be involved in the sperm transfer process; there was no appendix masculina on the second pair of pleopods as in many other decapods. The ovaries of prespawning females occupied much of the cephalothorax and reached to the 3rd abdominal segment. The oviducts were short and simple, without structures for sperm storage. We conclude that the male deposits a simple spermatophoric mass onto the posteroventral surface of the female and fertilization occurs externally as mature oocytes are subsequently spawned. This mode of sperm transfer and egg fertilization is ancestral within the decapod suborder Pleocyemata. As in some other animals, the relatively small size of the testes in S. hispidus may be related to the monogamous mating system, which may minimize selection for a large volume of sperm production.
The great diversity in various aspects of crustacean reproductive biology provides a rich source of characters that can help resolve phylogenetic relationships among the many groups of these arthropods (Bauer 1986, 1991). Descriptions of genitalia, sperm morphology and sperm transfer, and spermatophore storage mechanisms have been widely used for this purpose in recent years (Kronenberger et al. 2004; Mantelatto et al. 2009). The relationship of reproductive morphology and insemination mechanics is also crucial to an understanding of mating systems in different groups of animals (Soulsbury 2010). Additionally, an understanding of sexual behavior and the process of sperm transfer provides important information on the life history of crustaceans (Hazlett 1975; Wickler & Seibt 1981; Bauer 1986). Finally, a thorough knowledge of reproductive biology and mating behavior is essential for the successful aquaculture of species for human consumption or the “ornamental” species aquarium trade.
Mating behavior, pre- and post-copulation interactions, mode of sperm transfer, and success of insemination in marine crustaceans have been extensively investigated in recent decades. However, such information is still scarce compared with current knowledge on the same subjects in terrestrial arthropods (Bauer 1992, 2004, 2013). Within the decapod Crustacea, the function of various reproductive structures has been addressed by Yano et al. (1988), Bauer (1991, 1992), and Misamore & Browdy (1996) for Penaeoidea; Bauer (2004) for Caridea; Farmer (1974), Bauer (1986), and Waddy & Aiken (1991) for Astacidea; Hartnoll (1969), Diesel (1991), and McLay & López-Greco (2011) for Brachyura; and Hess & Bauer (2002) for Anomura.
According to Hinsch (1991), sperm produced in the testes of decapods is mixed with seminal fluid produced in the vasa deferentia and ejaculatory ducts, and reaches the exterior in variously formed aggregates called spermatophores. Spermatophores can vary in complexity. They may appear as a single sperm mass (a spermatophoric mass) that is deposited on the ventral surface of the sternum of females, as in most species of Caridea (Bauer 1986, 2004). Alternatively, spermatophores may be morphologically complex, as are the pedunculated structures in some species of Anomura (Mantelatto et al. 2009) or the highly ornamented and adhesive spermatophores of some species of Penaeoidea (Bauer 1991; Bauer & Min 1993). In the latter case, the spermatophores are usually deposited on or in a specific female structure, termed the thelycum, for temporary or more long-term storage prior to fertilization. Females of penaeoid species that have an open thelycum generally receive more complex external spermatophores, while females of species with a closed thelycum receive spermatophores with a less elaborate morphology that are stored within a seminal receptacle (spermatheca) (Bauer 1991; Misamore & Browdy 1996; Pérez-Farfante & Kensley 1997; Alfaro et al. 2003).
There are more than 70 described species in the decapod infraorder Stenopodidea (De Grave & Fransen 2011), but Stenopus hispidus (Olivier 1811) is the only species for which observations on mating behavior have been recorded (Zhang et al. 1998). However, the morphology of reproductive structures for this species has not been described in detail; the sperm mechanism is still controversial and poorly understood, and even general information about the reproduction of S. hispidus is scarce (Zhang et al. 1997; Chockley & St. Mary 2003), as it is for other stenopodidean shrimps (Goy 2010). Most studies on S. hispidus are related to its distribution or life history (Holthuis 1946; Kruczynski & Jenner 1969; Lukens 1978; Gregati et al. 2006; Chockley et al. 2008) and behavior (Johnson 1969; Jonasson 1987; Zhang et al. 1998).
It is known that, in nature, members of this species are typically found in breeding pairs (Brooks & Herrick 1891; Johnson 1977), and that male gamete transfer occurs after the female's molt in a series of continuous, successive breeding cycles (Young 1979; Zhang et al. 1998; Gregati et al. 2010). Balss (1944) and Bauer (1986) have argued that the process of sperm transfer in this species is similar to that of members of the Caridea, in which the male deposits a simple sperm mass on the sternum of the female and fertilization occurs externally during the extrusion of eggs. On the other hand, Zhang et al. (1998) suggested that both the deposition of sperm and fertilization may occur within the female genital pore.
The stenopodid shrimp S. hispidus has significant economic importance in the marine pet aquarium trade because of its striking coloration and ease of maintenance (Zhang et al. 1998; Calado 2008). These crustaceans are collected and traded around the world to supply a great demand. The species is distributed in the Indo-Pacific, Atlantic, and Red Sea regions (Holthuis 1946, 1993) and can be observed in a variety of reef habitats, including coral formations and crevices in rocks (Limbaugh et al. 1961; Colin 1978). In nature, mating pairs dwell in extremely restricted areas and exhibit strong agonistic behavior against other couples or solitary conspecifics (Limbaugh et al. 1961; Johnson 1969; Young 1979; Fletcher et al. 1995). In this species, the relationships between male and female are considered long-term, as the males guard the female for more than one reproductive cycle (Johnson 1969).
The objective of this study was, for the first time, to describe the functional morphology of the reproductive system of males and females of S. hispidus, with observations on mating behavior, copulation, and reproductive morphology, using various methods including video recording, and light and scanning electron microscopy. From these observations, we give our interpretation of sperm transfer in this species under laboratory conditions for comparison with that of other decapod species.
Methods
Collection and maintenance of specimens
Adults of Stenopus hispidus were obtained from Salvador, Bahia, Brazil from licensed ornamental marine fish traders. In the laboratory, the shrimps were separated by sex according to the descriptions by Johnson (1969) and Gregati et al. (2010). Incubating females were easily identified by the presence of embryos adhering to the pleopods, or the green color of developed (prespawning) gonads observed through the transparent carapace. Males could be identified by the presence of a short median spine on the ventral surface of each abdominal segment.
In the laboratory, 12 pairs of males and females of similar size were maintained in interconnected tanks (0.45 m×0.20 m×0.30 m) with seawater at a salinity of 35 ppt, a temperature of 26±0.5°C, and a photoperiod of 12 h light and 12 h dark. Each tank had a dark glass bottom, beach sand as substrate, and marine rock fragments to shelter the animals (see Gregati et al. 2010).
We made observations of salinity (with a hand refractometer), ammonia, nitrite, pH, and alkalinity every 2 weeks (Tropic Marin® titration tests). A monthly partial renewal of water (30%) was performed with filtered seawater treated with ultraviolet light. The shrimp were fed daily to excess with ornamental fish food (Tetra Bits® and Tetra Marine Color Flakes®) and pieces of muscle from shrimp, squid, and clams.
External morphology of the genital region
Six pairs of shrimps were used to describe the reproductive system and the morphology of the thoracic sterna and pleopods. The couples were selected based on visual monitoring of ovarian cycles; we chose females with obviously mature ovaries (dark green color). The selected shrimps were anesthetized in near-freezing seawater before removing the dorsal region of their cephalothorax to expose the gonads. The sterna of the females, the shape and location of the gonads of each sex, and all pleopods were traced on drawing paper using a SV6 Zeiss® stereomicroscope equipped with a camera lucida.
In addition, six pairs were used to analyze and describe the ultrastructure of reproductive organs. These individuals had their entire reproductive systems removed along with their thoracic sterna. This material was immediately fixed in 2.5% glutaraldehyde and 4% paraformaldehyde in 0.1 mol L−1 phosphate buffer at pH 7.3 (Karnovsky solution) for 48 h. Fixed material was then rinsed in the same buffer (three times 5 min each); post-fixed in 1% osmium tetroxide in the same buffer (30 min), dehydrated in an ascending sequence of ethanol solutions, and dried at critical point (CPD 020; Balzer Union®) with liquid CO2. Specimens were then glued to SEM stubs and coated with a 10 nm layer of gold using a MED 010 sputter coater (Balzer Union®). Observations were made with a scanning electron microscope (Quanta 200; FEI Company®) with voltages ranging from 10 to 20 kV, and digital images with a scale bar were recorded.
Observations on copulation and sperm transfer
All 12 pairs of shrimps and their reproductive cycles were monitored in the laboratory until the incubated larvae were released. Because the whole process of copulation and sperm transfer typically occurs at night, the couples were separated in the same aquarium with a removable plastic screen (1 cm mesh). Thus, male and female could maintain both visual and physical (with antennae and chelipeds) contacts through the plastic screen, but they could not copulate. In the morning, after verifying ecdysis of the female, the plastic screen was removed and the mating activities of the shrimps were recorded with a digital video camera (8.1 megapixels Sony®). This procedure was performed successfully nine times with different couples. The videos were edited with computer software (Windows Movie Maker® and Adobe Photoshop®) and are presented as photographs in the figures.
Shortly after copulation, the thoracic sterna of four newly mated females were observed using an SV6 Zeiss® stereomicroscope. The sterna were carefully scraped with an entomological needle and a small brush. Smears of removed material were mounted on glass slides under coverslips. The smears were then observed immediately with a microscope (Axioskop 2 Zeiss®) equipped with Nomarski differential interference contrast and phase contrast optics and equipped with an image capture system.
Results
Internal reproductive morphology
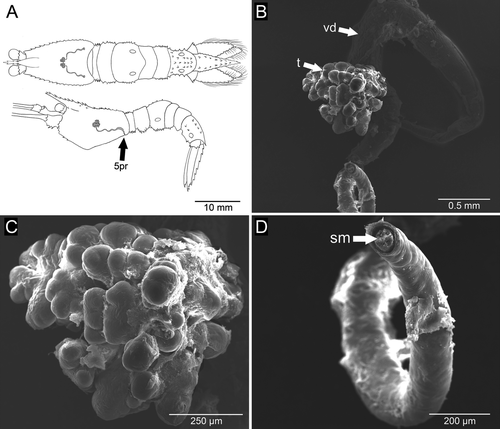
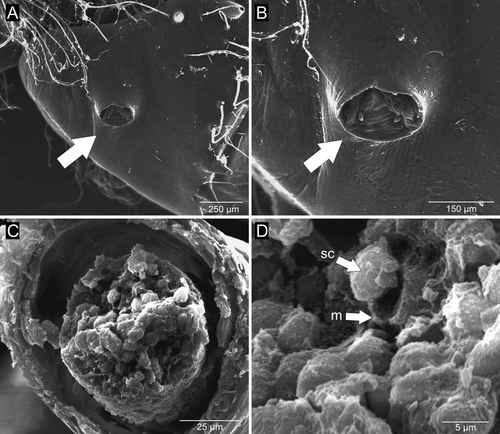
The male reproductive system consisted of a pair of testes, which were very small and difficult to see through the transparent carapace. The entire reproductive system was located in the central portion of the cephalothorax, in the region posterior to the stomach (Fig. 1A). The testes were lobed, light-colored structures ~1 mm in diameter (Fig. 1B,C). The vasa deferentia were slender, non-coiled tubes with a wider middle portion, and were ~4 mm in length (Fig. 1B,D). Each vas deferens originated in the median ventral portion of the corresponding testis and communicated with the outside by a small rounded genital opening (~180 μm in diameter) on the ipsilateral coxa of the 5th pair of pereopods (Fig. 2A,B). In the distal portion of the vas deferens, sperm cells of simple, unornamented elliptical form, ~7 μm in diameter, could be seen clustered and immersed in a matrix (Fig. 2C,D).
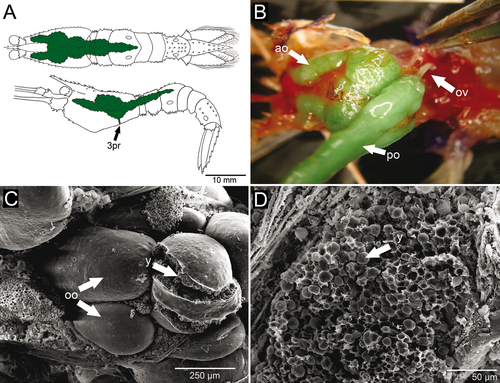
The female reproductive system consisted of a pair of ovaries that were easily visible through the transparent carapace. The ovaries were granular and located mainly in the dorsal portion of the cephalothorax, parallel and dorsal to the digestive tract. Anterior lobes reached the anterior region of the carapace, immediately behind the eyestalks; posterior lobes extended into the dorsal region of the abdomen, reaching the third abdominal somite (Fig. 3A). The total size of the ovaries, in both length and width dimensions, depended on the stage of ovarian development. In the most advanced stage of development, the ovaries were dark green and filled almost the entire carapace dorsally, as well as much of abdominal somites 1, 2, and 3. The oviducts were short and tubular (~3 mm in length and ~520 μm in diameter); their diameter was slightly larger than that of mature oocytes (Fig. 3B). The mature oocytes were visible and fragile, with prominent yolk granules (Fig. 3C,D). Each oviduct directly connected an ovary to the ipsilateral female gonopore; the left and right female gonopores were on the coxae of the 3rd pair of pereopods (third and largest pair of pereopods). Unlike the pore-like male gonopore, each female genital opening was a longitudinal slit that measured ~0.5 mm in length, and it was surrounded by many dense plumose setae (Fig. 4A,B).
External morphology of the genital region
In males, the cephalothoracic sternites had some plumose setae and very visible spines at their borders (Fig. 5A). The posterior cephalothoracic sternites of females were broader than those of males and were devoid of spines, with many plumose setae on their margins, especially at the junction with the coxae of pereopods and around the genital openings (“egg-guiding” setae) (Figs. 4B, 5B). In females, we observed grooves formed by the junction of cephalothoracic sternites. Males had a large easily observed central spine on each abdominal sternite. This large spine did not occur in females and thus was a convenient characteristic to easily distinguish males from females in both living and preserved individuals.
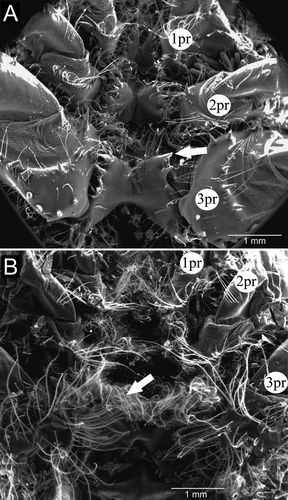
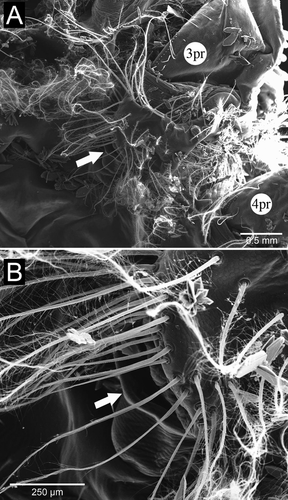
The pleopods were located on the ventral surface of the abdomen, one pair per abdominal segment 1–5, with uropods on the 6th (terminal) segment. In both sexes, the first pair was reduced, but the overall morphology of the remaining pairs was similar, with variations in size (Fig. 6). In males, the first pleopods were uniramous, with the proximal protopod of each bearing a spine and a distal flattened, spatulate ramus. The proximal portion of pleopods 2–5 had four short strong spines, sparsely plumose setae on the inner margin, and plumose setae on the outer edge. The distal portion had a pointed shape, with sparse marginal plumose setae. In general, pleopods 2, 3, 4, and 5 had globular protopods, armed with strong spines centrally aligned and one at the outer edge, along with some shorter setae, both simple and sparsely plumose. From the protopod of pleopods 2–5 arose a lateral exopod and a medial endopod, both flattened dorsoventrally, slightly sharp, with rows of short spines on the ventral surface and marginal plumose setae. The second pleopod, when folded under the cephalothorax, did not exceed the base of the fourth pair of pereopods. That same pleopod had a smooth proximal half of the inner margin of its endopod. We could not observe on any of the pleopods a structure resembling the appendix masculina of caridean shrimps and many other decapod crustaceans.
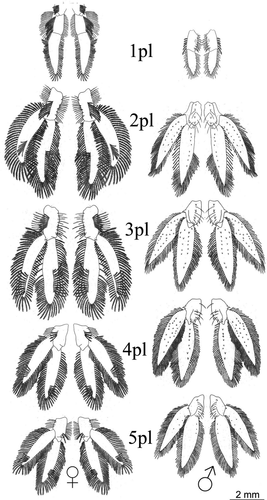
Unlike males, the protopod and distal ramus of the first pair of pleopods in females were devoid of spines, but had many long plumose setae, arranged in rows or sometimes in clumps. The distal ramus was pointed, with many long plumose setae across the border. Pleopods 2, 3, 4, and 5 had a protopod with a single spine, or no spine at all, and were sparsely populated with plumose setae arranged in rows. Both the exopod and endopod were dorsoventrally flattened and devoid of spines on their surfaces. All had a more rounded shape and more dense plumose setae across the whole margin. In this case, the second pleopod, when folded under the cephalothorax, reached the basal portions of the second pair of pereopods.
Copulation and sperm transfer
Sperm transfer occurred only when the female was in recent (≤12 h) postmolt. Females indicated receptivity to males by raising the abdomen and moving their pleopods rapidly in front of the male for a few seconds (Fig. 7A). During mating, the male placed itself perpendicularly to the female (Fig. 7B), and then moved to put its ventral pleon in front of and aligned with the female's ventral pleon (Fig. 7C). The male uses its first and second pereopods to touch the genital openings of the female. After that, the male seized the 5th pereopods of the female (Fig. 7C, white arrows). At this point, the male quickly turned its body, shifting to the abdomen versus cephalothorax position, but at a 45° angle (Fig. 7D). Sperm transfer apparently occurred in this position, with the process taking up to 11 s. During the transfer, the gonopores of male and female were very close, and the female agitated all of its pleopods rapidly, as did the male, at least those on the side facing the camera. The male pleopods from the other side could not be observed due to camera position. Once male and female separated, the female vigorously beat its pleopods three or four times, with two-second pauses.
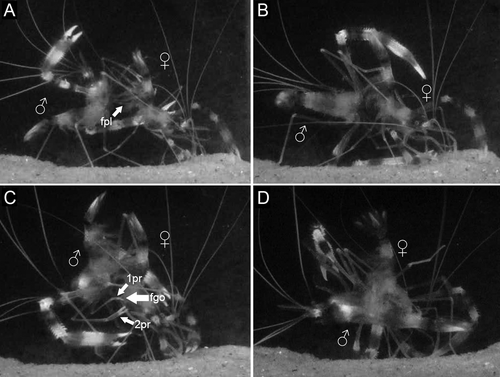
Sperm masses on newly mated females
In each newly mated female, the minute and transparent sperm mass was not visible upon unassisted visual inspection of the ventral thoracic surface. Smears of material scraped from the thoracic sterna of four newly mated females and placed on slides for observation with a compound microscope revealed elliptical sperm cells. Three of these females had small amounts of sperm, but one had a much larger sperm mass. After sperm mass removal from these females, spawning took place, but the attached eggs did not begin development and were aborted (removed) by the female within 72 h. All other non-manipulated females also spawned, but their attached eggs developed normally into embryos from which larvae later hatched.
Discussion
The general morphology of the reproductive system of Stenopus hispidus followed an anatomical pattern common to other groups of decapod “shrimps,” specifically, members of the Caridea and Penaeoidea. The major differences between these groups and S. hispidus regarding reproductive anatomy relate to the size and extent of the testes and, when mature, the size of the ovaries. The mature ovaries of S. hispidus, for example, had posterior parallel elongations into the abdomen, a common situation in Penaeoidea, but not in Caridea. In the Penaeoidea, the ovaries fill almost every available space in the cephalothorax and, as in Aristaemorpha foliacea (Risso 1826) and Farfantepenaeus brasiliensis (Latreille 1817), may extend dorsally along the entire abdomen parallel to the digestive tract (Levi & Vacchi 1988; Quintero & Gracia 1998). Caridean shrimps, in contrast, have their ovaries restricted to the cephalothorax, as described in the deep-water shrimp Alvinocaris muricola Williams 1988 and in freshwater species of the genus Macrobrachium (Valenti 1985; Bauer 2004; Ramirez-Llodra & Segonzac 2006). In some cases, such as Hippolyte inermis Leach 1815, the ovary extends posteriorly at most until the first abdominal somite (Cobos et al. 2005).
The male reproductive system of S. hispidus was restricted to the cephalothorax, as occurs in other groups of shrimps, but the much reduced size in S. hispidus is atypical among decapods. The general morphology in this case resembled that of the Caridea, which have testes in a position dorsal to the digestive tract and hepatopancreas, and which have vasa deferentia that are long simple ducts, often slightly or extensively coiled (Bauer 2004). In some Penaeoidea such as Litopenaeus setiferus (Linnaeus 1767), Trachypenaeus similis (Smith 1885), Farfantepenaeus duorarum Burkenroad 1939, Farfantepenaeus aztecus (Ives 1891), and Xiphopenaeus kroyeri (Heller 1862) the vas deferens can be anatomically differentiated into three clearly evident parts, the proximal, middle, and distal vas deferens ending in an ejaculatory duct (the terminal ampoule in older publications) (Bauer & Cash 1991; Bauer & Min 1993). This regional differentiation was not apparent for the vas deferens of S. hispidus.
The highly reduced testes of S. hispidus, relative to those of other decapods, may be related to the mating system of this species. Males occur in more or less monogamous pairs with females (Johnson 1969, 1977) and only mate when their female partner molts prior to spawning (every 25–26 d at 26°C; Gregati et al. 2010). The amount of sperm that males need to produce is small, and the reduced testes reflect this lower investment by males in sperm and seminal products. Similarly, in other animal groups, such as primates, rodents, and birds, the size of the testes and amount of sperm production is generally related to the mating system, with testes size and sperm production increasing from monogamous to polygamous (e.g., single-male harems; extra pair male mating) to promiscuous breeding systems (Harcourt et al. 1981; Diamond 1992; Soulsbury 2010).
In many decapod crustaceans, the endopods of the anterior two pairs of pleopods in males are modified from the more primitive paddle shape, which is appropriate for a swimming function. These initial two pairs of pleopods are located posterior to the male gonopores, and even small changes in the endopods of these pleopods are thought to be related to the act of inseminating the females (Bauer 1986; Zhang & Lin 2004). In representatives of the Stenopodidea, insemination may be different from species of many other groups of Decapoda, considering that anterior pleopods seem not to participate actively in the process of sperm transfer. In our video recordings of sperm transfer, all pleopods on one side of the male were clearly moving together and at the same rate, indicating use in maintaining male position and not in sperm transfer. Unfortunately, pleopods from the opposite side could not be filmed. Considering that males positioned their gonopores very closely to those of the females, one could infer that the deposition of a sperm mass is performed directly without the use of any pleopod copulatory organ. Only the first male pleopods have any morphologic differences from the posterior ones that might be related to the transfer of the sperm mass. The first pair of male pleopods is reduced and spatulate, while the second is morphologically similar to the other pleopods, in having a paddle shape and no male appendix as in other decapods.
In Dendrobranchiata (penaeoid and sergestoid shrimps), morphologic specializations for sperm transfer are generally complex and varied among taxa. In this group, the endopods of the first pleopods of males join to form a morphologically elaborate structure called a petasma, while the second pair has structures that are male appendices. Both structures may assist in the transfer of spermatophores directly to the thelycum of females (Pérez-Farfante 1975; Bauer 1986, 1991; Pérez-Farfante & Kensley 1997; Alfaro et al. 2003). Female penaeoid shrimps with an open thelycum do not exhibit a seminal receptacle. They instead have protuberances, hollows or grooves and occasionally plate-like extensions on thoracic sternites (XII to XIV) to which the external spermatophores are attached (Pérez-Farfante 1975; Bauer 1994).
In Pleocyemata, morphologic variation is even greater. In Brachyura, males have an injection system, in which extensions of the distal part of their vas deferens introduce seminal content into the base of the first pair of pleopods, which are modified into a tubular form (McLay & López-Greco 2011). The second pair of pleopods, also modified, serves as a piston rod, which pushes the sperm mass through the tubular form of the first pair of pleopods and directly into the female during copulation (Cronin 1947; Hartnoll 1969; Bauer 1986; Diesel 1991). In Astacidea, in general, each endopod from the first male pleopod forms half of a sperm channel, which can also be inserted into the female genital opening during copulation. In this case, the second pair of male pleopods has long appendages that propel the sperm mass to its destination (Farmer 1974; Bauer 1986; Waddy & Aiken 1991).
In Caridea, the endopods of the first pair of pleopods bear tiny coupling hooks (cincinnuli), while those of the second pair have a male appendix (appendix masculina) (Bauer 1976, 1986, 2004). Although these changes in anterior pleopods may be essential to the process of transferring sperm in some species of Caridea, such as Palaemonetes pugio Holthuis 1949, Heptacarpus sitchensis (Brandt 1851), and Rhynchocinetes typus Milne-Edwards 1837 (Bauer 1976, 1986; Berg & Sandifer 1984; Wicksten et al. 1996; Bauer & Holt 1998; Laubenheimer & Rhyne 2008; Martínez & Dupré 2010), Zhang & Lin (2004) showed experimentally that individuals of the shrimp Lysmata wurdermanni (Gibbes 1850) are able to conduct sperm transfer successfully even with their two anterior pleopods removed.
Phillips et al. (1980) and Bauer (1986) state that Palinura and a few Anomura do not follow the general trend of the Decapoda, and are without complex sperm transfer and storage structures. According to these authors, males of most species of palinurid lobsters do not have gonopods and the transferred sperm mass is in the form of tubes with sperm cells surrounded by protective and adhesive matrix, transferred directly from the male gonopores to female posterior thoracic sterna during copulation. Something similar occurs with the hermit crab Clibanarius vitattus (Bosc 1802). In this species, adhesive substances from the male gonopore attach the sperm mass directly to the surface of the female's sternum, without the necessity of gonopods to effect the transfer. In males of C. vittatus, the first pair of pleopods is absent and the second pair is composed of simple structures with no apparent role in sperm transfer (Hess & Bauer 2002).
The fact that the sperm mass transferred to the female of S. hispidus is transparent and small makes it difficult to understand the mode of sperm transfer in this species. Probably for this reason, Zhang et al. (1998), while analyzing newly mated females of this species, could not observe the sperm mass directly attached to the cephalothoracic sternites or in their grooves, even with a stereomicroscope. These authors suggested that the spermatophore could be deposited inside the female genital pore and thus that fertilization occurs internally in the female, as reported in the shrimp Crangon crangon (Linnaeus 1758), a caridean with sperm transfer and storage supposedly atypical among carideans (Boddeke et al. 1991). In fact, simple visual inspection of the sternites of females of S. hispidus failed to reveal an attached sperm mass. However, smear preparations of material scraped from the sterna of newly mated females and observed microscopically did reveal deposited sperm, thus supporting the conclusion of Balss (1944) that both the deposition of sperm and fertilization occur externally in S. hispidus.
The sperm mass in members of the Caridea is simple and, as in Macrobrachium rosembergii De Man 1879, is attached to the female cephalothoracic sternites among their posterior pereopods, through mucoid substances surrounding the sperm cells (Chow 1982; Bauer 1986, 2004). In general, caridean females do not have structures for storage of sperm. Except for C. crangon, in which the male purportedly deposits the sperm mass directly into the female oviduct (Boddeke et al. 1991), only members of the families Processidae have structures similar to a closed thelycum, and an Atyidae species shows a modification to the sternum, homologous to an open thelycum. In all these cases, storage is only for a short period of time, not exceeding a reproductive cycle (Correa & Thiel 2003).
Representatives of Stenopodidea have a low level of morphologic complexity for transferring sperm, as in most caridean shrimps, which do not use any copulatory organ. In Stenopodidea, besides the matrix in which the sperm cells are immersed and which is evident in the final portion of the vas deferens, the large number of dense plumose setae on the sternum of females can also aid in the adhesion of the sperm mass until the moment of fertilization. Some of these setae (“egg-guiding”) may serve another function, that is, to direct the eggs posteriorly toward the abdomen, under which they will be attached during spawning (Tóth & Bauer 2007). Their presence supports the hypothesis of external fertilization in this group. The rapid beating of the pleopods shown by each female of S. hispidus just after copulation might aid in fixing the sperm mass to thoracic setae or in the cavities between the sternites, as its second pair of pleopods covers the entire sternum and reaches the base of the most anterior pereopods. Thus, mature oocytes can be fertilized as they are spawned from the gonopores, and they are subsequently moved to the pleopods for their final attachment and incubation, as occur in most species of Caridea in which the process has been described (Bauer 1986, 2004; Correa & Thiel 2003; Zhang & Lin 2004).
Although much information about the transfer of sperm in Stenopodidea can be obtained through study of morphology, especially with the use of scanning electron microscopy, additional studies must still be performed. In addition to observations on other species of Stenopodidea, pleopod ablation experiments, as carried out by Bauer (1976), Wicksten et al. (1996), Zhang & Lin (2004), and Martínez & Dupré (2010), as well as serial histologic sections of the oviducts of newly mated females, as performed by Boddeke et al. (1991), can be used to further test hypotheses about sperm transfer and fertilization proposed here, based on our observations in this study.
Acknowledgments
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for providing laboratory facilities, to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for international cooperation fellowship Brazil/Argentina (CAPES-MINCYT BR12/217), and to Pró-Reitoria de Pesquisa da UNESP (PROPe) for funding the contact between the first author and Dr. Raymond Bauer. Part of the data presented here was obtained during the doctoral thesis of the first author (CAPES doctoral fellowship, Brazil). We are also grateful to the Electron Microscopy Center, UNESP for use of their facilities. This is the Contribution No. 156 of the University of Louisiana, Lafayette, Laboratory of Crustacean Research. The shrimps in this study were obtained in accordance to Brazilian laws concerning wildlife sampling.




