Chaetal arrangement and chaetogenesis of hooded hooks in Lumbrineris (Scoletoma) fragilis and Lumbrineris tetraura (Eunicida, Annelida)
Abstract
As the structure and arrangement of chaetae are highly specific for annelid species and higher taxonomic entities, we assume that rather conservative information guarantees formation of specific chaetae. Each chaeta of an annelid is formed within an ectodermal invagination, and the modulation of the apical microvilli pattern of the basalmost cell of this invagination determines the structure of the chaeta. Any hypothesis of the homology of chaetae could thus be tested by examining the process of chaetal formation. Investigations into the ultrastructure and formation of hooded hooks in different capitellids and spionids revealed that these chaetae can be homologized. The hood of each of their hooded hooks is formed by elongation of two rings of microvilli peripheral to the chaetal anlage, which give rise to the inner and outer layers of the hood. The hood layers are well separated and surround an empty space. Superficially similar hooded hooks are described for certain Eunicida. Presently available cladistic analyses suggest that the hooded hooks of eunicidans evolved independently of those in Capitellidae and Spionidae. Compared with the latter two families, we therefore expected to find differences in chaetogenesis of the hooded hooks in the eunicids Lumbrineris (Scoletoma) fragilis and Lumbrineris tetraura (Lumbrineridae). This was the case. In these eunicidans, the hood was formed by the bisected apical wall of the chaetoblast right after the mid-apical section of the chaeta had been sunk deeply into the chaetoblast during its formation. The apical wall generated a brush of microvilli that preformed the hood. Because the microvilli of the hood showed some accelerated differentiation, they soon merged with those of the slowly growing setal shaft to form the broad manubrium of the hooded hook in lumbrinerids. Our study confirms the predicted differences in chaetogenesis of the superficially similar hooded hooks of capitellids and spionids compared with those of eunicids.
Chaetae of Annelida are chitinous extracellular structures that are formed within an ectodermal pouch or chaetal follicle (Bouligand 1967; Schroeder 1984; Specht & Westheide 1988; Hausen 2005). The chaetal follicle consists of a few follicle cells and a single basal chaetoblast. The pattern of the apical microvilli of the chaetoblast is modified constantly, while N-acetyl-glucosamine is released between the bases of the microvilli. This material polymerizes extracellularly, forming the chaeta; thus, the definitive structure of the chaeta is primarily caused by changes in the pattern of chaetoblast microvilli, i.e., by dynamic microvilli (O'Clair & Cloney 1974). Chaetae are considered an autapomorphy of annelids, and reconstructions of the ancestral annelid pattern (bauplan) concur that early annelids bore simple capillary chaetae (Struck 2011; Struck et al. 2011). The dynamic developmental mode of chaetae allows for the formation of a plethora of different chaetal types, and these different chaetae are of immense functional importance, e.g., in defense, locomotion, burrowing, and anchoring of the body to the tube (Merz & Woodin 2006). Arrangement and structure of the chaetae are so conserved in annelid species and supraspecific taxa that chaetation has become an important source of diagnostic characters for taxonomists. As the dynamic structure, orientation, and number of microvilli of the chaetoblast determine chaetal structure, these factors must be strictly regulated to guarantee that chaetae are identical within an individual, a population, or a species. Regulation of these factors appears to be conservative enough that certain chaetae, once evolved, can be passed on to descendants and thus become characteristic of supraspecific taxa. Studies into chaetogenesis of uncini in certain “sedentary” polychaetes revealed that the structure of these chaetae results from a uniform process of chaetogenesis (Arenicolidae and Maldanidae: Bartolomaeus & Meyer 1997; Bobin 1944; Tilic, unpubl. data; Psammodrilidae and Oweniidae: Meyer & Bartolomaeus 1996; Pectinariidae; Terebellidae, Serpulidae, Sabellidae: Bartolomaeus 1995, 1998, 2002); thus, chaetogenesis in these cases seems to contain some phylogenetic signal. Studies of chaetogenesis also support the hypothesis of homology between uncini and the hooded hooks of capitellids and spionids (Hausen 2001, 2005; Bartolomaeus et al. 2005). In members of these two families, hooded hooks are found in the abdominal chaetigers. These hooded hooks resemble uncini whose apical portion is enclosed by a hood, consisting of an outer lamella and an inner lamella. Studies of the formation of the hood support the assumption of homology of spionid and capitellid hooded hooks (Hausen & Bartolomaeus 1998; Hausen 2005).
Similar hooded hooks, however, have also been described in Lumbrineridae, a taxon that clearly belongs to a clade called the Errantia or Aciculata, which consists of at least Eunicida and Phyllodocida (Rouse & Fauchald 1997; Struck et al. 2014; Weigert et al. 2014). Both in a molecular phylogenetic analysis of Eunicidae (Zanol et al. 2010) and in a recent revision of the taxonomy of Eunicidae based on morphological characters (Zanol et al. 2013), lumbrinerids are considered basally branching Eunicida and therefore taken as outgroups for unraveling the phylogeny of the Eunicidae. On the light microscopic level, the hooded hooks of Lumbrineridae appear to be similar to those of Capitellidae and certain Spionidae, as they also consist of a large spine that is surmounted by smaller ones. The main axis of the larger spine is perpendicular to the manubrium, and a large hood surrounds the spines (Hilbig 1989). The structural similarities between the hooded hooks of Capitellidae and Spionidae to those of Lumbrineridae are either the result of convergent evolution, or the result of an early origin during annelid evolution, with subsequent loss or modification in many different lineages. As the latter scenario appears to be unlikely, we expected striking differences in the mode of chaetogenesis that reflect the assumed convergent evolution of hooded hooks in Lumbrineridae on the one hand, and Capitellidae and Spionidae on the other. Recognizable differences in mode of chaetogenesis would also support the value of chaetogenesis as a phylogenetic character. To test this idea, we analyzed chaetogenesis in Lumbrineris (Scoletoma) fragilis Müller 1766 and Lumbrineris tetraura Schmarda 1861. Details of chaetal arrangement and the position of the growth zone were used to corroborate the hypothesis of a convergent evolution of hooded hooks in Lumbrineridae, Spionidae, and Capitellidae.
Methods
Transmission and scanning electron microscopy
Specimens of Lumbrineris tetraura and Lumbrineris fragilis were collected from sandy areas underneath and between smaller stones at the rocky shore close to Plozevet and in Concarneau (Brittany, France). For fixation for transmission electron microscopy (TEM), we used 2.5% glutaraldehyde buffered in 0.1 mol L−1 sodium cacodylate at room temperature for 1 h. Ruthenium red was added to the fixative, and the animals were dissected prior to fixation. After having been rinsed in the same buffer, the animals were postfixed in 1% OsO4 in 0.1 mol L−1 sodium cacodylate for 1 h, dehydrated in an acetone series, and embedded in Araldite. The chaetal follicle and the formative sites of six chaetigers of a young individual of L. tetraura were sectioned into a complete series of silver-interference colored sections, stained with uranyl acetate and lead citrate, and analyzed using Zeiss EM 10B and Hitachi H500 transmission electron microscopes. The course of formation of the hooded hooks was reconstructed from electron microscopical data from different formative sites of L. tetraura using Fiji (1.45b) (Schindelin et al. 2012)/TrakEM (Cardona et al. 2012) for cell identification, and Amira (4.0) for 3D visualization.
For scanning electron microscopy (SEM), specimens of L. tetraura and L. fragilis were fixed in Bouin's fluid, dehydrated in an alcohol series (during this step, the animals were sonicated to remove debris and sand particles from the chaetae), and critical point dried with CO2 in a Balzers critical point dryer. After dehydration, they were sputter-coated with gold (Balzers Sputter Coater) and examined using Novoscan and Hitachi scanning electron microscopes.
Confocal laser scanning microscopy
Specimens used for confocal laser scanning microscopy (CLSM) were fixed in 4% paraformaldehyde. The chaetigers were dissected to separate single parapodia or a single segment. Isolated parapodia and segments were permeabilized in four 5-min changes of phosphate buffered saline (PBS) with 0.1% Triton X-100 (Fisher Scientific). The parapodia were then stained overnight at 4°C with TRITC–phalloidin at a dilution of 1:100. After staining, parapodia were rinsed in three quick changes and subsequently in two 10-min changes of PBS with 0.1% Triton, and one 10-min rinse in PBS without Triton. Smaller parapodia were mounted on poly-l-lysine-coated coverslips, and larger ones were directly placed in hollow-ground slides. The samples were quickly dehydrated in isopropanol (2 min each in 70%, 85%, 95%, 100%, 100%), cleared in three 15-min changes of Murray Clear, and mounted in Murray Clear. Slide preparations were sealed with nail polish.
Light microcopy, histology, and 3D reconstruction
Chaetae were isolated from pieces of living specimens of L. tetraura by incubation in 5% NaOH for 2–3 h. The chaetae were rinsed in distilled water, mounted on slides, and examined using Nomarski differential interference contrast with an Olympus BX-51 microscope. Their structure was digitally recorded with an Olympus camera (Olympus cc12).
For reconstruction of the chaetal sac, specimens of both species were relaxed for 1.5–2 h in a 1:1 mixture of 7% MgCl2 and seawater. They were subsequently fixed in 1.25% glutaraldehyde buffered in 0.05 mol L−1 phosphate buffer with 0.3 mol L−1 NaCl for 1.5–2 h. Afterward, the specimens were postfixed in 1% OsO₄ in a 0.05 mol L−1 phosphate buffer. Before embedding, the specimens were dehydrated via an ascending acetone series. They were then transferred via propylene oxide to Araldite. The specimens were fragmented into smaller pieces within the resin. Polymerization was started with benzyldimethylamine (BDMA). Serial 1-μm sections were prepared using a diamond knife (Diatome Histo Jumbo) on a Leica Ultracut S ultramicrotome, following the method described by Blumer et al. (2002). The sections were stained with toluidine blue (1% toluidine, 1% sodium tetraborate, and 20% saccharose) and mounted in Araldite. The semithin sections were analyzed with an Olympus BX-51 and photographed with an Olympus camera (Olympus cc12) equipped with the dot slide system (2.2 Olympus, Hamburg). The images were aligned with IMOD (Boulder Laboratories: Kremer et al. 1996) and IMOD-align (http://www.evolution.uni-bonn.de/mitarbeiter/bquast/software). The software 3ds max 13.0 was used for 3D modeling of the chaetae. The histological images were imported as surface materials (discrete) and the chaetae were modeled using standard cylindrical or conic objects. When necessary, these were modified as NURBS (Nonuniform rational B-Splines)-surfaces. The outline of the chaetal follicle was created using NURBS-curves on selected section planes.
Data repository and voucher material
To allow data transparency, all of the aligned serial sections used for 3D modeling are freely accessible in the morphological database, MorphDBase (Grobe & Vogt 2009). Series of semithin sections for L.tetraura can be found at https://www.morphdbase.de?E_Tilic_20131009-M-10.1, and for L. fragilis at https://www.morphdbase.de?E_Tilic_20131009-M-12.1 and https://www.morphdbase.de?E_Tilic_20131009-M-11.1. Aligned TEM sections showing chaetogenesis can be found at https://www.morphdbase.de?E_Tilic_20131009-M-13.1, https://www.morphdbase.de?E_Tilic_20131009-M-14.1, and https://www.morphdbase.de?E_Tilic_20131009-M-15.1. Conspecific individuals of both species studied were collected at the same sampling site and deposited at the Zoological Museum of the University of Göttingen as voucher material.
Results
Parapodial structure and the arrangement of chaetae
Each parapodium has a small and rounded prechaetal lobe, and a larger postchaetal lobe. Macroscopically, the parapodia of both species studied are uniramous, but series of semithin sections and subsequent 3D-reconstruction of the chaetal sac of Lumbrineris fragilis (Fig. 1A–G) and Lumbrineris tetraura (Fig. 2A–G) revealed that their notopodia are extremely vestigial: a dorsal group of small chaetae, embedded inside the parapodia in both species (Figs. 1G, 2G), originate from their own chaetal sac, independent of that of the neuropodia. Due to their position and their origin we regard them as notopodial chaetae, which have been internalized. Although the notopodial chaetae were much smaller and thinner than the neuropodial chaetae, they could be seen by confocal laser scanning microscopy (CLSM: Fig. 3A–C). As only the neuropodial chaetae protrude to the outside of the body, the following description refers to the neuropodial chaetae.
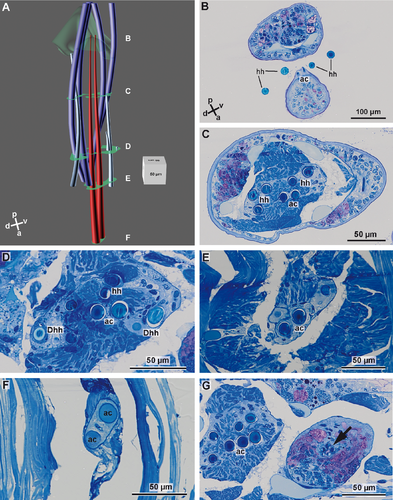
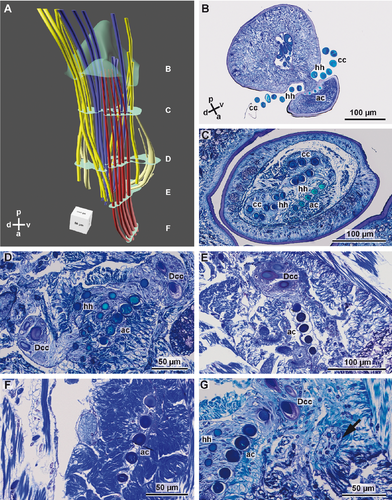
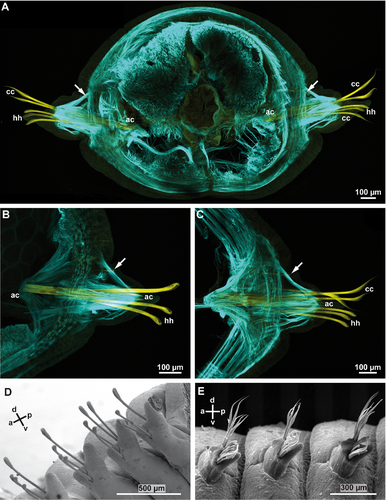
In L. fragilis, the neuropodial chaetation was consistent throughout the body; all chaetigers only bore ~4 hooded hooks. In L. tetraura, anterior and posterior parapodia differed in this respect. Anterior parapodia bore seamed capillary chaetae as well as hooded hooks, whereas hooded hooks were the only chaetae in posterior parapodia (Fig. 3B,C). In both species, the chaetae arose from a small rim that was transverse to the longitudinal body axis (Fig. 3D,E). Internally the structure of the chaetal sac was influenced by the insertion of parapodial and chaetal muscles (Fig. 3A–C). The outline of the chaetal sac thus became extremely irregular and folded a few micrometers below the parapodial surface, so that groups of chaetae appeared to be isolated within the parapodium (Figs. 1C, 2C–E). Nonetheless, all chaetae of one parapodium originated from a single chaetal sac; within each chaetal sac, hooded hooks, capillary chaetae, and acicula were aligned in a single dorsoventrally oriented row, sometimes in a zig-zag pattern. The aciculae did not protrude from the cuticle, were inserted much more deeply in the parapodia than the remaining chaetae, and were always located posterior to the row of capillary chaetae and hooded hooks (Figs. 1A,B, 2A,B, 3). In both species, new chaetae were formed continuously in each chaetiger, irrespective of the position and thus of the age of the parapodium. Each chaetal sac contained two formative sites. In L. fragilis, newly forming hooded hooks are shown in the 3D reconstruction of chaetae in a frontal segment (Fig. 1A). In a 3D reconstruction of a corresponding segment, L. tetraura only shows newly forming capillary chaetae, due to the heterogenous chaetation of the anterior segments (Fig. 2A). The number of acicula gradually increased during the chaetogenesis (Fig. 4A–C). In L. tetraura, the anteriormost segments sometimes contained up to five acicula, while in L. fragilis, there were maximally two acicula in one parapodium (Figs. 1A, 2A).
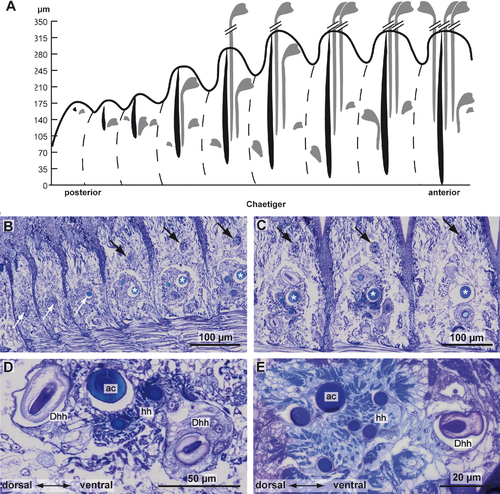
Serial semithin sections of the posteriormost segments in L. tetraura allowed a better insight into chaetogenesis, as the specimen studied still had an active growth zone, so that the course of chaetal formation could be followed by studying the developing segments anterior to the growth zone (Fig. 4A–E). Both neuropodial and notopodial chaetae could be seen from the youngest segment onward, although chaetogenesis in notopodia appeared to be a little bit delayed. Chaetogenesis always started with the formation of an acicula inside a small basiepidermal chaetal sac. During further development, the chaetal sac extended into deeper tissue layers and enlarged while additional chaetae were formed inside. This was especially evident in neuropodia, while in the notopodia, the chaetal sac remained small and the tiny acicula therein remained the only chaeta for a long time. Later, a few additional small chaetae were added. In neuropodia, the acicula increased rapidly in diameter and the first hooded hooks started to develop inside the chaetal sac (Fig. 4A,B). Their formation took place dorsally and ventrally to the acicula, so that each chaetal sac contained two formative sites. The dorsal formative site was always next to the acicula, and the ventral one clearly offset at the ventral edge of the chaetal sac. Chaetogenesis alternated between both sites and finally gave rise to a row of chaetae. During this process, different stages of chaetogenesis could be found in a single chaetal sac (Figs. 1A, 2A, 4C,D). Depending on the position of the formative site, the oldest chaetae were found in different positions relative to the aciculae. Those formed by the dorsal formative site were located at the dorsal edge; those formed by the ventral formative site were found next to the aciculae and thus in a central position in each row of chaetae. In general, the diameter of each acicula was much larger than that of the hooded hooks (Fig. 5A,B). The posterior ten chaetal sacs did contain a single acicula, but further anteriorly a chaetal sac could contain up to four aciculae (Fig. 2A).
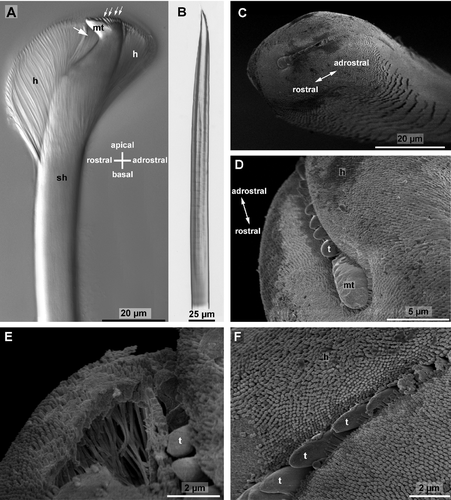
Ultrastructure of hooded hooks
The hooded hooks possessed a large spine or main tooth that was almost perpendicular to the main axis of the manubrium or shaft. Light microscopy showed several small canals inside the main tooth and their characteristic bending toward the manubrium (Fig. 5A). Several smaller spines or teeth surmounted the main tooth adrostrally; they were aligned in a single row (Fig. 5C,D). A large hood surrounded these apical structures; its larger portion was rostral, and its smaller portion was adrostral (Fig. 5C,E). A small apical slit allowed a view of the tip of the main tooth and some of the smaller teeth by SEM. Rostrally this slit ended beneath the main tooth, whereas it was much deeper on the adrostral side. The hood was not empty; it was filled with several irregularly arranged rods (Fig. 5E) that were embedded in an amorphous matrix. Each rod consisted of an electron-dense wall and an electron-lucent core. Toward the periphery their arrangement was more regular. Here several rows of electron-dense rods formed the outer surface (layer) and the inner surface (layer next to the shaft) of the hood. As they extended beyond the outer surface and inner surface, the hood was covered by densely arranged knobs (Fig. 5C,F). The rods become less regularly arranged toward the center of the hood. During preparation for SEM, the inner matrix shrank, so that the space between the rods appeared empty (Fig. 5E). The inner layer of the hood also consisted of densely arranged electron-dense rods. This inner layer was isolated from the manubrium just below the rostrum, and merged with the outer layer on either side of the rostro-adrostral axis of the chaeta. With Nomarski interference contrast optics, the margin of the inner layer of the hood was clearly visible beneath the rostrum (Fig. 5A). Hartmann-Schröder (1996; p. 268) misinterpreted this margin as subdistal tooth.
Chaetogenesis
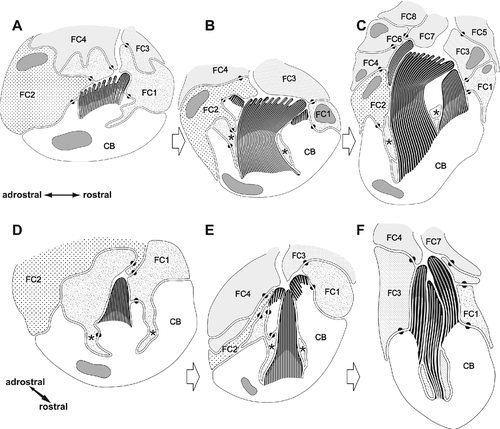
Chaetogenesis is a continuous process. Due to our methods (TEM of fixed material), this process was inferred from a series of different stages. Three of them are illustrated in this article, one showing the formation of the teeth (Fig. 6A–D), the second showing the beginning of the formation of the hood (Fig. 6E–H), and a third showing completion of the hood (Fig. 7A–D). For comparative reasons, formation of an acicula (Fig. 7E) and a reconstruction of lumbrinerid hooded hook chaetogenesis (Fig. 8A–F) are shown as separate figures.
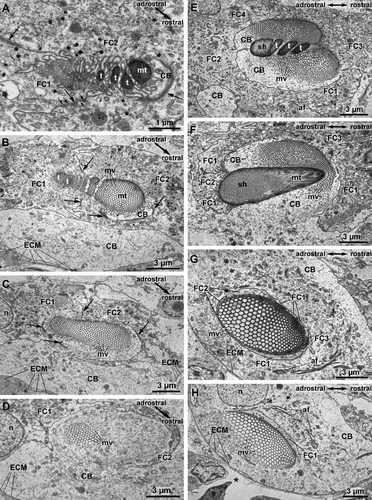

Chaetogenesis of hooded hooks can be divided into two steps, because the formation of the hood is spatially and temporarily separated from the development of the remaining apical section of the chaeta. In the following description, chaetogenesis of the apical teeth and the adjacent part of the manubrium will, thus, be separated from the formation of the hood. In terms of topology, we therefore will distinguish an inner anlage that gives rise to the spines and apical manubrium from an outer anlage that gives rise to the hood.
Formation of hooded hooks occurred within an ectodermal invagination that was continuous with the exterior and filled with some extracellular material. This compartment was surrounded by five prospective follicle cells; a single large cell was at the base of this cavity. All cells were interconnected by adluminal adhaerens junctions that linked an inner subapical net of actin filaments (Fig. 6A). Chaetogenesis started when a few small, ellipsoid clusters of microvilli appeared on the surface of the chaetoblast. These microvilli extended into the extracellular compartment surrounded by the follicle cells; each cluster of microvilli was isolated from the adjacent one by protrusions of the first basalmost follicle cells. While chaetal material that was released between the bases of the microvilli polymerized, additional clusters of microvilli were generated that were composed of a lesser number of microvilli than the previous ones. These were added to the existing clusters along a rostro-adrostral gradient, so that the oldest clusters were situated rostrally. During this process, additional microvilli arose peripherally to each cluster and enlarged it until it consisted of 2–5 rows of 9–11 mv. Each cluster gave rise to a single apical spine. As the initially formed clusters were always larger than the subsequently formed ones, the apical spines decreased in size toward the adrostral edge of the chaetal anlage (Fig. 6B). After the final number of apical spines was achieved, addition of further microvilli reduced the space between the clusters and led to a merger of all microvilli (Fig. 6C,D). When formation of the apical spines was completed, all spines were aligned along the rostro-adrostral axis of the anlage. By that time microvilli had begun to withdraw from the spines, and the canals left by them were refilled by electron-dense material. Formation of the inner anlage, which would become the apicalmost hooked part of the chaeta, was now complete. Additional electron-dense material released from vesicles of the first two follicle cells formed an enamel that covered the irregular surface of the chaeta (Figs. 6C, 7D). This material was produced inside Golgi stacks. The first two follicle cells and the chaetoblast interdigitated such that a protrusion of the follicle cells deeply extended into the chaetoblast and surrounded the inner anlage (Figs. 6C,D, 8A,D).
During this initial phase of chaetogenesis, the inner anlage continuously sank into the chaetoblast. First, second, and third follicle cells that enwrapped the inner anlage during formation followed this movement and formed a small cytoplasmic ring that surrounded the anlage and separated it from the chaetoblast (Fig. 6C,G). This ring was connected to the follicle cells with small rostral and adrostral cytoplasmic bridges that pierced the chaetoblast (Fig. 8B,E). The chaetoblast thus formed lateral walls on either side of the rostro-adrostral axis of the inner anlage; the walls surmounted the inner anlage. The chaetoblast now started to generate large clusters of microvilli on top of these lateral walls, and initiated formation of the outer anlage that would become the hood (Figs. 6E, 7D). As these microvilli extended beyond the inner anlage, chaetal material released between the bases of the newly formed microvilli formed a cap-like structure that extended beyond the inner anlage on either side of its rostro-adrostral axis (Fig. 8B,E). Both cap-like structures remained adrostrally separated by the bridge-like connection between the cytoplasmic ring and perikarya of the first, second, and third follicle cells (Fig. 6F). The rostral gap was caused by the position of the third follicle cell in a similar manner (Fig. 6E). In fully differentiated hooded hooks, thus, an apical slit indicated the former position of the follicle cells (Fig. 5C). At this stage, an inner cluster of microvilli forming the inner anlage and two groups of outer microvilli forming the outer anlage could be discriminated (Figs. 7C, 8E,F).
During further development, additional outer microvilli were formed to broaden the outer anlage of the hood. Finally, both microvilli clusters fused on the rostral side, forming a horseshoe-shaped patch of microvilli (Fig. 7A,B). The adrostral cleft was caused by the position of the first and the third follicle cells (Fig. 7B). Compared with the inner anlage, release and polymerization of chaetal material were accelerated in the outer anlage. This led to structural differences between hood and shaft in the fully differentiated hooded hook. The latter was more compact and stained much more electron densely than the hood, where electron-dense fibers, which marked the former position of the microvilli, appeared loosely connected by electron-lucent material (Fig. 7A,B,D). Merging of microvilli of the inner anlage reduced its diameter and initiated formation of the chaetal shaft or manubrium (Fig. 8C,F). When by differential deposition of chaetal material the outer and inner group of microvilli were finally on the same level, the first and second follicle cells began to retract (Fig. 7B). Thus, a large gap remained between the hood and the hooked part, which is characteristic for fully differentiated hooded hooks.
The microvilli of the outer anlage were now on the same level as those of the inner anlage, and surrounded them (Fig. 7C). Both merged during further development, although both could still be discerned for a while as their microvilli differed in diameter. Deposition of chaetal material between the bases of the microvilli and their continuous withdrawal proceeded, and the manubrium became longer. No significant modification of the microvilli pattern occurred in this phase of chaetogenesis. During elongation, the newly formed seta was pushed toward the surface, and finally became visible externally.
Formation of aciculae was much simpler. The basal chaetoblast continuously added microvilli to a central core group of microvilli. While chaetal material was continuously released between the bases of the microvilli, the diameter of the aciculum enlarged. The small tip and the rapid increase in diameter can be seen in isolated aciculae (Fig. 5B). When the final diameter was reached, no further change in the microvilli pattern of the chaetoblast occurred; the diameter of the chaeta remained constant for its entire length. Electron-dense vesicles released their contents into the small gap between chaetoblast and chaetal anlage to form an electron-dense enamel around the acicula (Fig. 7E).
Discussion
In Eunicida, the notopodia are either partially or completely reduced (Beesley et al. 2000: 89; Tilic & Bartolomaeus 2014). In both of the analyzed species of Lumbrineris, chaetal elements of the rudimentary notopodia were still present. In Eunice torquata Quatrefages 1866, the notopodium is reduced to a dorsal cirrus, which still contains chaetal elements. In Lysidice ninetta Audouin & Milne-Edwards 1833, however, no reduced chaetal elements of the rudimentary notopodia are present (Tilic & Bartolomaeus 2014). All species of the Eunicida possess at least hooded hooks in addition to seamed capillary chaetae; in addition, other kinds of chaetae have evolved within this group (Tilic & Bartolomaeus 2014).
Hooded hooks are also described from spionid, magelonid, and capitellid polychaetes (Hausen 2005). The hooded hooks in species of all three groups are basically arranged in double rows, but always possess a single formative site within a parapodium. This formative site is always located at the ventral edge of the notopodial chaetal sac and at the dorsal edge of the neuropodial chaetal sac (Hausen & Bartolomaeus 1998 and literature therein). In both lumbrinerid species studied here, hooded hooks and capillary chaetae are aligned in a single row, which may show a zig-zag pattern. Each row and each chaetal sac contains a ventral and a dorsal formative site. To find out whether this pattern is characteristic for all Eunicida will be part of our ongoing studies into chaetogenesis in this group.
Hooded hooks are described from all lumbrinerid species (Carrera-Parra 2006). It is remarkable that in Lumbrineris tetraura and Lumbrineris fragilis there are two formative sites in each chaetal sac. Although the process of chaetogenesis has not been described in other lumbrinerids, the process described here for L. tetraura and L. fragilis is assumed to be characteristic of Lumbrineridae. Provided this assumption is true, the similarities between lumbrinerid hooded hooks and those of spionids, magelonids, and capitellids (Hausen 2005) result from different processes (Table 1). After summarizing the course of hooded hook chaetogenesis in both groups as well as that of hooked chaetae and uncini in related taxa, we will return to this point.
| Lumbrineris spp. (Lumbrineridae, Eunicida) | Capitella capitata (Capitellidae) | Scolelepis squamata (Spionidae, Spionida) | |
|---|---|---|---|
| Hooded hook substructures | |||
| Main tooth | Present | Present | Present |
| Smaller teeth | Surmount main tooth | Surmount main tooth | Surmount main tooth |
| Smaller teeth number | At least ten | More than ten | One |
| Smaller teeth position | Single adrostral row | Several adrostral rows | Adrostral to main tooth |
| Shaft | Present | Present | Present |
| Hood | Peripherally condensed rods | Inner and outer lamellae | Inner and outer lamellae |
| Hood compartment | Discontinuously filled with chitinous rods | Electron-lucent (empty) with a few fibrils | Electron-lucent (empty) |
| Hood surface | Electron-dense knobs | Electron-dense knobs | Electron-dense knobs |
| Hood opening | Adrostral slit | Rostral slit | Rostral pore |
| Hooded hook formation | |||
| Main tooth | Group of microvilli | Group of microvilli | Group of microvilli |
| Smaller teeth | Group of microvilli | Single microvillus each | Group of microvilli |
| Shaft | Group of microvilli | Group of microvilli | Group of microvilli |
| Hood | Two lateral groups of microvilli | Several crescent rows of microvilli | Two crescent rows of microvilli |
| Hood compartment | Absent | Between rows of microvilli | Between rows of microvilli |
| Fusion with shaft | Entire hood | Inner and outer hood lamellae sequentially | Inner and outer hood lamellae sequentially |
Hooded hooks, hooked chaetae, and uncini in other annelids
In a series of papers, it was argued that the course of chaetogenesis substantiates the hypothesis of a homology of hooked chaetae and uncini in arenicolids, maldanids, terebellids, and sabellids and hooded hooks in spionids, magelonids, and capitellids (summarized in Hausen 2005). In arenicolids, maldanids, terebellids, and sabellids, the uncini or hooked chaetae consist of a large spine or rostrum (main fang, main tooth) that is surmounted by several smaller spines (teeth) that together comprise the capitium (Bartolomaeus 2002; Hausen 2005). The main axis of the shaft or manubrium is always perpendicular to that of the rostrum. Below the rostrum the manubrium is enlarged to form a subrostral process. In hooded hooks of capitellids and spionids, this apical section is enwrapped by a hood consisting of two chitinous lamellae. Uncini, hooked chaetae, and hooded hooks are aligned in a single or double row within a chaetal sac. In all species of these two taxa studied, chaetogenesis occurs in a single formative site in each sac. Chaetogenesis of uncini and hooked chaetae always starts with a group of microvilli that are a template for the rostrum. Continuous release of N-acetyl-glucosamine at the base of the microvilli forms the rostrum. Each of the subsequently formed larger microvilli is a template for a tooth of the capitium. During formation of these larger microvilli, the axis of the chaetoblast shifts so that the spines of the capitium surmount the rostrum after uncini formation has been completed. When the axis of the chaetoblast is shifting, additional microvilli are generated below the rostrum; these fuse with those that formed the rostrum and capitium. Merging of microvilli generates the shaft of the chaeta. In capitellids, magelonids, and certain spionids, this structure is altered by an apical hood enwrapping the rostrum, capitium, and the apical portion of the uncini (Hausen 2005). These chaetae are traditionally called hooded hooks. Microvilli that appear on the surface of the chaetoblast after the axis of the chaetoblast begins to shift are the template for the hood. As the main tooth is perpendicular to these microvilli, they form an adrostral, horseshoe-shaped group so that a rostral pore (Spionidae) or slit (Capitellidae) remains after the hood is completed. During formation, the microvilli split into two layers, an inner and an outer one. These layers are templates for the inner lamella and the outer lamella of the hood that surrounds rostrum and capitium in Capitella capitata (Fabricius 1780) (Capitellidae) and the bifid tip of the Scolelepis squamata Müller 1806 (Spionidae). Merging of the inner layer and the manubrium precedes fusion of the outer layer and the shaft. Due to developmental correspondences, a general homology between uncini and hooded hooks of capitellid species has been proposed (Hausen & Bartolomaeus 1998; Schweigkofler et al. 1998; Bartolomaeus et al. 2005; Hausen 2005). Subsequent studies into the chaetogenesis of additional spionids and magelonids showed some differences, but largely confirmed this pattern (Hausen 2001, 2005) (Table 1).
Conclusions
There are several structural and developmental differences between the hooded hooks in the lumbrinerid species studied and those of spionids and capitellids. These concern the formation of the apical portion of the chaetae and the formation of the hood (summarized in Table 1). While in Capitella capitata and certain spionids only the rostralmost tooth of the hooded hook, the rostrum, is preformed by a group of microvilli, those teeth that surmount the rostrum are preformed by a single large microvillus each. In Lumbrineris fragilis and Lumbrineris tetraura, all teeth of the hooded hook are formed at the same time; there is no delay in their appearance. Their hood is formed after the apical portion of the hooded hook has been completed and it is formed by a homogeneous group of microvilli that arise from that part of the chaetoblast which extends beyond the apical inner part of the chaeta. There are no outer and inner lamellae formed from different groups of microvilli as in spionids and capitellids. An adrostral slit in the hood is caused by follicle cells during the course of formation, while the rostral cleft or pore in capitellid and spionids is caused by the position of the rostrum. Formation of the hood then is accelerated relative to the remaining chaeta so that both inner apical portion and hood finally merge and form the manubrium in lumbrinerids. Merging of the inner hood and the outer hood in spionids, magelonids, and capitellids, however, is sequential (Hausen & Bartolomaeus 1998; Schweigkofler et al. 1998; Hausen 2005). These differences argue for a convergent evolution of hooded hooks in the lumbrinerids and the other taxa mentioned.
Differences between similar structures either result from transformations of an ancestral structure, or from convergent evolution. Differences alone, thus, do not decisively indicate convergent evolution; the latter must be the most parsimonious explanation in the context of a phylogenetic tree. According to morphological data, Eunicida, Phyllodocida, and Amphinomida form a monophyletic taxon called Errantia or Aciculata, characterized by internal chaetae (aciculae) and antennae among other traits (Rouse & Fauchald 1997; Bartolomaeus 1998). A taxon Errantia consisting of Phyllodocida, Eunicida, Amphinomida, and Orbiniidae is monophyletic in recent molecular analyses by Struck and colleagues (Struck 2011; Struck et al. 2011, 2014). Weigert et al. (2014) could confirm the monophyly of Errantia, if Amphinomida were excluded. Hooded hooks are absent in species of Phyllodocida, Orbiniida, and Amphinomida, but present in species of the Eunicida. Provided that the mode of formation of hooded hooks of L. fragilis and L. tetraura is characteristic of all lumbrinerids and also for eunicids, the most recent tree in Weigert et al. (2014), as well as the trees of Rouse & Fauchald (1997) and Struck et al. (2011, 2014), clearly favors the assumption that hooded hooks in Eunicida are not homologous to those in spionids and capitellids. According to these trees, it is more likely that hooded hooks evolved at least twice in annelids, corroborating our conclusion that the differing course of chaetogenesis of lumbrinerid hooded hooks reflects convergent evolution with eunicidan hooded hooks.
Acknowledgments
We thank Renate Feist, Christina Förster, Claudia Müller, and Tatjana Bartz for technical assistance. We also thank Björn Quast, Patrick Beckers, and Jörn von Döhren for their support. Our thanks are also due to the Laboratoire de Biologie Marine in Concarneau for hospitality during our collection trips. The study was financially supported by the German research foundation (DFG BA 1520/2).




