The anatomy of Lepetella sierrai (Vetigastropoda, Lepetelloidea): implications for reproduction, feeding, and symbiosis in lepetellid limpets
Abstract
The Lepetelloidea, a clade of small limpet-shaped gastropods, can be used as a case study in continental margin and deep-sea diversification. Lineages in this clade have been found associated with a combination of different substrates, including hydrothermal vents, seeps, wood, whale carcasses, polychaete tubes, chondrichthyan egg cases, seagrass rhizomes, algal holdfasts, crab carapaces, and sponges. Members of one lepetelloidean family, Lepetellidae, live on or inside empty tubes of members of the polychaete genus Hyalinoecia. The detailed morphology of a Mediterranean species, Lepetella sierrai Dantart & Luque 1944, was reconstructed in three dimensions from serial semi-thin sections and compared with that of eleven other members of Lepetellidae. The hermaphroditic lepetellid limpets possessed a ciliated seminal groove, distinct testis and ovary with a common distal gonoduct, and a seminal receptacle containing mature sperm. A unique alimentary tract, with huge esophageal pouches, no true stomach, an extensive multilobed midgut, and short intestine, was present. Additionally, a bacteriocyte system throughout the entire mantle rim was revealed via light and transmission electron microscopy. This is the first recognized evidence for intracellular microbial symbiosis in lepetelloidean limpets. Semi-thin sections showed evidence of a parasite, potentially a chitonophilid copepod, penetrating the body wall of the limpet. Hypotheses about reproductive biology, feeding, and symbiosis are presented based on anatomical features and knowledge of the habitat described herein.
Many metazoan taxa include lineages that have become specialized for life in chemosynthetic and biogenic communities. Molluscs, in particular, have repeatedly invaded these habitats. However, there have been few major diversifications (Kiel 2010). The Lepetelloidea is a clade of small, limpet-shaped vetigastropods that inhabit a high diversity of chemosynthetic or biogenic substrates in the deep sea. Members of Lepetelloidea have been found associated with different substrates, including hydrothermal vents or cold seeps, sunken wood, whale carcasses, fish bone, polychaete tubes, empty chondrichthyan egg cases, seagrass rhizomes, algal holdfasts, crab carapaces, sponges, or some combination of these (Haszprunar 1988a and references therein). Previous researchers have identified nine families within the Lepetelloidea based on unifying morphological characters (Moskalev 1978; Hickman 1983; Marshall 1983, 1987; McLean 1985; Haszprunar 1987, 1988a,b,c, 1989; McLean & Haszprunar 1987; Haszprunar & McLean 1996; Lesicki 1998). Kano et al. (2013) clearly showed that it is difficult and often misleading to reconstruct phylogeny from anatomy in lepetelloidean limpets. Recent molecular data have supported few of the previously described clades, and identified others that will require formal description following the acquisition of additional molecular and morphological data (Kano et al. 2013). With comprehensive morphological data in the context of a well-resolved phylogeny, an integrative and comparative approach can be used to better understand the diversification and ecological differentiation of the group as a whole. Many lineages within Lepetelloidea have had their morphology examined in detail (Haszprunar 1987, 1988a,b,c, 1998; Haszprunar & McLean 1996), but several gaps remain, including tube-dwelling lepetellid limpets.
Lepetellidae was initially erected based on external and radular morphology and is now also supported by molecular data (Kano et al. 2013). Prior to the present study, information on internal morphology was largely lacking, except for the radula, which is unusual in form. De Rayneval et al. (1854) described the first lepetellid limpet as Patella laterocompressa based on fossil material from the Pleistocene of Italy. Verrill (1880, 1881) and Dall (1882) added details on external morphology and the radula. Verrill introduced the genus Lepetella (type species: Lepetella tubicola Verrill & Smith in Verrill 1880), and Dall (1882) introduced the family Lepetellidae. Both had previously reported on the highly specialized habit of lepetellid limpets, which feed exclusively on the tubes of the polychaete genus Hyalinoecia Malmgren 1867, which consist largely of sugar-phosphate polymers (e.g., Graham et al. 1965). However, more than 20 years passed until Thiele (1908) presented the first notes on the anatomy of this family. Thiele placed the Lepetellidae near the Cocculinidae, Bathysciadiidae, and Addisoniidae. This opinion was repeated in Thiele's (1909) overview on the “Cocculinoidea” and also later in his “Handbuch” (Thiele 1929–31). Subsequently, the systematic position of the Lepetellidae remained unstudied and unquestioned for several decades.
Finlay (1927) introduced the genus Tectisumen (type species: Cocculina clypidellaeformis Suter 1908) on the basis of differences in shell morphology. Dell (1956) also used shell morphology in recognizing Tecticrater (type species: Cocculina compressa Suter 1908). However, neither genus was accepted by Warén (1972) or Moskalev (1978), because they considered shell shape to be highly variable, and the radulae of members of both genera were identical. Warén (1972) reported brood protection in Lepetella cf. laterocompressa; however, the “brood” later turned out to be parasitic copepods (Huys et al. 2002). Moskalev (1978) studied a large number of specimens (>180 of L. tubicola and 14 of T. lypidellaeformis) and even performed some histological sectioning, but did not report on lepetellid anatomy in detail. Histological work by Zharkova (1978) confirmed earlier observations of Verrill (1880) on the presence of eyes in L. tubicola.
Although Moskalev's radular drawings were based on scanning electron microscopy (SEM), Hickman (1983) first published scanning electron micrographs of lepetellid radulae, and she described the unique characters of the lepetellid radula. Warén (1991) published a scanning electron micrograph of a lepetellid protoconch, and Dantart & Luque (1994) published scanning electron micrographs of shells, radulae, and soft bodies of Iberian species. Mifsud (1996) provided the first photograph of living L. laterocompressa.
On the basis of a morphological cladistic analysis of major gastropod groups, Ponder & Lindberg (1997) argued that Cocculiniformia (Cocculinoidea & Lepetelloidea; cf. Haszprunar 1988a) is polyphyletic. Lepetelloidea are now included in the Vetigastropoda, and Cocculinoidea are a separate clade. This determination has subsequently been supported by molecular phylogenetic analyses that included members of Cocculinoidea, Lepetelloidea, and other vetigastropod groups, and is now generally accepted (Kano 2008; Aktipis & Giribet 2010, 2012).
Preliminary descriptions of lepetellid anatomy have been presented by Haszprunar (1988a) and Haszprunar & McLean (1996). Here, we present a detailed description of the anatomy and histology of the Mediterranean species Lepetella sierrai Dantart & Luque 1994 as an exemplar of lepetellid anatomy, and visualize it in a three-dimensional (3D) interactive reconstruction. We also present evidence for microbial symbiosis in this taxon, discuss anatomical variation among other lepetellids, and provide additional information on lepetellid parasites. The morphological characters, parasitism, and symbiosis are evaluated and interpreted in an ecological context.
Methods
Material studied
Specimens belonging to 12 tube-dwelling lepetellid taxa were accumulated by GH from various museums and individuals (see below) and investigated by means of serial sections. Several specimens were collected on the BALGIM (Benthos Alboran Golfe Ibéro−Marocain) expedition. For more information and details on collection stations, see Salas (1996) and Ramil & Vervoort (1992). For species determination and taxonomy, see Dantart & Luque (1994).
Tectisumen clypidellaeformis Suter 1908
Two juveniles, one early-stage adult, and three late-stage adult specimens from the National Museum of New Zealand (without detailed information on source locality) were serially sectioned (for details see below). The single sample contained 10 large and 48 very small specimens together with somewhat corroded empty tubes of Hyalinoecia, which had round depressions formed by the gastropods.
Lepetella espinosae Dantart & Luque 1994
One juvenile in poor condition and two adult paratypes in good condition from the type locality were sectioned. According to Dantart & Luque (1994), this is probably the species repeatedly referred to as Lepetella laterocompressa (De Rayneval, Van der Hecke & Ponzi 1854), a taxon based on Pleistocene subfossils from Italy (Monte Mario). However, because species identity in lepetellids cannot be established on shells alone, and there are four extant species with similar shells in the Mediterranean region (L. sierrai, L. espinosae, L. barrajoni, and L. “from Banyuls”), the name L. laterocompressa should be restricted to the original sample.
Lepetella sierrai Dantart & Luque 1994
A juvenile, an early-stage adult (parasitized), and two late-stage adult (parasitized) paratypes from the type locality (station 168A) in the Bay of Biscay were sectioned. Three additional adults (not parasitized) from station 185A on the Fauna II expedition were also examined (see Dantart & Luque 1994 for details).
Lepetella barrajoni Dantart & Luque 1994
Two paratypes were sectioned from the type locality (Bay of Biscay, off Vizcaya, Spain: 43°25.29′–4325.09′N, 231.05′E, station 151A: 82–86 m; 22-06-91; MNCN 15.05/5233).
Species A (Lepetella cf. tubicola). We examined the single original section series from the Humboldt-Museum in Berlin (see 5), on which Thiele's (1908) anatomical paper was based.
Species B (Lepetella cf. laterocompressa). Three specimens of different sizes and varying in their reproductive status (collected by A. Warén from Calvi, Corsica) were sectioned. Warén (letter of 29 June 1987) described the animals in this collection as follows: “1 tube with 1 on outside, 3 on inside, 4 on inside + 1 very young (0.3 mm), 110 m, just off Calvi, slit with numerous ascidians. 8 branchial lamellae at right side of pallial cavity. 2 halfgrown specimens, 0.9 and 1.1 mm had numerous eggs attached individually by stalks in pallial cavity. No side flaps on snout. Snout very extensile, anterior edge very finely crenulated. Pallial edge minutely and irregularly fringed. Foot very mobile, sucker-shaped. Tentacles 3-times as long as when retracted, quite cylindrical. No eyes.”
Species C. BALGIM CP18 (36°48′N–09°31′W; 1578 m, 30.5.1984), also BALGIM CP99, CP69, CP106, CP65. Two adult specimens were sectioned.
Species D. BALGIM CP 108 (36°48′N–09°31′W; 1578 m, 30.5.1984); the same species was also found at BALGIM CP99 and CP10. Warén (letter of 10 June 1987) described these as having “tentacles always contracted; smooth mantle margin.” Two specimens were sectioned.
Species E. BALGIM CP 106 (36°05′N–08°05′W; 1906 m, 10.6.1984. Warén (letter of 29 June 1987) described these as “very small, similar to [Species C], but different shells.” Three specimens from a single sample were sectioned.
Species F. BALGIM CP 84 (33°45.4′N–08°31.9′W; 345 m, 6.6.1984). Warén (letter of 29 June 1987) described these as “shell flatter and the gill is conspicuous.” Five specimens (two adults and three juveniles of various sizes) were sectioned.
Species G (Lepetella cf. laterocompressa). These were collected from a depth of 130 m off Ras-il-Pellegrin, Western Malta, Mediterranean. One adult specimen was collected alive (Mifsud 1996, fig. 4, p. 27), being “found attached to pieces of dead algae stalks and decaying roots of Posidonia on a muddy substrate” (Mifsud in letter of 19 Oct 1996). It should be noted that Posidonia is an unusual substrate for this species, and further investigation is necessary to determine whether this species also has an affinity for plant substrates such as this.
Lepetella “from Banyuls.” Live specimens were collected in tubes of Hyalinoecia from Banyuls-sur-Mer, France, during June 1996 and June 1999. Serial sections were cut of a juvenile, an early-stage adult, and two late-stage adults; some individuals were parasitized by copepods. Some tissues were thin-sectioned and examined by transmission electron microscopy.
Serial sectioning
The early-stage adult and late-stage adult specimens of T. clypidellaeformis and Species C were serially sectioned by conventional methods. It was possible to separate the intact shells from the bodies. The bodies were dehydrated in an ethanol series, then embedded in Paraplast. Series of transverse sections (5 μm thickness) were made. Staining was by Haidenhain's Azan method (Romeis 1989, p. 501). Thiele's (1908) sections of Species A were obviously stained by hemalaun–eosin, and the staining was still in good condition.
The juveniles of T. clypidellaeformis and all remaining specimens were treated differently. In these instances, the shells were dissolved by using Bouin's fluid; this treatment caused some additional postfixation. After washing in 70% ethanol (brought to basic pH by adding a drop of ammonia), the animals were stained in Safranin (1% in 80% ethanol) to facilitate handling of the tiny specimens. After embedding in Araldite or Spurr's (1969) resin, semithin sectioning (transverse, 2 μm thickness) was performed with glass (Ralph) knives. Resin blocks were coated on one side with contact cement to facilitate ribbon formation (Ruthensteiner 2008). The plastic was not removed from sections. Staining was by Regaud's (Romeis 1989, p. 365) iron–hematoxylin or (with better results) by methylene blue (Richardson et al. 1960). Slides were embedded in resin to prevent weakening of the stain over at least 20 years.
Transmission electron microscopy
After relaxation in an isotonic solution of magnesium chloride, specimens of Lepetella “from Banyuls” were prefixed in 2.5% glutaraldehyde in 0.1 mol L−1 phosphate buffer (pH 7.3) for about 20 d. After additional rinses in buffer, postfixation was performed in 1% osmium tetroxide solution in buffer for 2 h, again followed by rinsing in buffer. After dehydration in an ethanol series, the specimens were embedded in epoxy resin. Staining of 70–80 nm ultrathin sections was by uranyl acetate and lead citrate.
Three-dimensional reconstruction
One section series of L. sierrai was selected for 3D reconstruction with Amira® software following the methods outlined by Ruthensteiner (2008). The section series was prepared using a specimen from the empty tubes of Hyalinoecia collected at a depth of 95 m in Banyuls-sur-Mer, France, in June 1996. Every second section in the series was photographed, except for the occasional broken or obstructed section; these were skipped. Images were preprocessed for 3D reconstruction in Adobe Photoshop by converting to eight-bit grayscale, auto-scaling, unsharp masking, and resampling to 1600×1200 pixels, a resolution sufficient for the reconstruction of most histological details. The images were renamed with sequential numbers and imported as an image stack into Amira®. The body surface and each individual organ were traced and labeled manually by interpolating through subsets of sections that were consistent or changed shape gradually. All labeling was checked by eye, and sections distorted or broken by scratches were corrected using interpolation. The set of contour lines for each organ and the body surface were separately rendered to create a set of 3D surface models of the body surface and all organs. Snapshots of various organ systems and perspectives were taken in 2D, and the 3D model was embedded in a portable document format (pdf) file following Ruthensteiner & Heß (2008) and Ruthensteiner et al. (2010).
Graphic reconstructions
All graphic reconstructions were based on nearly transverse serial sections. In most cases (dorsal views), the structures and organs were projected vertically onto a horizontal plane, and a transverse line represented each section. In the case of Paraplast-embedded specimens, the midlines of the mantle border, nerve cords, and radular cartilages served as reference lines from which measurements were taken. In the case of resin-embedded material, the blocks were symmetrically trimmed (by a Reichert TM-60 trimming machine) to use the edges of the whole sections as reference lines, enabling very exact measuring in all views. After some jagged lines had been smoothed, the graphic reconstructions were directly used for illustrations, except for some shading and semi-schematic patterning that was added. Photographs of sections were taken to show anatomical and histological details.
Results
The primary morphological descriptions presented below are based on observations of Lepetella sierrai. Differences in anatomy from L. sierrai that were observed in members of other lepetellid species are also described, and summarized in Table 1.
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lepetella tubicola | 0 | ? | ? | ? | ? | 0 | 1 | ? | ? | ? | ? | ? | 0 |
| L. espinosae | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 |
| L. sierrai | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 2 |
| L. barrajoni | 1 | 1 | ? | 1 | ? | 0 | 1 | x | ? | ? | ? | ? | 2 |
| L. “from Banyuls” | 1 | 1 | 1 | 1 | 0 | 0 | 1 | x | 1 | 0 | 2 | 3 | 2 |
| Sp. A | 0 | 1 | 1 | 2 | 1 | 0 | 1 | x | 0 | 0 | 2 | 0 | 2 |
| Sp. B | 2 | 0 | 1 | 1 | 1 | 0 | 1 | x | 0 | 1 | 2 | 0 | 2 |
| Sp. C | 1 | 2 | 0 | 0 | 0 | 2 | 3 | x | 0 | 0 | 0 | 2 | 2 |
| Sp. D | 2 | 0 | 1 | 2 | 0 | 1 | 2 | x | 0 | 0 | 2 | 0 | 2 |
| Sp. E | 1 | 0 | 0 | 2 | 1 | 1 | 2 | x | 0 | 1 | 2 | 0 | 2 |
| Sp. F | 2 | 0 | 1 | 1 | 0 | 0 | 2 | x | 0 | 0 | 2 | 0 | 2 |
| Tectisumen clypidellaeformis | 2 | 0 | 0 | 0 | 1 | 2 | 0 | x | 1 | 0 | 1 | 1 | 1 |
External morphology
All known lepetellids are “symmetrical limpets,” i.e., there is no trace of juvenile coiling in the early teleoconch. The protoconch is a coiled-over cup with a fused tip and distinct lateral bulges as in other lepetelloid families (fig. 6B of Warén 1991; A. Warén, unpubl. data). The external body shape of the animals is also bilaterally symmetrical (Fig. 1).
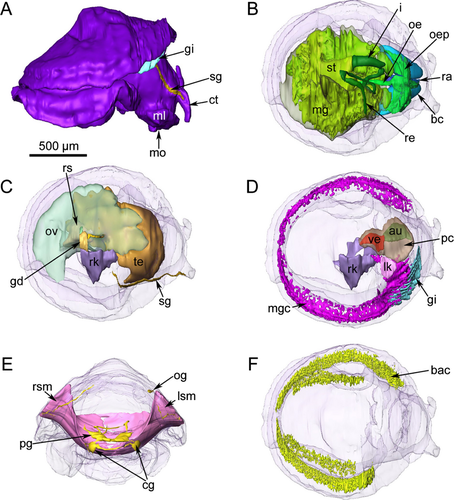
Lepetella sierrai had a large head with two anteriorly situated slightly papillate cephalic tentacles; the ventral, slit-shaped mouth was flanked by medium-sized oral lappets. From the outer base of the right cephalic tentacle, a seminal groove extended backward along the right side of the neck, reaching the right side of the mantle cavity (Fig. 1). Eyes were lacking.
The pedal sole had two distinct zones: (1) a densely ciliated zone of very elongate cells around (2) the flat epithelium of the central zone, which was nonciliated. A pedal gland was not present, but there were laterally situated sole glands. Lepetella sierrai also had a single epipodial tentacle in a posterior median position (Fig. 2A) (cf. fig. 51 of Dantart & Luque 1994).
Interspecific variation
The central sole of the foot was not (Species B, D, F) or only sparsely (Tectisumen clypidellaeformis, Species C) ciliated; conditions in Species A could not be clearly determined. The dorsal side of the pedal flaps consisted of distinct mucous cells in Species F. In Species C, lappets of the esophageal pouches reached into the lateral flaps of the pedal sole.
Mantle margin and cavity
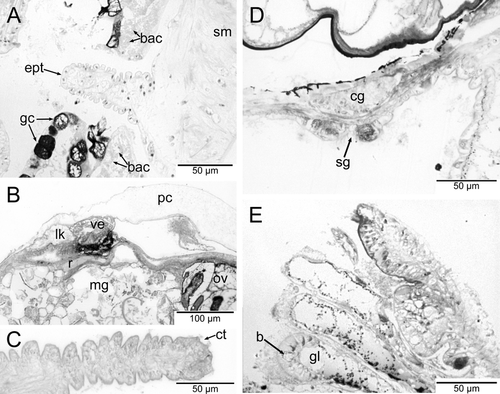
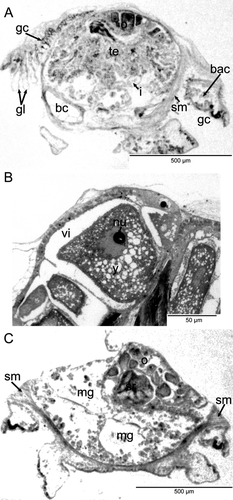
The mantle had small papillae and a dense region of glandular cells along its margin, surrounding the animal in a horseshoe shape. These cells stained a distinctive pink color (toluidine blue), rather than the typical blue staining of the other cell types. A dense network of bacteriocytes was positioned directly below these glandular cells in the same, symmetrical, horseshoe pattern (Fig. 1).
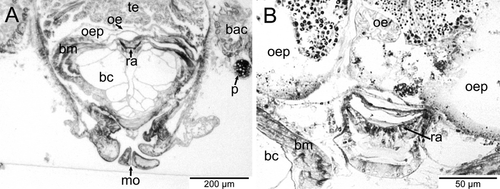
The mantle cavity was asymmetrical and quite deep on the left side. The pericardium was situated in the left anterior roof of the mantle cavity and contained a single auricle anteriorly and a ventricle posteriorly (Figs. 1, 2B). The posterior part of the ventricle enclosed the rectum, which curved to the right side and opened via an anal papilla into the mantle cavity. The left kidney was a small organ in the right anterior roof; it lay alongside the pericardium adjacent to where the auricle met the ventricle. The distal part of the rectum was directly posterior to the left kidney and the anal opening was further right. To the right of the anus, the anterior chamber of the right kidney opened into the mantle cavity and a glandular zone continued forward from this opening to the anterior end of the right shell muscle. The external ciliated seminal groove extended up the right cephalic tentacle and connected to the right dorsal anterior part of the right kidney opening (Figs. 1, 2C,D). A hypobranchial gland was lacking.
The gill leaflets occupied the space in the mantle cavity reaching from the central anterior-most part of the mantle cavity and back one-third the length of the animal into the right side of the mantle cavity. The gill in the reconstructed specimen had ten leaflets. The gill was equipped with short pockets (“sensory bursicles”) at its efferent axis (Figs. 1, 2E).
Interspecific variation
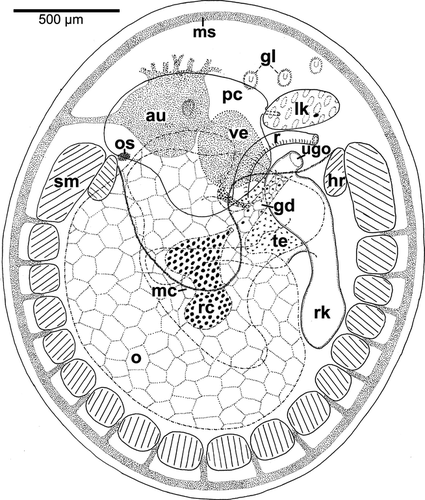
Species C was somewhat exceptional, as the gill leaflets were here reduced to short ciliated spots providing water circulation, but still equipped with bursicles (Fig. 3). In T. clypidellaeformis, the heart was positioned in the central area, whereas the anterior left area was occupied by the “sinus venosus” (Fig. 4A). In Species D, the kidney–rectum complex was shifted to the right and somewhat detorted so that the left kidney was situated anteriorly of the rectum, and the urogenital opening was placed more posteriorly.
Muscle systems
The shell muscle was a compact horseshoe-shaped structure penetrated by nerves (see Haszprunar 1985, 1988d). Smooth muscle fibers were oriented longitudinally and met mid-ventrally in the foot, with clear intercrossing of these fibers (Fig. 1).
Two distinct head retractor muscles had their insertion areas at the inner anterior ends of the shell muscle. They followed the cerebral connective nerves and connected in the center, immediately dorsal to the buccal apparatus (Fig. 1). Cross-striated buccal muscles surrounded and supplied the buccal apparatus.
Interspecific variation
In Species B, only the anterior portions of the shell muscles were thick, whereas the posterior portion and the ventral intercrossing area were very weakly developed. Most remaining species had thicker shell muscles that were compact and only penetrated by nerves. Species C, however, showed a shell muscle divided by blood sinuses like Patellogastropoda or Cocculinoidea (Fig. 3).
Heart and excretory system
The heart was monotocardian (one auricle and one ventricle) and rested within a large, flattened pericardium located in the left anterior mantle roof. The auricle was positioned on the left side of the pericardium, while the ventricle was posterior and slightly more to the right. The posterior part of the ventricle surrounded the rectum. The aortic vessel was very short, and opened into the body sinuses.
Lepetella sierrai had two kidneys. The left one was substantially smaller than the right one, was situated in the roof of the mantle cavity in front of the rectum, and was connected with the pericardium via a thin, short, and densely ciliated duct. The right kidney occupied the space between the gonads and extensive midgut. It had a much thinner epithelium than the left kidney and did not connect to the pericardium, but it shared an opening in the mantle cavity with the gonoduct (Fig. 1).
Interspecific variation
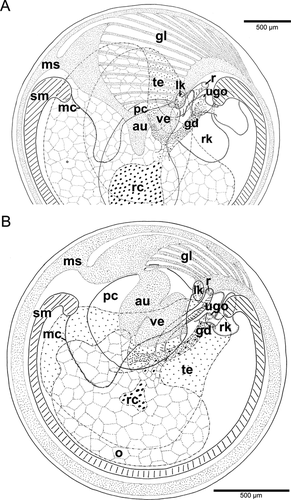
In all species except T. clypidellaeformis, the main portion of the heart was located in the mantle roof (Fig. 4A). In Species B, the bloodstream passed the kidneys, then surrounded the anterior end of the right shell muscle, and was oxygenated by passing the mantle sinus, the gill leaflets, or the mantle roof. The bloodstreams united immediately before entering the auricle anteriorly left. In Species C, the bloodstream passed the right kidney, then extended between the shell muscle bundles, and formed a large mantle sinus, which entered the auricle at the left side. A second stream was filtered by the left kidney, then spread over the mantle roof and collected in numerous small sinuses entering the auricle anteriorly.
Due to the well-developed gill, the heart conditions differed in late-stage adults of T. clypidellaeformis (Fig. 4A). The heart was mainly viscerally situated and showed a somewhat hypertorted orientation in that the auricle–ventricle axis was nearly horizontal to the left kidney. In addition, the pericardium had a large but depressed pouch, which reached far backward. Early-stage adults had their heart much more pallially situated and lacked the posterior pouch of the pericardium (Fig. 4B). Azan staining suggested that the blood was oxygenated mainly in the subpallial cavity, in the mantle roof, bypassing the gill leaflets (if present). The anterior right leaflets released their blood into the anterior portion of the mantle sinus, whereas the blood of the central (truly pallial) leaflets was collected in an efferent sinus proper. Both streams united in a large “sinus venosus” before entering the auricle.
Reproductive system
Adults of L. sierrai were simultaneous hermaphrodites. The gonad occupied over half of the visceral mass and possessed distinct testicular and ovarian tissue in separate chambers. The maximum egg diameter observed was 230 μm; all eggs near this size contained substantial quantities of yolk.
In L. sierrai, the testis began directly posterior to the buccal apparatus and occupied the left side of the body. All stages of spermatogenesis were observed within the testis (Fig. 5A). The ovary was situated more posteriorly and occupied the right dorsal side of the body (Fig. 5B). Between the testis and ovary sat a seminal receptacle containing mature spermatozoa (Figs. 1, 5C). The oviduct and receptacular duct joined initially and then met the vas deferens to form a common hermaphroditic gonoduct (Fig. 1). The gonoduct itself was a simply ciliated tube and lacked special glands or vesicles. It extended forward to the right and opened into the distal portion of the right kidney forming a common urogenital opening into the mantle cavity. The urogenital pore opened into the right posterior corner of the mantle cavity, and from there a glandular groove extended forward a short distance reaching the anterior end of the right shell muscle. It was continued by a simple ciliated groove, which extended forward along the right neck to reach the outer side of the right cephalic tentacle (Figs. 1, 2D). Aside from the ciliary groove, the right cephalic tentacle resembled the left one.
Interspecific variation
In Species B, the smallest of the three examined specimens showed only a testis with ripe sperm, but no trace of an ovary; in the intermediate-sized specimen, both gonads were present, including ripe gametes; and in the largest specimen, only traces of a testis were detected, whereas the ovary was enormously developed. In Species A, C, D, and E, only adults were studied, which had both gonads well developed, and the same can be stated for Species F and all stages of T. clypidellaeformis (Fig. 4), although in the early-stage adult, the testis was relatively much larger than in the late-stage adults (Fig. 4B). There were no differences between the species with respect to their copulatory organs. In one specimen of Species C, mature sperm were found in the mantle cavity, but we could not determine whether these were auto- or allosperm.
Thiele's (1908, p. 88, his fig. 16) description of the genital openings of Species A must be corrected. Thiele's “gonoduct” (labeled gd) is the distal chamber of the right kidney, and Thiele's “receptaculum” (labeled rec) is the genital duct. Nonetheless, a quite large seminal receptacle was indeed present in the right side of the mantle cavity of Species A. It contained a mass of dense sperm, which was not orientated. The seminal receptacle opening was to the right of the urogenital opening.
Alimentary tract
The voluminous buccal cavity was lined by a cuticularized epithelium. Jaws and salivary glands were lacking, and the sublingual pouch was shallow and lacked a subradular sense organ or distinct glands. A ciliated dorsal food channel extended up from the mouth between two large buccal cartilages. Prominent cross-striated muscles, the fibers of which looked hollow in cross-section, supported the buccal cartilages (Fig. 6A). The horizontal muscle inserted ventrally and a thick sphincter muscle surrounded the mouth opening. The anterior end of the radula extended vertically up the dorsal food channel and met the esophagus near the top of the channel (Fig. 6B). An additional small cartilage was located ventral to the radula, which extended posteriorly and downward, ending where the midgut began. The radula was described from SEM images by Dantart & Luque (1994). The radular sheath was quite short and the radular cecum, which was short but broad and depressed, containing many small odontoblasts, was situated between the posterior ends of the cartilages. The anterior esophagus had huge lateral pouches, which folded up and extended backward along the top of the buccal cartilages (Fig. 6A,B). After a short distance, the enlarged esophagus was narrowed abruptly and extended backward as a thin tube, approaching the ventral side of the body. The epithelium of the posterior esophagus consisted of few vacuolated and ciliated cells. The lumen was very narrow. Running backward at the very ventral side, the esophagus finally entered the stomach region, which was situated in the center of the animal (Fig. 1).
The stomach could not be clearly delimited, but was combined with a rather complex multilobed midgut with extensive folding in the lumen. The stomach region lacked a gastric shield, cecum, sorting areas, and typhlosole, and could be defined only by the entrance of the esophagus and the emergence of the intestine. The stomach region and midgut epithelium were very flat and not ciliated. There were two distinct anterior lobes of the midgut that were thin walled compared with the rest of the organ. Several openings into the midgut from the stomach region were present. The extensive midgut occupied the ventral posterior body cavity. Small particles of food were evident in the lumen of the gut, but the material was not very dense (Fig. 5C).
At the anterior left side of the stomach region, the intestine emerged as a short, narrow tube with a flat and simply ciliated epithelium; a typhlosole was not present. After a short loop at the ventral side of the body, the intestine extended dorsally near the posterior end of the buccal apparatus (Fig. 1). It emerged anterior to the gonoduct and transitioned into the rectum extending to the right along the posterior edge of the left kidney. After having penetrated the posterior part of the heart ventricle (Fig. 2B), the rectum extended to the right side and opened via a papillate anus into the right mantle cavity.
Interspecific variation
In Species A, B, D, and F, the esophageal pouches were somewhat smaller than in T. clypidellaeformis, where they occupied the anterior third of the animal's body (Fig. 4A). In Species C, these pouches were enormously developed and ran laterally backward, filling free space between the shell muscle bundles and in the lateral pedal flaps (Fig. 3).
Nervous system
The weakly concentrated lepetellid nervous system had a hypoathroid (adjacent pleural and pedal ganglia) cerebropedal nerve ring enclosing the buccal apparatus, and a streptoneurous visceral loop (Figs. 1, 7). The cerebral ganglia were laterally situated and interconnected by a long commissure. Anteriorly, they sent out thick but simple tentacular nerves into the cephalic tentacles. An optic nerve could not be detected. Ventrally, three main nerves emerged from the cerebral ganglia that supplied the region of the mouth. The posterior-most nerve had a common origin with the nerve supplying the oral lappet region. Immediately beneath the cerebral ganglia, the buccal connectives emerged. They extended downward and then upward between the buccal muscles to meet the buccal ganglia. The latter were laterally situated at the line of the emergence of the esophagus and were interconnected by a long commissure.

The connectives to the pleural and pedal ganglia were nearly equal in length. The pedal system lacked pedal cords, and the large pedal ganglia were interconnected by two commissures only, a very thick anterior and a very thin posterior one. Furthermore, there were no anterior pedal nerves as is usual in vetigastropods. Aside from the pedal nerves, which supplied the pedal sole, each pedal ganglion sent out a shell muscle nerve immediately below the emergence point of the mantle nerve. The statocysts were situated immediately above the anterior pedal commissure. The pleural ganglia were positioned laterally and somewhat above the pedal ganglia. Thick mantle nerves emerged laterally from the pleural ganglia and penetrated the anterior bundles of the shell muscle to eventually reach the mantle region, where they formed a nerve net surrounding the whole animal.
Whereas the anatomy of the cerebropedal ring and the osphradial ganglion could be studied in all species investigated, the details of the visceral loop could only be resolved in Species F (Fig. 7). In that species, from the left pleural ganglion the visceral loop started to the right side leading under the radular sheath and the right pleural ganglion. The subesophageal ganglion was small, elongated, and quite indistinct, being situated above the posterior pedal commissure. From there, the visceral loop extended further to the right, then upward along the right dorsoventral shell muscle, finally looping to the left along the posterior end of the mantle cavity. The visceral ganglion was long but dorsoventrally compressed, reaching from the region of the urogenital opening to the point of entrance of the rectum into the heart ventricle. From this latter position, two nerves emerged, supplying the urogenital chamber and the rectum. The visceral loop continued anteriorly along the downward bending intestine and reached the supraesophageal ganglion after a short distance. This ganglion was situated near the center of the animal and was very small and indistinct and was embedded in the gonad. From this position, a long and very thin connective was oriented between gonad and viscera to the left. At the anterior end of the left shell muscle this connective entered the mantle roof, then extended anteriorly. After a short distance it formed the osphradial ganglion. From the supraesophageal ganglion the visceral loop continued downward, surrounding the posterior portion of the right buccal cartilage, and finally terminated in the right pleural ganglion.
Sense organs
Lepetella sierrai had two short lateral cephalic tentacles. The right tentacle was modified with a ciliated seminal groove. The cephalic tentacles were seemingly smooth, but showed small papillae with ciliary tufts at the tips in the sections using SEM (Fig. 2C,D). A single thick nerve innervated them.
Eyes, paired epipodial tentacles, and subradular organ were lacking. Statocysts were located directly dorsally to the pedal ganglia and included several small statoconia, which were in contact with very thin fibers within the statocyst's capsule. There was no distinct osphradial epithelium, but a single osphradial ganglion was present in the left anterior mantle roof.
Metazoan parasites
An unidentified parasite was observed in the body of the serially sectioned specimen of L. sierrai. Between the body wall and mantle tissue a round mass of foreign tissue was found (Fig. 6A). A narrow piece of tissue from this mass penetrated the body wall and internally gave rise to a highly branched structure within the limpet's body. No diagnostic appendages or distinct organs associated with this mass were observed in the histological sections, so the identity of this parasite is unknown. However, it is possible that the observed instance of parasitism is an early stage of the parasitic chitonophilid copepods observed by other researchers in this species (Dantart & Luque 1994; Huys et al. 2002), similar to the “vermiform endosome” stage seen in lepetodrilid limpets by Tunnicliffe et al. (2008). Although the sections are not clear, there does appear to be a “rootlet system” (sensu Huys et al. 2002) and what may be a small female body on the outside of the body wall.
Microbial symbiosis
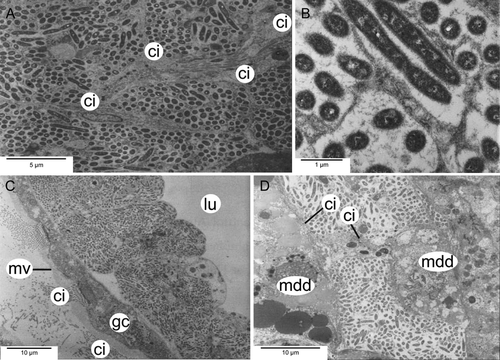
A dense network of bacteriocytes in the mantle margin and pedal tissue of L. sierrai extended in a horseshoe shape that almost completely surrounded the entire animal (Fig. 1). Observations by TEM (Fig. 8; Michalak & Haszprunar, unpubl. data) of Lepetella “from Banyuls” showed that the bacteriocytes form an epithelial network with a common but narrow lumen. However, connections to the alimentary tract, other organs, or to body sinuses were not observed in the serial sections. These bacteriocytes have previously been identified as “glandular” areas, but TEM confirmed their identity as microbial host cells (Fig. 8).
Discussion
Discrimination of species
As shown in Table 1, all putative species available were uniquely characterized by a combination of various characters and thus may represent distinct lineages. However, little is known about the interspecific variability of lepetellid soft bodies, and only three putative species show clear anatomical autapomorphies. Species C had complex papillae at the mantle margin, cartilages consisting of many small cells rather than large ones, reduced gill leaflets, and huge esophageal glands. Lepetella “from Banyuls” showed unique esophageal pouches with vesicles. Tectisumen clypidellaeformis had cartilages consisting of medium-sized cells, and an optic nerve, but no eyes. As there are many undescribed species of lepetellids, further systematic work will benefit greatly from the examination of molecular data.
Comparison to other members of Lepetelloidea
The most distinctive morphological and ecological differences that have been observed among members of the Lepetelloidea are presented in Table 2. Comparisons including more traits and higher level taxa have been made and discussed elsewhere (Haszprunar 1988a, 1998; Haszprunar & McLean 1996). Preliminary molecular phylogenetic data presented by Kano et al. (2013) indicates that the tube-dwelling Lepetellidae discussed here are monophyletic and quite derived within the Lepetelloidea, indicating a comparatively recent specialization to polychaete tubes as a substrate in this group. Here, we focus on particular life history and ecological hypotheses for Lepetella.
| Genus | Substrate | Esophageal pouches | Gastric shield | Midgut | Symbiosis evidence | Seminal receptacle | Rectum penetrates heart | Gonoduct | Bursicles | Shell muscles | Common urinogenital opening | Oral lappets | Jaws |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lepetella | Hyalinoecia tubes | Large | Absent | Large, multilobed | Bacteriocytes in mantle rim and periphery of foot | Present | Yes | Hermaphroditic | Yes | Symmetrical | Present | Present or absent | Absent |
| Pseudococculina | Wood | Present | Present | 2-lobed, common opening | Unknown | Absent | Yes | Hermaphroditic | Yes | Symmetrical | Present | Present | Present |
| Osteopelta | Whale bone | Glands with duct | Present | 2-lobed, 2 openings | Unknown | Absent | Yes | Gonochoristic | No | Symmetrical | Separate openings | Absent | Present |
| Addisonia | Elasmobranch egg cases | Glands with duct | Absent | No stomach, enlarged intestine | Suspect “glandular” tissue of gills (GH obs.) | Present | No | Gonochoristic | No | Asymmetrical | Separate openings | Absent | Absent |
| Pyropelta | Hydrothermal vents | Present | Present | Unmodified | Unknown | Absent | Yes | Hermaphroditic | Yes | Symmetrical | Present | Absent | Present |
| Bathyphytophilus | Seagrass rhizomes | Large | Present | 2 large glands | “Grape-like” structures on posterior esophagus | Absent | Yes | Hermaphroditic | Yes | Asymmetrical | Present | Absent | Absent |
Feeding ecology and symbiosis
Lepetella species inhabit the inner and outer walls of empty tubes of polychaete worms from the genus Hyalinoecia. The tubes are composed of a sugar-phosphate polymer called onuphic acid (Graham et al. 1965). Most of the Lepetella specimens we examined were collected from tubes brought up in dredges of muddy continental shelf/slope sediment. The microhabitat of the tube provides a semi-hard substrate for the limpet, and is also a likely source of nourishment. Other cocculiniform limpets inhabiting similar biogenic microhabitats, such as Tentaoculus, which lives on crab carapaces and shows a typical vetigastropod gut, are presumed to be feeding on the microbial and/or fungal community on the substrate. Lepetella specimens have been observed on both the outside and inside of Hyalinoecia tubes, and it has been noted by A. Warén (unpubl. data) that shell shape differs depending on whether they are situated on the inside or outside of the tube. Thus, shell characters are not particularly useful for taxonomy in this group. Lepetella has extensive modifications to the alimentary tract, including a characteristic radula (Dantart & Luque 1994), large esophageal pouches, and an extensively folded and multilobed midgut rather than a typical stomach with gastric shield. Additionally, round depressions where tube material has been removed were clearly evident when limpets were removed (G. Haszprunar, unpubl. data). We hypothesize that the alimentary tract has been highly modified to cope with the digestion of the sugar-phosphate polymer tube. The lepetellid radula, which is a synapomorphy for the family Lepetellidae, is specialized to work as a coarse file on the tubes rather than simply as a scratching brush as in most other members of Lepetelloidea, which have radulae modified to scrape off surface film. Yet, we doubt that lepetellids can subsist on the nutrition they receive from the onuphic acid tube alone, and it is possible that they also ingest the biofilm that is assumed to be decomposing the tube.
The presence and location of an extensive system of bacteriocytes suggest the possibility of chemosynthetic pathways as well. Despite an extensive search, we could not find any connection of the bacteriocyte network to the gut; its major part is situated in the mantle rim. This suggests a likely exchange with the environment within the Hyalinoecia tube or directly surrounding it. Although studies of the oxygen and sulfide profiles inside and surrounding the tube have not been performed, studies on the burrows of the lugworm, Arenicola marina, show a steep shift from an oxygenated microenvironment near the surface of the burrow to an anoxic one with high but frequently fluctuating levels of free hydrogen sulfide a few millimeters further into the burrow (Wetzel et al. 1995). If a similar oxygen-sulfide gradient exists within the empty tubes of Hyalinoecia, this would be the ideal environment for sulfide-oxidizing chemoautotrophic symbionts. Confirmation of bacteria in the mantle rim tissue by TEM has only been obtained in Lepetella “from Banyuls.” However, all other Lepetella specimens examined by histology have the same “glandular” tissue that has now been recognized as a bacteriocyte system in the mantle rim. Additional research is needed to better understand the mode of nourishment and the role of this symbiosis in the biology of this limpet group.
Reproductive behavior
All examined Lepetella specimens possess an ovotestis with spatially distinct areas of testis and ovary. They are designated as simultaneous hermaphrodites, except for Species B, for which there is evidence for protandric hermaphroditism. Given limited samples, it is difficult to determine the mode of reproductive development, and the hypothesis of protandry in Species B is based on only three specimens varying in size, the smallest having mostly testis and little ovary, the medium-sized specimen having nearly equally sized testis and ovary, and the largest one having a much larger ovary and reduced testis. It is possible that protandry is a common reproductive strategy among lepetellid limpets; however, further study of a wider range of sizes in each species is needed.
Given the high yolk content of the eggs, it is very likely that larval development is lecithotrophic, as in all other studied Vetigastropoda. The ciliated seminal groove on the right cephalic tentacle, which is observed in all specimens, is evidence that the cephalic tentacle has been modified as a copulatory organ for internal fertilization. Further evidence for internal fertilization is the presence of a distinct seminal receptacle containing only mature spermatozoa, suggesting these are allosperm. The testis and ovary show continuous gametogenesis, suggesting that these animals have nonseasonal reproduction. Hermaphroditism, internal fertilization, and nonseasonal, continuous spawning are all traits that increase fertilization probability, given the meeting of two conspecifics. These traits are also present in several other deep-sea vetigastropod groups (see Kano 2008). Given the patchiness and small size of Hyalinoecia tube substrata, and the low densities of limpet populations on the tubes, it is not surprising to find these traits in Lepetella as well.
Parasitism
The first recognized parasites in Lepetella were chitonophilid copepods whose eggs were originally identified as brooded limpet eggs by Dantart & Luque (1994) and subsequently investigated in detail and identified as copepods by Huys et al. (2002). Distinct, round, white eggs can be seen in the mantle cavity when this parasite is present. The female copepod's body is external to the host's body, but it attaches by a rootlet system. Dwarf males subsequently attach to the females and fertilized eggs are produced. It is possible that while we did not observe the distinctive eggs, we may have observed an early stage of this parasitism with just a small female body with rootlets extending into the limpet. This parasitism does not seem to be uncommon in Lepetella specimens, and has also been recognized and beautifully photographed in lepetodrilid limpets (Tunnicliffe et al. 2008). However, we cannot exclude the possibility that the parasitic copepods we observed are members of another family, the Splanchnotrophidae (see e.g., Anton & Schrödl 2013). Preservation was not good enough to confirm the identity of the parasite, and further study of this parasitic relationship will require additional well-preserved specimens, preferably for molecular data.
Acknowledgments
We are most grateful to Anders Warén (Swedish Museum of Natural History, Stockholm) for providing most of the material studied. Further material was generously provided by the Australian Museum Sydney, Louis Dantart (Universidad de Barcelona) and Angel Luque (Universidad Autónoma de Madrid), and Constantine Mifsud (Malta). We thank the staff of the Malacology Department of the Museum für Naturkunde, Berlin, for the loan of Thiele's sections. For sectioning and photographing, we thank Bernhard Ruthensteiner (ZSM), Eva Lodde (ZSM), and Heidemarie Gensler (LMU Munich). Bernhard Ruthensteiner also trained JJ concerning 3D reconstruction and visualization. We would also like to thank the German Academic Exchange for awarding JJ a research grant that made her visit to Munich and this collaboration possible. We thank David Lindberg for helpful comments on the manuscript. We also thank editors Louise Page and Bruno Pernet, Anders Warén, and an anonymous reviewer for improving the quality of the manuscript with their constructive reviews.




