Effectiveness of XBB.1.5 Vaccines Against Symptomatic SARS-CoV-2 Infection in Older Adults During the JN.1 Lineage-Predominant Period, European VEBIS Primary Care Multicentre Study, 20 November 2023–1 March 2024
The members of the VEBIS Primary Care Vaccine Effectiveness Group are acknowledged at the end of the article.
Funding: Funding for this project was received from the European Centre for Disease Prevention and Control (ECDC) implementing Framework Contract ECDC/2021/019 ‘Vaccine effectiveness, burden and impact of COVID-19 and influenza’. The funder of the study had a role in the study design, data interpretation and the review of the manuscript, but no role in data collection or data analysis. The corresponding author had full access to all the data in the study, and final responsibility for the decision to submit for publication was by consensus.
ABSTRACT
We estimated XBB.1.5 vaccine effectiveness (VE) against symptomatic SARS-CoV-2 infection among adults aged ≥ 65 years during the 2023/2024 JN.1 lineage-predominant period in a European multi-country test-negative case–control study at primary care level. We estimated VE adjusted by study site, age, sex, chronic conditions and onset date. We included 220 cases and 1733 controls. The VE was 48% (95% CI: 12–71), 23% (95% CI: −11–48) and 5% (95% CI: −92–56) among those with symptom onset 1–5, 6–11, and ≥ 12 weeks after vaccination, respectively. XBB.1.5 vaccine provided short and moderate protection against JN.1 symptomatic infection.
1 Introduction
During the 2023/2024 season, the SARS-CoV-2 JN.1 lineage, a sublineage of BA.2.86, rapidly displaced XBB lineages. As of 30 April 2024, > 90% of SARS-CoV-2 viruses sequenced globally were JN.1 or its descendants [1]. JN.1 harbours the L455S substitution in the receptor binding site in the spike protein, which may be associated with immune escape [2].
In the autumn/winter 2023/2024 COVID-19 vaccination campaigns in Europe, the XBB.1.5 vaccine was recommended in most countries, comprising over 97% of vaccines administered [3]. Older adults were a priority group in these campaigns, as well as those with medical risk conditions and healthcare workers. In certain European countries, the XBB.1.5 vaccines have also been used for spring 2024 campaigns.
Regular monitoring of COVID-19 vaccine effectiveness (VE) is important to understand the performance of the vaccine in light of evolving variants and underlying population characteristics, including by time since vaccination.
The VEBIS (Vaccine Effectiveness, Burden and Impact Studies) project is a European multicentre test-negative case–control study. We estimated autumn/winter 2023/2024 COVID-19 VE against medically attended symptomatic SARS-CoV-2 infection in older Europeans at primary care level during a period of JN.1-predominance.
2 Methods
2.1 Study Design, Data Collection and Key Definitions
Eleven study sites in 10 European countries participated in the VEBIS primary care study: Croatia, France, Germany, Hungary, Ireland, the Netherlands, Portugal, Romania, Spain (national), Spain (Navarre region) and Sweden.
Methods have been described elsewhere [4, 5]. Briefly, participating physicians selected all or a systematic sample of patients with acute respiratory infection (ARI) for swabbing and inclusion in the study. Samples were tested by RT-PCR for SARS-CoV-2 and influenza. Those testing positive for SARS-CoV-2 were cases, and those testing negative were controls. Demographic (age and sex) and clinical (symptoms, dates of symptom onset and swabbing) information were collected. COVID-19 vaccination information was collected through vaccine registry linkage, electronic health record look-up and self-report. Site-specific information on patient recruitment, case definitions, 2023/2024 COVID-19 vaccination campaign information and data sources are specified in Table 1. We defined a patient as vaccinated if they received 2023/2024 COVID-19 vaccine on or after the start of their country/region-specific autumn 2023/2024 vaccination campaign and ≥ 7 days before symptom onset. To estimate VE, we defined the reference group of ‘unvaccinated’ individuals as those patients who did not receive 2023/2024 COVID-19 vaccine either within the study period before recruitment or during the 180 days prior to the start of the 2023/2024 COVID-19 vaccination campaign. Study sites sequenced all or a random sample of SARS-CoV-2 viruses below a specified cycle threshold (Ct) value.
| Study site | Start of autumn 2023 national COVID-19 vaccination campaigns | Recommendation for COVID-19 vaccination for all adults over this age (years) | Case definition used for recruitment of patientsb | Data source for COVID-19 vaccination information | JN.1 lineage-predominant period start (≥ 60%)c |
|---|---|---|---|---|---|
| France | 2 October 2023 | 65 | Sentinelles ARI | GP interview (self-report) | 11 December 2023 |
| Germany | 18 September 2023 | 60 | ARI | Medical records, patient's certificate of vaccination, GP interview (self-report) | 18 December 2023 |
| Hungary | 1 October 2023 | 60 | EU-ARI | Primarily from the National Immunisation Registry (83.5%), or from GP records or self-report | 8 January 2024 |
| Ireland | 2 October 2023 | 50 | EU-ARI | Data linkage to vaccine registry | 11 December 2023 |
| The Netherlands | 2 October 2023 | 60 | EU-ILI or EU-ARI | GP interview (self-report) | 11 December 2023 |
| Portugal | 29 September 2023 | 60 | EU-ARI | Vaccine registry look-up by GPs | 20 November 2023 |
| Spain, national | 25 September 2023 | 60 | EU-ARI | Data linkage to vaccine registry | 4 December 2023 |
| Spain, Navarre region | 16 October 2023 | 60 | EU-ILI | Data linkage to vaccine registry | 4 December 2023 |
- Abbreviations: ARI, acute respiratory infection; EU, European Union; ILI, influenza-like illness; VEBIS, Vaccine Effectiveness, Burden and Impact Studies.
- a Eight study sites were included in the 2023/2024 COVID-19 VE analysis in the JN.1 lineage-predominant period (providing ≥ 10 COVID-19 cases): France, Germany, Hungary, Ireland, the Netherlands, Portugal, Spain (national) and Spain (Navarree region).
- b EU-ARI: sudden onset of symptoms and at least one of four respiratory symptoms (cough, sore throat, shortness of breath, coryza) and a clinician's judgement that the illness is due to an infection; EU-ILI: sudden onset of symptoms and at least one of four systemic symptoms (fever or feverishness, malaise, headache or myalgia) and at least one of three respiratory symptoms (cough, sore throat, or shortness of breath); Sentinelles ARI: sudden onset of fever (or feverishness) and respiratory signs; the ARI case definition in Germany includes patients with at least one of the following four symptoms: fever, cough, coryza or sore throat.
- c Due to low samples sequenced by week, GISAID data for Hungary were imputed using data from the seven countries sharing a border (Austria, Croatia, Romania, Serbia, Slovenia, Slovakia and the Ukraine).
For analysis, we restricted to those patients swabbed within 10 days of symptom onset, meeting the EU-ARI case definition (sudden onset of symptoms and at least one of four respiratory symptoms [cough, sore throat, shortness of breath and coryza]), with complete and consistent key variable information, eligible for XBB.1.5 vaccination in the 2023/2024 season. We excluded patients with non-JN.1 or descendant characterised viruses and excluded study sites with fewer than 10 cases or controls each.
We determined the start of the JN.1 lineage-predominant week from the week in which ≥ 60% of sequenced viruses were JN.1 viruses, by country, as reported by GISAID [6]. If countries sequenced a low number of viruses on a weekly basis, sequences in the neighbouring countries were used as a proxy (Table 1). The end of the study period was at the time of last study site data extraction, up to 1 March 2024.
2.2 Statistical and VE Analyses
We pooled individual patient data from each study site and described the data according to baseline characteristics of cases and controls. In a primary analysis, we restricted to those aged 65 years and older in order to better compare to the literature and in a secondary analysis to those who were part of the age-specific target group for vaccination in each country, with ages ranging between 50 and 65 years and older (Table 1).
Using logistic regression, we estimated VE as (1 − odds ratio) * 100, with study site as fixed effects and adjusting for a priori confounders of age, sex, onset date and presence of at least one of four commonly collected chronic conditions. We used the Akaike information criterion to select the best functional form of the continuous variables age and onset date, modelled as either a restricted cubic spline with three to five knots, categorical or continuous variables. We estimate VE overall and by time since vaccination using cut-offs of 1–5, 6–11 and ≥ 12 weeks.
All analyses were performed in R software Version 4.3.2 (R Project for Statistical Computing).
Using the 10 events per parameter rule of thumb, we re-analysed the data using Firth's penalised logistic regression if the number of parameters in the model exceed the number of cases (or controls) divided by 10 [7]. If the VE estimates of standard and Firth's logistic regression differed by > 10%, we excluded the estimates, assuming bias due to overfitting.
2.3 Sensitivity Analyses
We estimated VE defining a person as vaccinated if they received vaccination 14 days or more before symptom onset, rather than 7 days, in order to better compare to the literature. We also used monthly cut-offs for time since vaccination: 7–29, 30–59, 60–89 and ≥ 90 days.
3 Results
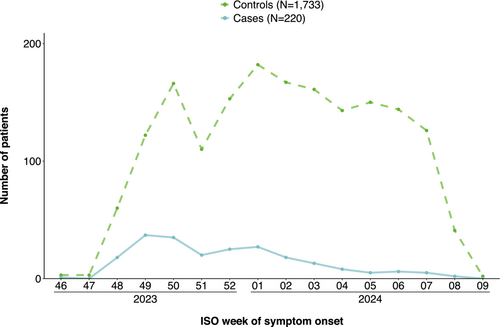
We included 1953 patients aged 65 years and older, of which 220 were cases and 1733 controls. Our study period ranged from 20 November 2023 to 1 March 2024 (Figure 1). The median age of both cases and controls was 74 years (Table 2). Among cases, 34% (75/220) were vaccinated against COVID-19 in the 2023/2024 autumn/winter campaign vs. 43% (753/1733) of controls (Table 2). Vaccine brand was known among 562 of 828 vaccination patients (68%), of these 561 (100%) were vaccinated with Comirnaty and one with Spikevax.
| Variables |
Number of COVID-19 cases (%) n = 220 |
Number of test-negative controls (%) n = 1733 |
|---|---|---|
| Median age (IQR) | 74 (69–80) | 74 (69–80) |
| Age groups (years) | ||
| 65–69 | 57/220 (26) | 471/1733 (27) |
| 70–79 | 107/220 (49) | 823/1733 (47) |
| ≥ 80 | 56/220 (25) | 439/1733 (25) |
| Female | 140/220 (64) | 1005/1733 (58) |
| Presence of four commonly collected chronic conditionsa | 159/220 (72) | 1248/1733 (72) |
| Received COVID-19 vaccination as part of the autumn/winter 2023/2024 campaign | 75/220 (34) | 753/1733 (43) |
| Number of COVID-19 doses received at time of autumn/winter 2023/2024 campaignb | ||
| 1 | 0/75 (0) | 1/747 (0) |
| 2 | 0/75 (0) | 2/747 (0) |
| 3 | 2/75 (3) | 15/747 (2) |
| 4 | 5/75 (7) | 95/747 (13) |
| 5 | 59/75 (79) | 542/747 (73) |
| 6 | 6/75 (8) | 48/747 (6) |
| 7 | 3/75 (4) | 43/747 (6) |
| 8 | 0/75 (0) | 1/747 (0) |
| Missing | 0 | 6 |
| Median number of days between last COVID-19 vaccination received in the autumn/winter 2023/2024 campaign and symptom onset (IQR) b | 54 (45–77) | 67 (47–87) |
| Influenza virus RT-PCR positive | 11/220 (5) | 329/1721 (19) |
| Missing | 0 | 12 |
- Abbreviations: IQR, interquartile range; VEBIS, Vaccine Effectiveness, Burden and Impact Studies.
- a Diabetes, heart disease, chronic lung disease, and immunodeficiency.
- b Out of all those vaccinated in the autumn/winter 2023/2024 COVID-19 vaccination campaigns.
The VE among those aged ≥ 65 years was 28% (95% CI: 2–48) overall and 48% (95% CI: 12–71), 23% (95% CI: −11–48) and 5% (95% CI: −92–56) at 1–5, 6–11 and ≥ 12 weeks after vaccination, respectively (Table 3).
| Analysis | TSV | Cases | Median TSV in days among cases (IQR) | Controls | Median TSV among controls (IQR) | VE (95% CI) |
|---|---|---|---|---|---|---|
| Adults aged ≥ 65 years | Unvaccinated | 145 | — | 980 | — | — |
| Overall | 75 | 54 (45–77) | 753 | 67 (47–87) | 28 (2–48) | |
| 1–5 weeks | 18 | 25.5 (20.25–38) | 143 | 29 (20–36) | 48 (12–71) | |
| 6–11 weeks | 46 | 55.5 (49–72.25) | 397 | 63 (53–74) | 23 (−11–48) | |
| 12–20 weeks | 11 | 96 (90.5–106) | 213 | 99 (90–110) | 5 (−92–56) | |
| Older adults part of the age-specific COVID-19 vaccination campaigna | Unvaccinated | 219 | 1574 | |||
| Overall | 92 | 55 (46–76) | 920 | 66 (47–86) | 26 (3–44) | |
| 1–5 weeks | 20 | 24 (20–38) | 178 | 29 (20–36) | 51 (20–71) | |
| 6–11 weeks | 59 | 56 (50.5–72) | 491 | 63 (53–74) | 17 (−15–40) | |
| 12–20 weeks | 13 | 96 (89–111) | 251 | 99 (90–110) | 10 (−64–54) |
- Abbreviations: CI, confidence interval; TSV, time since vaccination; VE, vaccine effectiveness; VEBIS, Vaccine Effectiveness, Burden and Impact Studies.
- a The age-specific recommendation for 2023/2024 COVID-19 vaccination was among adults aged ≥ 50, ≥ 60 or ≥ 65 years, depending on study site (see Table 1).
In our secondary analysis among older adults who were part of the countries' age-specific target group for vaccination, there was overlap with the ≥ 65-year-old age group analysis, with 71% of cases and 69% of controls aged ≥ 65 years. The VE point estimates differed by ≤ 6%, depending on analysis (Table 3).
In the sensitivity analysis with a patient considered as ‘immunised’ after 14 days post-vaccination, instead of 7 days, all VE point estimates differed by < 2% (Table S1). When using monthly cut-offs for time since vaccination, the VE point estimates were 40% (95% CI: −15–72), 28% (95% CI: −9–53), 29% (95% CI: −15–58) and 5% (95% CI: −110–61) at 7–29, 30–59, 60–89 and ≥ 90 days after vaccination (Table S2).
4 Discussion
Our results indicate that XBB.1.5 VE against JN.1 symptomatic infection among adults aged ≥ 65 years medically attended at primary care level was initially moderate at 48% 1–5 weeks after vaccination, but declined quickly to 23% within 6–11 weeks, with little effect from 12 weeks after vaccination onwards, although precision was low, particularly from 12 weeks onwards.
We are not aware of other outpatient-based studies among older adults estimating symptomatic COVID-19 VE due to JN.1. Among studies of persons aged ≥ 18 years, Caffrey et al. report a VE of 31% within 60 days of vaccination and 20% within 61–133 days [8]. Link-Gelles et al. report a VE of 49% among a study population with median delay of 80 days since vaccination [9]. Our results suggest a potentially lower VE with more time since vaccination than those studies, although comparison is difficult without similar groupings for time since vaccination in the other studies. Additionally, due to low sample size and precision among those with 12 weeks since vaccination, it is difficult to conclude from our study if the decline in VE by time since vaccination is moderate or rapid from its peak.
We previously reported within the VEBIS primary care study a VE of 45% (95% CI: 26–60) within 1–5 weeks of vaccination among adults in the older age target group in the early 2023/2024 season (September 2023 to January 2024), when the majority of viruses were likely to be XBB and its descendants [10]. When linking epidemiological and sequencing data, the XBB lineage-specific VE in the same study and age group was 63% (95% CI: −2–90) within 1–5 weeks of vaccination. Comparison to the current VE of 51% (95% CI: 20–71) against JN.1 within the same population and time since vaccination suggests no difference in VE or at most a modestly higher VE against XBB than against JN.1. Neutralisation studies indicate that JN.1 has more immune escape compared to XBB, although the drop in neutralisation titres are moderate [11]. VE in other studies suggest potentially greater differences in VE between XBB and JN.1 than we observe [9, 12, 13]. More studies on VE against symptomatic infection in the XBB and JN.1 lineage-predominant periods among older adults are needed to help understand if our results are due to small sample size and/or differing population characteristics or if XBB.1.5 vaccine may work similarly against XBB and JN.1 lineages within a very short time after vaccination.
As with all observational studies, our study is subject to residual and unmeasured confounding. Additionally, the low sample size of cases in the JN.1 period limits the precision around our estimates. VE among those vaccinated within 12–20 weeks of symptom onset was particularly imprecise, and inferences should be made with caution. Due to low numbers and in particular low vaccine coverage among the younger target group for vaccination (< 50–64 years, depending on country-specific recommendations), we were unable to provide estimates in this group within the JN.1 period. Our previous work suggests VE may be higher in this younger target group [10]. The Spanish study site dominated in our study (73%, data not shown). Although we are not aware of any differences in terms of vaccine brands or schedules used in Spain compared to other countries, we cannot exclude heterogeneity among countries in terms of previous SARS-CoV-2 infection, which may result in heterogeneity in VE. We determined the JN.1 lineage-predominant period using external surveillance data, as sample size was too small for an analysis using within-study sequencing data. Using a 60% cut-off may have resulted in contamination of our estimates with non-JN.1 viruses, although mainly in the early study period, as the proportion JN.1 viruses increased rapidly.
A strength of our study is to be able to provide COVID-19 VE results in the outpatient setting, where there are limited studies. Our long-standing platform can provide VE results across seasons, which is useful for comparison. Ongoing monitoring of VE is key in the context of the WHO recommendation of JN.1 vaccines in the autumn/winter 2024/2025 vaccination campaigns [14] and the diversification of JN.1 viruses into sublineages.
In conclusion, our results suggest XBB.1.5 vaccines provide short and moderate protection against symptomatic infection with JN.1 lineages among older patients, similar to the VE against predominantly XBB lineages earlier in the season. Careful timing of COVID-19 vaccination campaigns is needed to ensure optimal protection of vulnerable populations.
Author Contributions
Lore Merdrignac: conceptualisation, formal analysis, visualisation, methodology, writing – review and editing. Charlotte Laniece Delaunay: conceptualisation, formal analysis, methodology. Nuno Verdasca: conceptualisation, data curation, formal analysis, writing – review and editing. Lorena Vega-Piris: conceptualisation, investigation, methodology, writing – review and editing. Joan O'Donnell: conceptualisation, investigation, methodology, writing – review and editing. Noémie Sève: conceptualisation, investigation, methodology, writing – review and editing. Camino Trobajo-Sanmartín: conceptualisation, investigation, methodology, writing – review and editing. Silke Buda: conceptualisation, investigation, methodology, writing – review and editing. Mariëtte Hooiveld: conceptualisation, investigation, methodology, writing – review and editing. Ana Paula Rodrigues: conceptualisation, investigation, methodology, writing – review and editing. Gergő Túri: conceptualisation, investigation, methodology, writing – review and editing. Neus Latorre-Margalef: conceptualisation, investigation, methodology, writing – review and editing. Ivan Mlinarić: conceptualisation, investigation, methodology, writing – review and editing. Mihaela Lazar: conceptualisation, investigation, methodology, writing – review and editing. Marine Maurel: conceptualisation, methodology, visualisation, data curation, writing – review and editing. Daniel Castrillejo: conceptualisation, investigation, methodology, writing – review and editing. Charlene Bennett: investigation, methodology, writing – review and editing. Marie-Anne Rameix-Welti: conceptualisation, investigation, methodology, writing – review and editing. Iván Martínez-Baz: conceptualisation, investigation, methodology, writing – review and editing. Ralf Dürrwald: conceptualisation, investigation, methodology, writing – review and editing. Adam Meijer: conceptualisation, investigation, methodology, writing – review and editing. Aryse Melo: conceptualisation, investigation, methodology, writing – review and editing. Beatrix Oroszi: conceptualisation, investigation, methodology, writing – review and editing. Tove Samuelsson Hagey: conceptualisation, investigation, methodology, writing – review and editing. Sanja Kurečić Filipović: conceptualisation, investigation, methodology, writing – review and editing. Frederika Dijkstra: conceptualisation, investigation, methodology, writing – review and editing. Veronica Gomez: conceptualisation; investigation, methodology, writing – review and editing. Sabrina Bacci: conceptualisation, project administration, funding acquisition, writing – review and editing. Marlena Kaczmarek: conceptualisation, project administration, methodology, funding acquisition, writing – review and editing. Esther Kissling: conceptualisation; writing – original draft, methodology, project administration, writing – review and editing. The VEBIS Primary Care Vaccine Effectiveness Group: investigation, data curation.
Acknowledgements
All study teams are very grateful to all patients, general practitioners, paediatricians, laboratory teams and regional epidemiologists who have contributed to the studies. We acknowledge the huge contribution by the VEBIS Primary Care Vaccine Effectiveness Group: Croatia: Ivana Ferenčak, Bernard Kaić, Maja Ilić and Vesna Višekruna Vučina, Croatian Institute of Public Health, Zagreb; Katica Čusek Adamić, Institute of Public Health, Varaždin County, Varaždin; Mirjana Lana Kosanović Ličina, ‘Dr Andrija Štampar’ Teaching Institute of Public Health, Zagreb; Danijela Lakošeljac, Teaching Institute of Public Health, Primorje-Gorski kotar County, Rijeka; Ivana Mihin Huskić, Teaching Institute of Public Health, Osijek-Baranja County, Osijek; Diana Nonković, Teaching Institute for Public Health, Split-Dalmatia County, Split. France: Thierry Blanchon, Caroline Guerrisi, Titouan Launay and Aubane Renard, Sorbonne Université, INSERM, Institut Pierre Louis d'épidémiologie et de Santé Publique (IPLESP UMRS 1136); Marie Chazelle, Alessandra Falchi and Shirley Masse, Laboratoire de Virologie, Université de Corse-Inserm; Vincent Enouf and Sylvie van der Werf, Centre National de Référence Virus des Infections Respiratoire (CNR VIR), Institut Pasteur. Epiconcept: Alain Moren, Anthony Nardone and Angela MC Rose, Epiconcept, Paris. Germany: Luise Goerlitz, Annika Erdwiens, Ute Preuss and Kristin Tolksdorf, Department for Infectious Disease Epidemiology, Respiratory Infections Unit, Robert Koch Institute; Barbara Biere, Djin-Ye Oh, Janine Reiche and Marianne Wedde, National Reference Centre for Influenza, Robert Koch Institute. Hungary: Judit Krisztina Horváth, Krisztina Mucsányiné Juhász, Katalin Krisztalovics and Csaba Luca, National Laboratory for Health Security, Epidemiology and Surveillance Centre, Semmelweis University, Budapest; Katalin Kristóf, Institute of Laboratory Medicine, Semmelweis University, Budapest. The Hungarian study team works as part of the National Laboratory for Health Security Hungary (RRF-2.3.1-21-2022-00006) supported by the National Research, Development and Innovation Office (NKFIH). Ireland: Lisa Domegan and Adele McKenna, HSE Health Protection Surveillance Centre, Dublin; Jeff Connell, National Virus Reference Laboratory, Dublin; Michael Joyce, Olga Levis and the Irish sentinel GP network, Irish College of General Practitioners, Dublin; The Netherlands: Lynn Aarts, Mariam Bagheri, Danytza Berry, Sanne Bos, Sharon van den Brink, Dirk Eggink, Rianne van Gageldonk-Lafeber, Gabriel Goderski, Liz Jenniskens, Femke Jongenotter, Marit de Lange, Tara Sprong, Anne Teirlinck and molecular pool technicians, National Institute for Public Health and the Environment (RIVM), Bilthoven; Nivel Primary Care Database–Sentinel Practices team, Ruud van den Broek, Safira Wortel, Ruben van der Burgh, Cathrien Kager, Mayra Klinkhamer, Bart Knottnerus, Marloes Riethof, Nienke Veldhuijzen, participating general practices and their patients, Nivel, Utrecht. Portugal: Vitor Borges, Licínia Gomes, Camila Henriques, Daniela Dias, Débora Pereira, Pais de Lacerda, Susana Maia Silva, Paula Pinto and Cristina Bárbara, Epidemiology Department, Instituto Nacional de Saúde Doutor Ricardo Jorge, Lisbon. Romania: Maria Elena Mihai, Alina Ivanciuc, Catalina Pascu, Iulia Bistriceanu, Sorin Dinu, Mihaela Oprea, Olivia Timnea and Adrian Jidovu, ‘Cantacuzino’ National Military Medical Institute for Research and Development, Bucharest; Rodica Popescu, National Institute of Public Health, Bucharest. Spain: SiVIRA surveillance and vaccine effectiveness group. Spain: Navarre: Itziar Casado, Aitziber Echeverria, Camino Trobajo-Sanmartín, Manuel García Cenoz and Guillermo Ezpeleta, Instituto de Salud Pública de Navarra – IdiSNA, CIBERESP, Pamplona; Ana Navascués, Miguel Fernández-Huerta and Carmen Ezpeleta, Hospital Universitario de Navarra – IdiSNA, Pamplona. Sweden: Annasara Carnahan (epidemiology team), Emmi Andersson, Eva Hansson-Pihlainen, Elin Arvesen, Nora Nid, Anna-Lena Hansen and Lena Dillner (influenza virus surveillance team) and the NGS platform, Public Health Agency of Sweden, Stockholm, Sweden.
Participating laboratories submitted their sequences to GISAID (www.gisaid.org) for easy sharing with the central laboratory in Lisbon.
Ethics Statement
Official ethical approval was not required in Spain or the Netherlands, as this study was classified as routine care/surveillance. Other study sites received local ethical approval from a national or regional review board: Croatia: (class 030-02/23-01/1); France: 471393; Germany: EA2/126/11; Hungary: IV/1885-5/2021/EKU; Ireland: ICGP2019.4.04; Portugal: no registration number given; Romania: CE199/2022; Sweden: 2006/1040-31/2 revised Drn 2021-02791.
Consent
Patient consent was not required in Ireland or Spain. Verbal consent was required for all other study sites, with the exception of Germany and Hungary, where written consent was required.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.