Therapeutic monoclonal antibodies in allergy: Targeting IgE, cytokine, and alarmin pathways
This article is part of a series of reviews covering Effector Functions of Antibodies in Health and Disease appearing in Volume 328 of Immunological Reviews.
Summary
The etiology of allergy is closely linked to type 2 inflammatory responses ultimately leading to the production of allergen-specific immunoglobulin E (IgE), a key driver of many allergic conditions. At a high level, initial allergen exposure disrupts epithelial integrity, triggering local inflammation via alarmins including IL-25, IL-33, and TSLP, which activate type 2 innate lymphoid cells as well as other immune cells to secrete type 2 cytokines IL-4, IL-5 and IL-13, promoting Th2 cell development and eosinophil recruitment. Th2 cell dependent B cell activation promotes the production of allergen-specific IgE, which stably binds to basophils and mast cells. Rapid degranulation of these cells upon allergen re-exposure leads to allergic symptoms. Recent advances in our understanding of the molecular and cellular mechanisms underlying allergic pathophysiology have significantly shaped the development of therapeutic intervention strategies. In this review, we highlight key therapeutic targets within the allergic cascade with a particular focus on past, current and future treatment approaches using monoclonal antibodies. Specific targeting of alarmins, type 2 cytokines and IgE has shown varying degrees of clinical benefit in different allergic indications including asthma, chronic spontaneous urticaria, atopic dermatitis, chronic rhinosinusitis with nasal polyps, food allergies and eosinophilic esophagitis. While multiple therapeutic antibodies have been approved for clinical use, scientists are still working on ways to improve on current treatment approaches. Here, we provide context to understand therapeutic targeting strategies and their limitations, discussing both knowledge gaps and promising future directions to enhancing clinical efficacy in allergic disease management.
1 INTRODUCTION
Since the discovery of immunoglobulin E (IgE) as the antibody isotype responsible for immediate hypersensitivity reactions1-3 our understanding of the network of cells and cytokines that drive allergic pathology has greatly expanded.4 These insights have been derived from basic research and confirmed by clinical programs targeting core pathways in the allergic cascade. This review will introduce key therapeutic targets in allergic disorders and the insights gained from these interventions, with a focus on monoclonal antibodies.
Defining the etiology of allergy has remained a challenge in part because of the diverse stimuli capable of driving type 2 inflammatory responses. Allergic disorders have overlapping inflammatory patterns with parasitic diseases and have thus been framed as the dysregulation of immune responses evolved to expel parasites or neutralize noxious agents at the host-environment interface like the airway, gut and skin.5 Within this framework the evolution of allergic pathology can be broken down into several stages. First, after the loss of epithelial integrity, exposure to allergens initiates adaptive immune responses6, 7 (Figure 1). Insult to epithelial barrier tissues triggers rapid local inflammatory responses driven by the release of the alarmins IL-25, IL-33, and TSLP from the epithelium and proximal immune cells. These alarmins work in concert to induce dendritic cells expression of OX40-ligand and type 2 cytokines,8, 9 activate ILC2s to secrete IL-13 and IL-5, and ultimately promote T helper 2 cells (Th2) development and eosinophil recruitment.10 Antigen activated mature Th2 cells then serve as a key source of the canonical type 2 cytokines, including IL-4, which is essential for promoting B cell class switching and the production of allergen-specific IgE. In the periphery, allergen-specific IgE is bound by basophils and mast cells where it can trigger rapid inflammatory responses, including anaphylaxis, upon allergen re-exposure. Ultimately the aforementioned cascade of events must be considered in the context of the chronic nature of allergy, which is marked by numerous allergen exposures and prolonged inflammation. Therefore, in many cases, the allergic mediators listed above act to perpetuate and reinforce pathology at multiple levels via multiple cell types as discussed below.
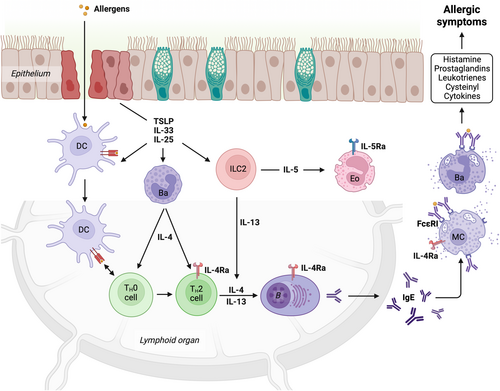
1.1 Alarmins
Epithelial cells, fibroblasts, and immune cells positioned at barrier surfaces are able to secrete the alarmins TSLP, IL-25, and IL-33 in response to danger signals or cellular damage.11 TSLP release can be triggered by mechanical stress, proteases, and a host of pathogen associated molecular patterns (PAMPs).12 IL-25 is both constitutively released by keratinocytes and can be induced by pathogen proteases and PAMPs in epithelial tissues.13 In contrast the alarmin IL-33 is constitutively expressed in the nucleus of epithelial cells and other cells and is primarily released following cellular damage. IL-33 released from cells can be further processed by host or exogenous proteases into highly active isoforms.14, 15 Once released these alarmins are not required for the clonal expansion of T cells in response to antigen exposure, but they are critical for subsequent Th2 polarization,16 and the blockade of all three alarmin pathways greatly attenuates local type 2 inflammation and tissue remodeling in models of parasitic infection and allergy.17
Similar to the diverse molecular mechanisms that initiate alarmin release, the alarmins themselves exhibit significant structural variability. TSLP is a member of the IL-2 family of cytokines and signals through a heterodimer of TSLPR and IL-7Ra,18 IL-33 is a member of the IL-1 cytokine family and signals via binding to ST2 (IL-1RL1) and subsequent recruitment of the IL-1 receptor accessory protein (IL-1RAcP),19 and IL-25 is a member of the IL-17 family and binds with two IL-17RB subunits to form a complex which subsequently engages IL-17RA to drive downstream signaling.20
Despite their molecular diversity, alarmins appear to have evolved partially redundant pathways to induce inflammation at barrier surfaces in response to environmental stimuli, and to trigger similar downstream events, such as the production of IL-13 from ILC2s.17 This redundancy could be one reason that the most profound effects on type 2 inflammation occur when multiple alarmin pathways are suppressed. However, individual targeting of alarmin pathways has been reported to be sufficient in reducing type 2 inflammation in many models of allergic disease.21
1.2 Type 2 cytokines
The canonical type 2 cytokines IL-4, IL-5 and IL-13 are produced by multiple innate and adaptive immune cells. IL-4 is primarily secreted by basophils and Th2 cells, while IL-13 is predominantly expressed by Th2 cells and ILC2s and can be quickly induced by alarmin mediated activation of ILC2s.22-24 IL-4 signals through type I receptor complexes composed of the IL-4 receptor (IL-4Ra) and common gamma chain (ɣc) or type II receptor complexes composed of heterodimers of IL-4Ra and IL-13 receptor 1 (IL-13Ra1).25 In contrast IL-13 can signal via type II receptors mentioned above as well as the IL-13Ra2 receptor, which can negatively regulate IL-13 function and has also been implicated in IL-13 mediated upregulation of TGF-β1 expression in fibrosis.26 Although these cytokines share some common receptor components, their biologic functions are diverse. For example, IL-4 depletion greatly impairs Th2 development and IgE production owing in part to distribution of type I receptors on lymphocytes, while IL-13 depletion does not impact IgE production but does limit tissue fibrosis and goblet cell hyperplasia.25 Importantly class switch recombination to IgE requires IL-4. It has recently been suggested that allergen-specific IgE reservoirs are sustained by memory IgG1 memory B-cells that continually class switch to express IgE.27 IL-5, produced by activated ILC2s, Th2 cells, and other cells, recruits and promotes the survival and proliferation of eosinophils in tissues. IL-5 signals via complexes of IL-5Ra and the common beta chain (βc),28 and targeting soluble cytokine and receptor have both been shown to effectively block IL-5 signaling.29
1.3 Immunoglobulin E (IgE)
IgE is best known for its key role in immediate hypersensitivity reactions upon allergen exposure and is the primary driver of allergen induced anaphylaxis. However, IgE plays an underappreciated role in facilitating antigen acquisition and presentation (IgE-FAP), amplifying adaptive immune responses, and triggering the release of multiple cytokines including the canonical type 2 cytokines and alarmins described above.
The biology of IgE is best understood in the context of its high-affinity receptor FcεRI, expressed as a stable tetramer (αβɣɣ) on mast cells and basophils,30 and as a trimer (αɣɣ) on dendritic cell (DC) subsets and monocytes, where it is steadily endocytosed and recycled.31 The FcεRI alpha chain (FcεRIα) binds IgE in a similar orientation as other Fc receptors, however the kinetics of IgE and FcεRI interactions are unique. FcεRI is the highest affinity immunoglobulin receptor,32-34 and the dissociation of IgE from this receptor is at least 10-fold slower than IgG1 from FcγRI35 and multiple orders of magnitude slower than other immunoglobulin Fc interactions. This allows IgE:FcεRI complexes to imbue mast cells in tissue with memory for antigens targeted by IgE. In human transfusion studies in patients with hypogammaglobulinemia, the half-life of exogenously provided IgE is only ~2 days in the absence of endogenous IgE, yet patient reactivity to allergens recognized by exogenous IgE persists for 50 days.36 Likewise, in IgE deficient mice, injected IgE is undetectable after 6 days in the blood, yet systemic allergen reactivity is detectable for well over a month.37
The remarkable stability of IgE:FcεRI complexes is compounded by the extreme sensitivity of basophils and mast cells expressing FcεRI. Paradoxically, partial loss of surface IgE in these cells can even increase effector cell sensitivity to stimulation via the remaining IgE receptor complexes,38, 39 and low levels of allergen-reactive IgE are sufficient to drive cellular activation.40, 41 Recent studies also suggest that allergen-reactive IgE is produced at high concentrations locally,38, 39, 42-44 exposing long-lived tissue resident mast cells to an abundance of allergen-specific IgE. This combination of high affinity binding, high cellular sensitivity, and local production of IgE in tissues works in concert to make rapid and complete inhibition of IgE challenging. Even in the presence of highly potent IgE inhibitors, which effectively eliminate free IgE from the serum, reactivity to allergens in skin prick testing persists for months.45 The durable sensitization of FcεRI bearing mast cell and basophils, and their subsequent activation, sustains an allergy permissive milieu with the release of cytokines and mediators that recruit, remodel, and sustain adaptive responses to allergens.
Beyond the sensitization of mast cells and basophils, trimeric FcεRI on plasmacytoid DCs (pDCs), has been shown to enhance antigen presentation and pDC activation.46, 47 Likewise, removal of IgE from pDC populations has been shown to enhance the generation of regulatory T cells in human peripheral blood mononuclear cell (PBMC) cultures.48 Prior studies have demonstrated that antigen induced activation and subsequent IL-4 release from mast cells can promote regulatory T cell (Treg) dysfunction, and IgE knockdown or inhibition can rescue Treg activity.49, 50 These studies suggest IgE signaling in pDCs can directly skew T cell responses. Therefore, engagement and activation of IgE receptor complexes on DC subsets may potentiate IgE focused antigen-specific responses, while blockade of this pathway may facilitate reestablishment of antigen tolerance. This agonistic effect of IgE on T-cell responses does not appear to be simply mediated by recruitment of antigen to DCs via IgE, as soluble monomeric IgE-antigen fusions that do not crosslink receptors induce systemic tolerance.51 Instead, these studies demonstrate that IgE not only potently traffics antigen to pDC subsets via high affinity binding to FcεRI, but that IgE crosslinking and signaling can work in concert to enhance, suppress, or skew T-cell responses via FcεRI on DCs and mast cells. This multifaceted nature of FcεRI allows the receptor to perpetuate the production of more antigen reactive T cells, and mediate terminal effector functions that drive anaphylaxis and allergy.
While the aforementioned studies highlight the emerging role of FcεRI in potentiating and regulating adaptive responses, the role of the low-affinity IgE receptor CD23 in antigen presentation and amplification of adaptive immune responses has also been extensively studied. Starting at sites of antigen exposure in the lung and gut, CD23 is expressed at the epithelium and facilitates bidirectional transcytosis of IgE and IgE:antigen complexes.52-55 In the tissue these immune complexes readily bind CD23 positive B cells and serve as extremely potent antigen sources. This effect, termed IgE-FAP, has been shown to amplify antibody responses by greater than 100-fold and dramatically enhance antigen specific T cell proliferation.56, 57 IgE-FAP is driven by IgE transport on CD23+ B cells to follicles in spleen, where IgE:antigen complexes can be captured by CD11c+ dendritic cells58, 59 and internalized, degraded, and presented to T cells.60 This diverse set of potential outcomes for IgE:antigen complexes is enabled by two distinct CD23 isoforms (CD23a and b) that act in concert with IgE and immune cells to surveille the lumen of the airway and gut and collect antigens for efficient antigen presentation. This underappreciated role of IgE serves to initiate and potentiate allergic disease in tissues and demonstrates that an effective IgE blockade should be able to inhibit IgE interaction with multiple receptor types across multiple tissues and physiologic spaces.
1.4 Other important regulatory receptors on allergic effector cells
Several other receptors including stem cell factor receptor (KIT) and sialic-acid-binding immunoglobulin-like lectins (Siglecs) are expressed on allergic effector cells. KIT is the principal receptor for stem cell factor (SCF) and plays central roles in multiple biologic processes including hematopoiesis as well as melanocyte, germ and gut cell development.61 It is a type III receptor tyrosine kinase which consists of five extracellular Ig-like domains and is activated via by soluble or cell bound dimeric SCF isoforms that promote receptor dimerization.62 Blockade of SCF binding to KIT or targeting of the fourth and fifth Ig-like dimerization domains of KIT, prevent KIT dimerization and suppress KIT signaling. KIT is expressed at very high levels on the surface of mast cells, is required for mast cell development and survival, and synergizes with other mast cell activating receptors to enhance mast cell responses.63
Siglecs are a diverse family of sialic-acid-binding lectins that are broadly expressed across many hematopoietic lineages and other cells. Siglecs primarily serve to modulate signaling via intracellular immunoreceptor tyrosine-based inhibitory (ITIM) motifs following ligation, crosslinking, or receptor co-localization,64 yet the biology of Siglec receptors and their glycan ligands is complex and capable of inducing a wide range of downstream events depending on the cellular context and distribution of their glycan ligands.65 Of the Siglec family, Siglec-8 and Siglec-6 have emerged as therapeutic targets in allergy as each are expressed mast cells and basophils among other cells.66 Ligation of Siglec-8 or 6 is primarily thought to stimulate ITIM mediated recruitment of phosphatases and subsequent suppression of multiple allergic pathways including IgE mediated FcεRI signalling,65 however antibody ligation of Siglec-8 has also been shown to induce apoptosis in eosinophils and promotes antibody-dependent cellular cytotoxicity/phagocytosis (ADCC/ADCP) of Siglec positive cells.67
2 THERAPEUTIC ANTIBODIES IN ALLERGY
Over the last two decades, the therapeutic use of monoclonal antibodies has gained significant momentum across various diseases, including allergic disorders. This advancement is largely due to the continuously increasing understanding of molecular and cellular mechanisms involved the allergic cascade, which has enabled the identification of specific targets for therapeutic intervention. While several monoclonal antibodies have been developed, only a few have been approved for clinical use in allergy management to date (Figure 2).
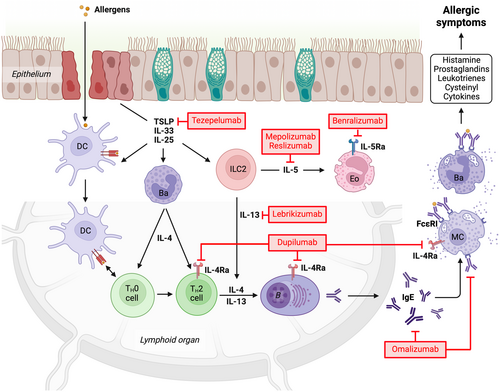
2.1 Classical anti-IgE antibodies
2.1.1 Omalizumab
The original conceptual framework for developing anti-IgEs as a treatment for allergies was based on defining three desired functional characteristics: (i) high-affinity IgE binding able to compete with FcεRI interactions; (ii) lack of binding to IgE:FcεRI complexes on mast cells or basophils to prevent receptor crosslinking and activation and (iii) lack of binding to IgE:CD23 complexes.68 Omalizumab was the first anti-IgE antibody approved for clinical use, although parallel clinical studies were contemporaneously carried out with another anti-IgE (CGP51901/CGP56901/TNX-901/talizumab) that did not advance into phase III clinical trials at that time.68 Omalizumab was developed from a murine anti-human IgE monoclonal antibody (MAE11) and was selected based on its binding to free IgE and lack of binding to IgE:FcεRI complexes on mast cells.69 Humanization of MAE11 was carried out by grafting MAE11 CDRs onto consensus human framework sequences with 5 human-murine framework mutations required to recapitulate MAE11 affinity.69 The resulting humanized antibody (rhuMAb-E25) was advanced into clinical trials for the treatment of allergic rhinitis and asthma70-73 and approved for therapeutic use in 2003 for moderate to severe asthma under the tradename Xolair. Xolair has since been approved for the treatment of chronic spontaneous urticaria (CSU) in 2014, allergic asthma in children in 2016, chronic rhinosinusitis with nasal polyps (CRSwNP) in adults in 2020, and food allergies in children and adults in 2024.
Omalizumab treatment in patients results in the reduction of free IgE levels and blocks IgE binding to mast cells and basophils, as expected from its competitive binding with FcεRI. Omalizumab forms immune complexes with IgE, with some preference to assemble into 3:3 hexamers rather than larger immune aggregates.68 These complexes are not cleared from serum and their formation results in unexpected increases in the total IgE in serum, which accumulates at up to six to ten times basal IgE levels. The accumulation of “neutralized” IgE in immune complexes that is still able to bind allergen may contribute to the protective therapeutic benefit of omalizumab.68 Omalizumab treatment also results in the downregulation of FcεRI levels on circulating basophils and dendritic cells,74-76 reducing their ability to be sensitized by IgE and activated by allergens. The downregulation of FcεRI in basophils likely occurs through two contributing mechanisms: (i) the intrinsic, more rapid degradative turnover of FcεRI in cells in the absence of IgE binding and (ii) the turnover and production of new basophils, also occurring in the absence of free IgE and therefore lacking stabilized FcεRI levels. While omalizumab treatment results in relatively rapid decline in free IgE levels, clinical benefit emerges over much longer periods, indicating that the absolute reduction in free IgE is a rather poor indicator of therapeutic efficacy.
Omalizumab engages an IgE epitope in the constant epsilon (Cε) 3 domains, adjacent to the binding site for FcεRIα,77, 78 with only 2 IgE amino acids in common between the omalizumab and FcεRI interaction sites. However, omalizumab binding to IgE sterically blocks FcεRI binding. In addition, one of the omalizumab epitopes is also buried by Cε2 domains in their folded-back, bent conformation. Binding of omalizumab interferes with this bent IgE conformation, which could also contribute to inhibiting FcεRI binding.78 Omalizumab also inhibits the binding of CD23, through significant overlap of their respective binding sites on IgE and through substantial steric clashes of the two IgE ligands.77 Although omalizumab was originally selected for therapeutic development because it competes with FcεRI but does not engage IgE:FcεRI complexes on effector cells, recent studies have demonstrated that omalizumab can form transient complexes and actively dissociate IgE from the receptor.79-81 Initially, this observation was only described for high omalizumab concentrations, which raised the question of how physiologically relevant the additional mode-of-action might be in vivo.79 However, additional experimentation has revealed that the mechanism was not only concentration but also time dependent.80 In other words, over prolonged exposure periods removal of IgE from FcεRI could even be observed at physiological omalizumab concentrations.
2.1.2 Ligelizumab
Ligelizumab is a more recent anti-IgE therapeutic candidate developed by Novartis that has its roots in earlier clinical studies. Ligelizumab is a high-affinity variant (KD ~ 17 pM) based on the CGP51901/CGP56901/TNX-901/talizumab series of anti-IgE antibodies developed in the 1990s. CGP51901 was a murine/human chimeric IgG1 antibody that underwent phase I and phase II clinical trials for allergic rhinitis and allergic asthma.68, 82-84 CPG56901 (TNX-901/talizumab) was a humanized variant of CGP5190185 that underwent additional Phase II clinical trials for the treatment of peanut allergy,86, 87 providing the first promising indication that anti-IgE therapeutics could be effective in treating food allergies. These studies showed that peanut allergic patients treated with TNX-901 exhibited an increase resistance to peanut exposure from roughly half a peanut to nine peanuts—a level of resistance that could protect against most accidental exposures. Although TNX-901/talizumab was not developed further, ligelizumab builds on these prior promising studies, with the expectation that its high affinity for IgE would lead to increased clinical benefit over both TNX-901 and omalizumab. This expectation follows naturally from the concept that the sequestration of free IgE by anti-IgEs would represent a key correlate of therapeutic efficacy. Surprisingly, this expectation for ligelizumab has not been borne out by multiple phase III clinical studies (in CSU and asthma),88, 89 raising important mechanistic questions about the pharmacologic basis of anti-IgE therapy.
Initially, ligelizumab showed significant promise in early phase II clinical trials for allergic asthma and CSU, consistent with its potential to outperform omalizumab due to its higher IgE binding affinity.45, 90 Compared to omalizumab, it led to greater suppression of free IgE as well as longer lasting suppression of IgE levels in patients. Surprisingly, these results and promising clinical observations have been followed by disappointing phase II and III clinical trials of ligelizumab in allergic asthma,89 CSU,88 chronic inducible urticaria (CIndU; NCT05024058) and food allergy (NCT04984876), although a long-term extension study is ongoing (NCT05678959) and a food allergy trial with higher dosing is planned for late 2024. While ligelizumab is more effective than omalizumab at blocking IgE-binding to its high-affinity receptor and more effectively suppresses free IgE in allergic patients, it is striking that this increased potency has not translated into improved therapeutic efficacy. These findings clearly challenge the original conceptual framework for anti-IgE therapeutic development.
Key mechanistic differences between ligelizumab and omalizumab have emerged through structural and functional studies of these antibodies, which have contributed to understanding the clinical observations.77, 78, 80, 89 Ligelizumab and omalizumab engage IgE through overlapping, but distinct, epitopes on IgE. While the omalizumab epitope lies within a single Cε3 domain,77, 78 the ligelizumab epitope is shifted such that ligelizumab binds IgE across two Cε3 domains.80 This difference in positioning on IgE of ligelizumab relative to omalizumab results in key differential structural and functional attributes. While ligelizumab binding more substantially interferes with FcεRI binding through steric blocking, IgE-bound omalizumab is offset to one side of the receptor complex in a position that has a substantially smaller volume of overlap with FcεRI binding. One functional consequence of this positioning difference is that ligelizumab exhibits no observable activity in transiently engaging IgE:FcεRI complexes and activating their dissociation.80 A second functional outcome of the ligelizumab binding epitope and Fab binding pose, is that ligelizumab is a significantly weaker inhibitor of IgE:CD23 interactions as compared to omalizumab.80, 89 The clinical studies of ligelizumab clearly indicate that increasing anti-IgE potency in blocking IgE:FcεRI binding does not provide a simple correlate for therapeutic efficacy. These additional functional differences between ligelizumab and omalizumab, due to the way in which they each engage IgE, could potentially account for the disappointing clinical impact of ligelizumab. On the other hand, while studies support a role for IgE:CD23 complexes in allergic asthma55, 91-94 and food allergies,52, 95-99 it is unclear whether this would be true for CSU and could explain ligelizumab's generally disappointing clinical outcomes. Further studies are needed to address these questions.
2.1.3 HAE1
HAE1 (PRO98498) was developed by Genentech as an affinity-matured, potential second generation anti-IgE therapeutic. HAE1 was engineered using a phage display approach yielding a variant with 9 amino acid differences that has an ~25-fold higher binding affinity compared to omalizumab (Fab KD ~0.6 nM vs. ~15 nM), as a result primarily of a slowed dissociation rate.100, 101 HAE1 showed more potent inhibition of IgE binding to FcεRI and inhibition of huFcεRI-RBL cell activation than omalizumab in vitro.100 Preclinical efficacy was further assessed by observing the suppression of free IgE in cynomolgus monkeys, and free IgE was used as a biomarker for pharmacodynamic behavior of HAE1 and its potential for therapeutic efficacy. HAE1 showed a greater ability to suppress free IgE levels, as compared to omalizumab, and resulted in the accumulation of higher levels of IgE:drug complexes.101 Modeling of the ability of HAE1 to suppress free IgE was used to guide the clinical development program, based on the expectation that the higher HAE1 affinity would allow for lower drug:IgE dosing ratios to achieve the desired level of free IgE suppression. However, we now have, in hindsight, substantial evidence from ligelizumab clinical trials that indicate that free IgE suppression is not the sole contributor to anti-IgE clinical efficacy. Phase I and Phase II clinical studies with HAE1 incorporated designs based on this foundation, but development of HAE1 was halted after two people developed hypersensitivity reactions during the Phase II studies.
At the time that the HAE1 development program was underway, the idea that omalizumab, or its derivatives, could potentially interact with and dissociate IgE:FcεRI complexes was antithetical to the founding concepts of the original anti-IgE drug development programs. Thus, it was not until over a decade later in studies that were specifically designed to re-engineer omalizumab disruptive activities that the impact of the HAE1 program on IgE:FcεRI complex dissociation was assessed.102 In these studies, omalizumab disruptive activity was re-engineered using a yeast-display approach, revealing a direct link between omalizumab binding affinity and disruptive potency (see below). At this time, it was discovered that HAE1 antibody could more potently dissociate IgE:FcεRI complexes and more rapidly desensitize human basophils than omalizumab. These observations indicate that HAE1 not only achieved increased potency in blocking IgE:FcεRI interactions, similar to ligelizumab, but it had the potential to more rapidly desensitize allergic effector cells and maintain potent CD23 blockade. It remains to be established whether these improved functional attributes, and in particular the more rapid desensitization of allergic effector cells, could have yielded a more effective and safe anti-IgE therapeutic.
2.2 Alternative approaches to targeting the IgE pathway
While targeting free serum IgE in allergic diseases has become a clinically validated strategy, it requires frequent dosing every 2–4 weeks depending on body weight and IgE levels of the patient. Furthermore, dosing limitations restrict the use of omalizumab in adults (>12 years) to patients with IgE levels <700 kU/L. These obvious constraints of the classical anti-IgE approach to blocking free serum IgE prompted researchers to investigate other possibilities, including direct targeting of the cellular source of IgE production, namely IgE-expressing B cells.103 Isotype switching to IgE in B cells leads to a transient expression of membrane IgE (mIgE).104, 105 Several strategies to target such IgE producing B cells by engaging mIgE or other specific IgE+ B-cell markers with monoclonal antibodies have been developed in various research programs. Even though none of these approaches have been approved for clinical use yet, we will highlight several interesting strategies here.
2.2.1 Quilizumab (h47H4)
Compared to soluble IgE, mIgE contains an additional 52 amino acid long domain, known as M1′ (or M1 prime, me.1, or CemX), located between the constant heavy chain 4 (CH4) domain and the C-terminal membrane-anchor peptide of the epsilon chain on human B cells.106 The monoclonal mouse anti-human IgE antibody 47H4, which recognizes the M1′ domain of mIgE, has been developed at Genentech. In an experimental allergy mouse model using transgenic mice carrying the human M1′ domain in the mouse IgE locus, 47H4 demonstrated a reduction in serum IgE levels and number of IgE-producing plasma cells in vivo.107, 108 The authors concluded that 47H4 most likely induces apoptosis in mIgE+ B cells. Following these studies, an afucosylated humanized version of the antibody, known as quilizumab, was generated.109 The removal of fucose from the IgG antibody increases binding affinity to FcγRIIIa, enhancing ADCC by NK cells and increasing quilizumab's efficacy to drive mIgE+ B cells into programmed cell death. A phase I clinical trial for allergic rhinitis (NCT01160861) and a phase II clinical trial for allergen-induced asthma (NCT01196039) revealed encouraging results, with quilizumab treatment reducing total and allergen-specific IgE levels by 35% and 40%, respectively.110 The treatment also improved clinical signs of allergen-induced asthma. Surprisingly, quilizumab treatment only partially reduced serum IgE levels and did not significantly improve clinical outcomes in subsequent phase II clinical trials with allergic asthma patients (NCT01582503)111 or CSU patients (NCT01987947).111 The unexpected results of these trials may be attributed to the infrequent and transient presence of short-lived mIgE+ B cells, along with increasing evidence that the memory of the IgE compartment originates in a CD23-positive IgG memory B cell pool (i.e., MBC2), which undergoes sequential and potentially repetitive isotype switching to IgE.112, 113 To our knowledge the quilizumab program is no longer being actively pursued.
2.2.2 Xmab7195
The anti-IgE antibody XmAb7195 is a humanized, affinity-enhanced variant of MaE11,114 the murine parental antibody of omalizumab, with an altered Fc-region to improve binding to the inhibitory receptor FcγRIIb implemented with the goal of accelerating clearance of anti-IgE:IgE immune complexes from the circulation. Initially developed at Xencor, XmAb7195 aims to neutralize free serum IgE and reduce IgE production by promoting the aggregation of FcγRIIb and mIgE on the B cell surface.115, 116 In a severe combined immunodeficiency (SCID) mouse model engrafted with human PBMCs, XmAb7195 decreased free IgE levels and prevented the formation of IgE-secreting plasma cells. The effectiveness of this dual-targeting strategy was also assessed in a phase Ia clinical trial with 72 healthy volunteers and individuals with high IgE levels (NCT02148744). Several subjects with high IgE levels (300–3000 kU/L) reached undetectable free IgE levels (<9.59 ng/mL = <4 kU/L) following single dose intravenous XmAb7195 infusion. Additionally, soluble free and total IgE, basophil surface IgE and FcεRI levels showed pronounced reductions. As for the most important adverse events, transient dose-dependent thrombocytopenia and one drug-related severe bronchospasm with trial discontinuation was reported. A subsequent phase Ib clinical trial (NCT02881853) for subcutaneous administration was conducted and completed in 2017. However, no results have been communicated to date. In February 2020 Aimmune Therapeutics acquired an exclusive license from Xencor to develop and commercialize the antibody (renamed to AIMab7195). While they planned to develop AIMab7195 as an adjunct treatment to oral immunotherapy, to explore treatment outcomes in patients with food allergies, Aimmune Therapeutics was subsequently acquired by Nestlé in October 2020.
2.2.3 UB-221 (8D6)
In 2012, a new monoclonal anti-IgE antibody named 8D6 was developed at United BioPharma, which was reported to bind the Cε3 domain of IgE with fourfold higher affinity than omalizumab (apparent KD ~59 pM versus KD ~230 pM, as measured with intact IgG antibodies). 8D6 has a unique binding feature in that it blocks the interaction between IgE and FcεRI but does not interfere with IgE binding to CD23.117 Similar to ligelizumab, 8D6 can recognize CD23-IgE complexes on the cell surface.80, 117 However, 8D6:IgE unlike ligelizumab:IgE complexes can still interact with CD23. In a recent follow-up study a humanized version of 8D6, termed UB-221, has been characterized and tested in a phase I clinical trial in patients with CSU.118 UB-221 exhibited the same binding characteristics as its murine predecessor. With higher affinity for free IgE compared to omalizumab, UB-221 showed equal effectiveness to ligelizumab in neutralizing IgE levels ex vivo in sera from patients with atopic dermatitis—some of which contained total IgE levels >10,000 kU/L (24 μg/mL). In vitro, UB-221 suppressed IgE-mediated degranulation of rat basophilic leukemia cells expressing human FcεRIα (i.e., RBL SX-38) with sevenfold higher efficacy than omalizumab. Additionally, UB-221 was shown to markedly reduce the synthesis of IgE in PBMC cultures from healthy donors stimulated with IL-4 and anti-CD40 antibody at both the protein and mRNA level. In direct comparison, this inhibition was superior to that achieved with omalizumab or ligelizumab. The exact mechanism of how UB-221 achieves inhibition of IgE production in B cells, however, remains elusive. The authors speculate that the effect might result from modulating the CD23 pathway possibly by directly cross-linking the receptor and thereby reducing IgE production. As CD23 lacks intracellular signaling domains more research is needed to confirm this hypothesis. In nonhuman primates and human IgE (IGHE) knock-in mice, single application of UB-221 rapidly reduced serum IgE levels to >90%. In the mice, IgE suppression lasted for ~14 days before IgE levels gradually rebounded back to baseline, whereas IgE levels in cynomolgus monkeys were maximally reduced for less than 1 day and reached baseline in <10 days. In the phase I, open-label, dose-escalation trial single intravenous infusion in CSU, UB-221 was found to be safe and well-tolerated. With an estimated serum half-life of 16–22 days at doses between 0.6 and 10 mg/kg, UB-221 led to a significant reduction in serum free IgE levels and a rapid, dose-dependent decrease in the weekly UAS7 scores, which measure disease symptoms. In summary, the current data warrants evaluation of UB-221 in further clinical trials.
2.2.4 IgETrap-Fc protein (YH35324)
IgETRAP is a fusion protein consisting of two extracellular domains of FcεRIα linked to a hybrid IgD/IgG4 Fc domain.119 While the extracellular FcεRIα domains were incorporated with the aim to bind and neutralize IgE, the engineered Fc-domain has been chosen to potentially reduce the likelihood of IgG1 Fc-mediated side effects such as ADCC, complement-dependent cytotoxicity (CDC), and IgG-mediated anaphylaxis. IgETRAP retains the neonatal Fc-receptor (FcRn) binding site, which is crucial for in vivo half-life extension. However, as its eight N-linked glycosylation sites might enhance clearance, IgETRAP was sialylated by co-transfecting a α-2,6-sialyltransferase in CHO DG44 cells, which led to capping of the glycans with sialic acids. Compared to omalizumab IgETRAP showed superiority in certain functional features. IgETRAP showed a 69-fold higher binding affinity for human IgE compared to omalizumab in surface plasmon resonance measurements, which was mainly driven by a slower dissociation rate. Using the human mast cell line LAD2, IgETRAP was more effective than omalizumab in inhibiting IgE-mediated degranulation. In cynomolgus monkeys, IgETRAP reduced free serum IgE levels more effectively and for a longer duration than omalizumab. A single dose of IgETRAP decreased IgE levels below the detection limit and maintained IgE suppression for 10 days, while omalizumab failed to fully reduce free IgE levels and its suppressive effect lasted only 3 days. In a mouse model of IgE-mediated passive systemic anaphylaxis, IgETRAP fully suppressed the allergic reaction as measured by drop in body core temperature.
Recently, a first in human study examining IgETrap in a randomized, double-blind phase I clinical trial to assess safety, tolerability, pharmacokinetics, and pharmacodynamics was conducted in individuals with atopy.120 In a first assessment, healthy or atopic adults with mild allergic conditions and IgE levels between 30 and 700 kU/L were treated with different doses IgETrap, omalizumab, or a placebo. In the second part of the study subjects with IgE levels >700 kU/L received either IgETrap or omalizumab. The study results indicate that treatment-emergent adverse events occurred in 38.5% of participants in the first part and 62.5% in second part of the study. These events were primarily mild or moderate, with no serious adverse events, treatment discontinuations due to adverse effects, or anaphylaxis reported. IgETrap showed a dose-proportional increase in peak concentration and total exposure over the dosage range. The drug also significantly reduced free serum IgE levels, with a greater and longer-lasting effect compared to omalizumab. In conclusion, these data suggest that IgETrap is mostly safe and warrant further investigation as to whether IgETrap might be suitable to treat subjects with atopic conditions.
2.2.5 Lumiliximab (IDEC-152)
In the 1990's Fujiwara et al. observed that CD23 deficient mice have increased and sustained antigen-specific IgE levels compared to wild-type mice, while T and B cell development seemed normal.121 Their findings provided strong evidence that CD23 is involved in negative regulation of IgE synthesis. On the other hand, monoclonal antibodies against human CD23 have been reported to inhibit the production of IgE in IL-4 stimulated human PBMC cultures by several groups122-124 further substantiating the regulatory role of CD23 in this context. While anti-CD23 antibodies can potentially affect multiple aspects of the allergic cascade, different in vitro studies have suggested that crosslinking of membrane CD23 with an IgG receptor could be a key mode-of-action to downregulate IgE synthesis.125-127 In vivo, anti-CD23 antibodies inhibited antigen-specific IgE responses128 and blocked lung eosinophil infiltration in murine asthma models.91 A chimeric macaque/human anti-CD23 monoclonal antibody consisting of cynomolgus macaque variable regions and human IgG1 constant regions, known as Lumiliximab (IDEC-152), has been developed at IDEC Pharmaceuticals125 and tested in a phase I, singledose, dose-escalating clinical trial with allergic asthma patients.129 The antibody showed a favorable safety profile and dose-dependently decreased mean IgE levels. However, no clinical disease amelioration as measured by change in FEV1, was observed upon single dose application. Interestingly, the study found a reduction in total CD19+ B cell counts, since CD23 is not exclusively expressed on IgE-producing B cells. While the assessment of its use in allergic disorders was ceased after this phase I trial, lumilixumab was further tested in chronic lymphocytic leukemia (CLL), to deplete CD23+ B cells. However, this program has also been halted as the study failed to meet primary endpoints in a phase II/III clinical trial.130
2.3 Anti-cytokine receptor antibodies
2.3.1 Dupilumab (Dupixent)
Dupilumab is a humanized monoclonal IgG4 antibody against the alpha subunit of the interleukin-4 receptor (IL-4Rα), which is the common chain of the IL-4 and IL-13 receptor. The antibody has been co-developed at Regeneron Pharmaceuticals and Sanofi. To date, it has been approved for the treatment of several allergic and inflammatory conditions, including moderate-to-severe atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, prurigo nodularis and most recently for chronic obstructive pulmonary disease (COPD) with raised blood eosinophils. While the positive results of two phase III clinical trials with dupilumab in CSU have recently been published,131 it has already been approved as add-on therapy to standard-of-care H1 antihistamines for CSU in Japan. Dupilumab works by inhibiting the biological activity of both IL-4 and IL-13, which are key drivers of the Th2 response. By doing so, dupilumab targets multiple molecular and cellular mechanisms in the allergic cascade, which has been demonstrated in human in vitro culture systems and humanized mouse asthma models.132 Interestingly, dupilumab has been shown to prevent isotype class switching to IgE in B cells in vitro and to suppress IgE levels in vivo.132, 133 In that sense, it blocks IgE pathophysiology upstream of monoclonal antibodies directly targeting IgE. However, IgE suppression is less effective, incomplete and requires long treatment periods (1 year for approximately 70% reduction).132 While direct comparison with omalizumab in a prospective clinical trial is lacking, no clinically significant differences in efficacy outcomes were reported in indirect retrospective evaluations.134
2.3.2 Benralizumab (Faserna)
Benralizumab is a humanized, afucosylated, monoclonal IgG1 antibody that targets the IL-5 receptor alpha chain (IL-5Rα) on the surface of eosinophils and basophils.135 Developed by AstraZeneca's biologics research and development branch, MedImmune, it has been approved as add-on maintenance treatment of severe eosinophilic asthma. Besides blocking the biological activity of IL-5, benralizumab has been shown to drive eosinophils into apoptosis via ADCC.29 IL-5 is a crucial cytokine for the growth, differentiation, recruitment, and survival of eosinophils, which play a significant role in the pathophysiology of eosinophilic asthma and other allergic conditions. Benralizumab mediated elimination of these cells, reduces inflammation and helps to control asthma symptoms and exacerbations. Clinical studies have shown that benralizumab is highly effective in reducing eosinophil counts, improving lung function, and decreasing the frequency of asthma exacerbations in patients with severe eosinophilic asthma.136, 137 Its ability to target and deplete eosinophils makes it a powerful therapeutic option for patients with difficult-to-control asthma characterized by elevated eosinophil levels.
2.4 Approved anti-Th2 cytokine antibodies
2.4.1 Mepolizumab (Nucala) and Reslizumab (Cinqair)
Mepolizumab is a fully humanized monoclonal IgG1 antibody from GSK targeting IL-5. The antibody binds IL-5 with high affinity and inhibits its interaction with the IL-5 receptor, which is predominantly expressed on eosinophils. This approach has been shown to result in a reduction of eosinophil counts in blood and tissues.138 A single intravenous injection led to a remarkable reduction of blood eosinophils, which persisted for 30 days.139 While initial studies in asthma did not show the expected clinical effectiveness,140 Mepolizumab has in the meantime been approved for the treatment of severe eosinophilic asthma,141 eosinophilic granulomatosis with polyangiitis (EGPA), hypereosinophilic syndrome (HES) and as an add-on therapy to intranasal corticosteroids in CRSwNP. Clinical trials have demonstrated its efficacy in decreasing the frequency of asthma exacerbations,142 improving lung function,143 and managing symptoms in other eosinophilic conditions.144
Reslizumab is a fully humanized monoclonal IgG4 antibody also targeting IL-5 initially developed at Cephalon, which was acquired by Teva Pharmaceuticals in 2011. It is approved as add-on maintenance treatment of severe eosinophilic asthma.145 While a direct comparative in vitro study has demonstrated that reslizumab features an 11–26 fold higher affinity and neutralization potency for IL-5 than mepolizumab146 indirect comparisons of clinical outcomes did not show major differences between the two antibodies.
2.4.2 Tezepelumab (Tezspire)
Tezepelumab is a fully human monoclonal IgG2 antibody that inhibits the epithelial-cell–derived alarmin TSLP. The antibody which has been co-developed by Amgen and AstraZeneca binds TSLP with high affinity (~60 pM),18 preventing its interaction with the TSLP receptor complex. This blockade interrupts the signaling cascade that leads to the production and release of pro-inflammatory cytokines and chemokines, reducing inflammation and hyperresponsiveness in the airways. Tezepelumab is approved as an add-on maintenance treatment for patients with severe asthma, particularly those who are inadequately controlled with standard therapies such as inhaled corticosteroids and long-acting beta-agonists.147 Interestingly, tezepelumab is effective irrespective of baseline eosinophil counts.148 The findings that tezepelumab reduced blood eosinophil count as well as levels of fractional exhaled nitric oxide (FeNO) and IgE, indicate that the antibody is able to suppress multiple inflammatory pathways. The effect of tezepelumab on these biomarker levels may be related to decreased interleukin-5 and interleukin-13 levels.149 Reduction in total IgE levels may be linked to suppression of IL-4 and IL-13 concentrations, leading to less efficient B cell isotype class switching to IgE. Clinical trials have demonstrated that tezepelumab significantly reduces the rate of asthma exacerbations, improves lung function, and enhances asthma control compared to placebo.150 Further, a phase IIb clinical trial has recently demonstrated promising results for the treatment of CSU.151 These data support the concept that TSLP inhibition may have broader physiological effects than targeting individual type 2 cytokines.
2.4.3 Lebrikizumab (Ebglyss)
Lebrikizumab is a humanized IgG4 anti-IL-13 monoclonal antibody developed by Almirall and Eli Lilly. The antibody binds IL-13 with an affinity <10 pM.152 Lebrikizumab-bound IL-13 is still able to bind its cell surface receptors, but the antibody sterically prevents the heterodimerization of IL-4Rα/IL-13Rα1 (Type 2 receptor) and thus its downstream signaling. However, it does not affect the binding of IL-13 to the IL-13Rα2 decoy receptor.153 Lebrikizumab has been assessed for the treatment of both atopic dermatitis and asthma. Initial phase III clinical trial data in moderate-to-severe asthma did not demonstrate consistent efficacy.154 However, reanalysis in a well-defined type 2 asthma population with elevated blood eosinophils, elevated FeNO,155 reported a successful outcome with reduced asthma exacerbations. In two identically designed phase III clinical trials for atopic dermatitis, Lebrikizumab therapy demonstrated significantly improved skin clearance, itch, and interference of itch with sleep.156 Currently, its use is restricted to the treatment of atopic dermatitis.157
2.4.4 Antibodies in clinical trials for the treatment of allergy
Apart from the clinically approved monoclonal antibodies to treat different allergic conditions, additional drug candidates are currently undergoing clinical evaluation in a variety of allergic indications (Table 1). While some of them are directed against pre-validated targets such as IL-4R⍺, IL-5, IL-13 or TSLP, others explore new therapeutic routes for example, by (i) neutralizing the alarmin IL-33, an important driver of type 2 immunity, (ii) blocking inflammatory cytokine signaling involved in allergic itch (e.g., IL-31R), or (iii) preventing interaction between T cells (e.g., OX40) and TSLP-activated DCs (e.g., OX40L) to inhibit Th2 cell mediated immune responses. While this topic has been covered extensively in other reviews,170, 171 the table provides an overview of current efforts and strategies to develop more effective monoclonal antibody-based drugs for the treatment of allergies.
| Target/antibody | Clinical stage | Company | References |
|---|---|---|---|
| IL-4R⍺ | |||
| MG-K10 | Phase 2 in asthma and AD | Shanghai Mabgeek Biotech | NCT05382910, NCT05466877 |
| CM310 | Phase 2/3 in asthma, AD and CRSwNP | Keymed Biosciences | NCT05186909, NCT05715320, NCT05436275158 |
| AK120 | Phase 2 in asthma | Akeso | NCT05155020159 |
| Rademikibart | Phase 2 in asthma | Simcere Pharmaceutical | NCT06488755160 |
| IL-5 | |||
| Depemokimab | Phase 3 completed in asthma | GSK | NCT04718389161 |
| Varokibart | Phase 2 in asthma | Teva Pharmaceutical Industries | NCT04847674 |
| SHR-1703 | Phase 2 in asthma | Jiangsu Hengrui Pharma | NCT05522439 |
| IL-13 | |||
| Tralokinumab | Phase 3 in AD | Leo Pharma | NCT03526861162 |
| Cendakimab | Phase 3 completed in AD | Celgene/Abbvie | NCT04800315163 |
| IL-31R | |||
| Nemolizumab | Phase 3 completed in AD | Galderma | jRCT2031230174164 |
| IL-33 | |||
| Etokimab | Phase 2 completed in severe eosinophilic asthma and peanut allergy | Anaptys Bio | NCT02920021165 |
| Itepekimab | Phase 2 completed in asthma | Regeneron/ Sanofi | NCT03387852166 |
| Tozorakimab | Phase 2 in asthma | AstraZeneca | NCT04570657167 |
| TSLP | |||
| AIO-001 | Phase 2 ready in asthma | GSK/ Aiolos Bio | NCT06170827 |
| BSI-045B | Phase 2 in AD | Biosion | NCT05114889 |
| TQC2731 | Phase 2 in CRSwNP | Chia Tai Tianqing Pharmaceutical Group | NCT06036927, NCT05472324 |
| UPB-101 | Phase 2 in asthma, CRSwNP | Upstream Bio | NCT06196879, NCT06164704 |
| CSJ117 | Phase 2 in asthma | Novartis | NCT04410523168 |
| SHR-1905 | Phase 2 in asthma and CRSwNP | GSK | NCT05593250, NCT05891483 |
| CM326 | Phase 2 in asthma, AD and CRSwNP | Keymed Biosciences | NCT05774340, NCT05671445, NCT05324137 |
| OX-40 | |||
| Rocatinlimab | Phase 2/3 in asthma and AD | Amgen | NCT06376045, NCT05633355 |
| BAT6026 | Phase 2 in AD | Bio-Thera Solutions | NCT06094179 |
| OX-40L | |||
| Amlitelimab | Phase 2/3 in asthma and AD | Sanofi | NCT06033833, NCT06130566169 |
- Abbreviations: AD, atopic dermatitis; CRSwNP, chronic rhinosinusitis with nasal polyps.
2.5 Other therapeutic antibodies under development
2.5.1 Barzolvolimab (CDX-0159)
Barzolvolimab, also known as CDX-0159, is a humanized monoclonal IgG1 antibody designed to target KIT with high picomolar affinity and inhibit its function. While Celldex has developed its predecessor CDX-0158 (KTN0158) for the treatment of KIT-positive neoplasms such as advanced refractory gastrointestinal stromal tumors (GIST),172, 173 they subsequently started to implement CDX-0159 to target mast cells in allergic diseases. Compared to CDX-0158, CDX-0159 carries modifications in its Fc portion to abolish FcγR and C1q receptor binding to prevent unwanted FcγR-dependent mast cell activation potentially leading to infusion reactions. Further mutations (i.e., YTE) were introduced to increase the binding affinity for FcRn, reducing the rate of in vivo clearance and extending the antibody's serum half-life. CDX-0159 has been reported to inhibit SCF-dependent KIT activation in vitro.174 In a phase Ia clinical trial it demonstrated a systemic and prolonged mast cell ablation upon in healthy volunteers. Further CDX-0159 treatment reduced skin mast cells and substantially ameliorated disease activity in a phase Ib with chronic inducible urticaria and systemic dermographism patients.175 While a phase II clinical trial in CSU is currently still ongoing (NCT05368285), top line results on angioedema relief have been communicated.176
2.5.2 Lirentelimab (AK002) and other anti-Siglec antibodies
A humanized non-fucosylated monoclonal IgG1 antibody, AK002, targeting Siglec-8 has been developed by Allakos and analyzed for treatment of diseases associated with mast cell and eosinophil-driven inflammation. The ITIM-containing inhibitory Siglec-8 is selectively expressed on mast cells, eosinophils and basophils. Different studies have shown that AK002 depletes eosinophils by ADCC and prevents mast cell activation through inhibitory signaling via Siglec-8.177-180 Initially, AK002 showed safety, tolerability and bioavailability in a phase I clinical trial with healthy volunteers (NCT04324268). However, despite successful depletion of eosinophils (95–96%), Lirentelimab failed to meet primary endpoints in two phase II clinical trials with CSU (NCT05528861) and Atopic Dermatitis (NCT05155085) patients. While this Siglec-8 program with AK002 has been stopped, Allakos has developed the agonistic humanized IgG1 monoclonal antibody AK006 against Siglec-6. AK006 has been reported to induce strong inhibitory signaling in human mast cells181 and to trigger ADCP leading to a reduction in mast cell numbers in vivo.67 A phase I clinical trial in healthy volunteers and subjects with CSU has been initiated (NCT06072157).
2.5.3 Anti-Orai1 (DS-2741a)
ORAI1 is a transmembrane pore-forming subunit forming the hexameric structure of the calcium release-activated calcium (CRAC) channel. It regulates cellular uptake of calcium, which is critical for several biological processes in various cell types including activation of T cells and mast cells. While mutations in the Orai1 gene can cause severe combined immunodeficiency (SCID), characterized by impaired T cell function and a compromised immune system,182 Orai1 knockout mice exhibit reduced T cell cytokine production and mast cell activation.183 Thus, targeted approaches to specifically inhibit ORAI1 have been assessed. Daiichi Sankyo has developed DS-2741a, a humanized anti-ORAI1 IgG1 antibody, which attenuated bone marrow derived mast cell (BMMC) degranulation in vitro and the passive cutaneous anaphylaxis reaction in vivo in human Orai1 transgenic mice.184 Further, DS-2741a induced suppression of cytokines release triggered by store-operated Ca2+ entry in a variety of T helper subsets. In vivo, DS-2741a treatment resulted in an amelioration of dermatitis in a mouse model of mite antigen-induced atopic dermatitis.184 Since Orai1 is widely expressed, it remains to be investigated whether such an approach could be associated with overt unwanted side effects. Further studies are needed to assess the therapeutic potential of targeting ORAI1 in allergic diseases.
3 COMPARATIVE ANALYSIS OF THERAPEUTIC ANTIBODIES
The availability of many different biologicals for the treatment of allergic conditions (Table 2) enables the possibility of tailoring the therapy for a patient's disease phenotype/endotype in a personalized manner. At the same time, it requires a detailed diagnostic work-up and informed therapeutic decision-making based on well-established biomarkers. As reviewed in detail elsewhere,195 additional factors such as patient co-morbidities, practical aspects surrounding drug delivery (e.g., frequency of injection, location of administration, ability to receive delivery and properly store medication) and personal situation of the patient (e.g., age, work hours, caretaker demands and travel frequency) can influence the choice of the most suitable treatment. There are clear guidelines from EAACI196, 197 and GINA198 for the use of biologicals in asthma. Moreover, EAACI provided guidelines on the use of biologicals in CSRwNP199 and urticaria.200
| mAb | Target | Asthma | CSU | AD | CRSwNP | Food allergy | EoE |
|---|---|---|---|---|---|---|---|
| Omalizumab | IgE | Approved | Approved | Limited efficacy185 | Approved | Approved | Limited efficacy186 |
| Dupilumab | IL-4Rα | Approved | Approved (add-on)* | Approved | Approved | - | Approved |
| Benralizumab | IL-5R | Approved (add-on, severe eosinophilic) | Limited efficacy187 | Limited efficacy188 | Limited efficacy189 | - | Limited efficacy190 |
| Mepolizumab | IL-5 | Approved (add-on, severe eosinophilic) | - | - | Approved (add-on) | - | Limited efficacy191 |
| Reslizumab | IL-5 | Approved (add-on, severe eosinophilic) | - | - | - | - | Limited efficacy192 |
| Tezepelumab | TSLP | Approved (add-on) | Promising phase 2b151 | Limited efficacy193 | - | - | ODD |
| Lebrikizumab | IL-13 | Limited efficacy194 | - | Approved | - | - | - |
- Abbreviations: AD, atopic dermatitis; CRSwNP, chronic rhinosinusitis with nasal polyps; CSU, chronic spontaneous urticaria; EoE, eosinophilic esophagitis; ODD, orphan drug designation.
- * In Japan.
Patients with IgE-driven disease pathology benefit from omalizumab treatment across different allergic conditions, as indicated by its broad success and approval across indications (Table 2). The assessment of allergen-specific and total IgE levels can be an important indicator in this context. However, there are exceptions in which high or extreme levels of IgE were associated with worse treatment responses such as in AD.201 Furthermore, omalizumab has shown limited efficacy in AD, which might be due to the observation that these patients often exhibit non-IgE mediated aspects of chronic skin inflammation.
IL-4Rα targeting therapy with dupilumab can act at multiple points along the allergic cascade and has also shown clinical efficacy across multiple indications.202 It is recommended in conditions with prominent type 2 inflammation characterized by elevated blood eosinophils and/or increased fractional exhaled nitric oxide (FeNO). IL-4 and IL-13 share many biological actions, which can likely be attributed to the overlapping use of the IL-4Rα subunit and downstream activation leading to activation of signal transducer and activator of transcription (STAT) pathways. This molecular pharmacology is one reason why approaches targeting IL-4Rα chain, such as dupilumab, have proven more successful than isolated approaches that inhibit only IL-13 or IL-4.203
As the development, recruitment and survival of eosinophils is highly dependent on IL-5, blocking its biological activity with benralizumab, mepolizumab or reslizumab has proven to bring clinical benefit in patients with inflammatory eosinophil in the lung.204 However, these anti-IL5/5Ra biologicals target a narrower patient population as they were only approved as add-on maintenance therapy in severe eosinophilic asthma.
Consistent with basic disease models, therapeutic blockade of alarmin pathways has shown promise in settings where eosinophil recruitment and tissue remodeling are key drivers of pathology. TSLP targeted therapies modulate lung function, inflammation, and reduce exacerbation rates in allergic asthma.11 In contrast the same intervention has been less successful in atopic dermatitis (AD), where IL-13 induced tissue remodeling and IL-5 driven eosinophil recruitment are less central to disease pathophysiology.12, 151
Currently, there is only limited information available from head-to-head trials of approved biologicals in a specific allergic indication. A prospective direct comparison between dupilumab, mepolizumab, and reslizumab, however, has been performed in severe eosinophilic asthma.205 The study, which included 141 patients, reported comparable clinical outcomes regarding time-to-first exacerbation, exacerbation rate, forced expiratory volume in 1 s, and asthma control test score within a 12-month treatment period. An indirect comparison based on meta-analysis of 14 randomized clinical trials (RCTs) in uncontrolled persistent asthma reported greater reductions in annualized severe exacerbation rates for dupilumab as compared to benralizumab, mepolizumab, reslizumab, and omalizumab treatment.206 Additionally, a greater improvement in FEV1 was described for dupilumab at week 12, 24, or 52 than for the other biologics. In another indirect comparison between tezepelumab, mepolizumab, benralizumab, and dupilumab in eosinophilic asthma including 9201 patients from 10 RCTs, it has been reported that tezepelumab and dupilumab were associated with greater improvements in exacerbation rates and lung function than benralizumab or mepolizumab.207 Results from such indirect comparisons should, however, be interpreted with caution until there is reliable data available from direct head-to-head clinical trials.
4 FUTURE DIRECTIONS AND INNOVATIONS
The continued authorization of omalizumab for treating additional allergic indications, more than 20 years after its initial approval for asthma,72 underscores the pivotal role of IgE biology in a wide range of allergic diseases. With food allergy as the most recent example,208 many clinical investigations have demonstrated that targeting and neutralizing IgE remains one of the most important cornerstones of allergic disease management. Current clinical limitations for anti-IgE approaches such as dosing restrictions and frequency of injections, however, call for further improvements in this approach. Given the broad treatment success of omalizumab, we will particularly focus on future directions of anti-IgE treatment strategies in the next sections.
4.1 Molecular engineering of next-generation IgE inhibitors
Over time it has become evident that developing a next generation anti-IgE treatment that achieves clinical improvement over omalizumab anti-IgE treatment is more difficult than initially anticipated. This has especially been exemplified by the termination of the HAE-1 development program, and disappointing clinical results obtained with both ligelizumab and Xmab7195. For ligelizumab, two phase III clinical trials in asthma and CSU88, 89 with side-by-side comparison to omalizumab did not yield anticipated improvements in clinical outcome. These studies provide accumulating evidence that solely increasing the affinity of an anti-IgE antibody is insufficient to overcome current therapeutic limitations. Instead, the data rather suggest that other binding characteristics such as IgE epitope specificity and the ability to actively dissociate IgE from FcεRI might represent important contributors to clinical efficacy.
4.1.1 Improving existing anti-IgE approaches
With the development of an efficiency screen based on yeast display to simultaneously increase affinity and disruptive potency, we recently laid the experimental and conceptual foundation for the selection and engineering of potent omalizumab variants.102 In this work, we characterized a range of anti-IgE antibodies with distinct epitopes, affinities, and disruptive potencies. Clone C02 resulted from such a screen and showed high affinity as well as improved disruptive potency. It completely desensitized basophils from allergic donors within hours at therapeutically relevant doses ex vivo. Insertion of flexible Glycine linkers at each Fab elbow (VH/VL) in C02 (C02-H2L2) further increased its disruptive potency. Importantly, we observed that the dwell time of disruptive anti-IgE antibodies on intact IgE:FcεRI complexes prior to disruption is a critical parameter that correlates with their ability to safely remove IgE from human allergic effector cells. Another group has engineered an omalizumab variant, termed FabXol3, by structure guided insertion of three point mutations, two in the VL domain framework region and one in the C𝜅 domain.78, 209 FabXol3 featured slightly higher affinity than the unmutated omalizumab Fab and was more efficient in accelerating the dissociation of IgE-Fc from FcεRI. Overall, these findings illustrate the potential of kinetically active disruptive anti-IgE antibodies to enhance therapeutic efficacy of omalizumab. Furthermore, they provide a structural and conceptual framework for engineering optimized next-generation anti-IgE antibodies featuring both highly efficient neutralization and disruptive potency.
Another study described the development of an omalizumab biobetter, AB1904Am15, through the optimization of stability, immunogenicity, affinity and bioavailability.210 First the authors replaced two aspartic acid isomerization hotspots in the complementary determining regions (CDRs) and mutated multiple murine framework residues to the homologous human germline antibody sequence. Using yeast display technology in conjunction with a designed mutation library of each CDR in the variable domains they further screened for increased affinity. Additionally, the previously described YTE mutation211 was inserted into the Fc region of the antibody to prolong its serum half-life. The resulting anti-IgE antibody AB1904Am15 showed favorable biophysical properties with improved molecular stability. The twofold improved affinity resulted in enhanced in vitro inhibition of IgE binding to FcεRI and enhanced suppression of IgE induced histamine release in human FcεRI-expressing rat basophilic leukemia cells compared to omalizumab. Further, AB1904Am15 showed a more than twofold longer serum half-life in human FcRn transgenic mice. Finally, the optimized antibody outperformed the original omalizumab antibody in terms of free IgE suppression efficacy in a cynomolgus monkey asthma model in vivo. This study further demonstrated the possibilities of improving anti-IgE biologicals. Overcoming current limitations associated with molecular stability and dosing restrictions may hold great promise to optimize clinical efficacy.
4.1.2 Exploring novel mechanisms and alternative scaffolds
The first systematic description of active IgE dissociation from FcεRI using a disruptive IgE inhibitor has been published in 2012.79 In this study, we characterized the binding kinetics of an alternative scaffold molecule, the designed ankyrin protein (DARPin) E2_79, featuring this accelerated dissociation mechanism. As E2_79 engages a subset of ligand attachment points on IgE, which become exposed during partial IgE:FcεRI complex dissociation, it accelerates the intrinsic dissociation rate and fully removes IgE from the receptor. This work laid the conceptual foundation to develop more potent inhibitors through targeted screening approaches and molecular engineering. In subsequent studies, we generated bispecific DARPins, such as bi53_79, by genetic fusion79 and a bispecific antibody-like hybrid molecule between two DARPins and an IgG Fc-domain, KIH_E07_79, using the knobs-in-hole techniques.81 The latter example represents an interesting molecule as it integrates important functional features from the initially described disruptive DARPin with classical characteristics of IgG antibodies, including a potentially prolonged serum half-life. These studies highlighted the remarkable potency of KIH_E07_79 to neutralize free IgE, rapidly dissociate preformed IgE:FcεRI complexes, terminate IgE-mediated signaling in preactivated human blood basophils in vitro, and shut down pre-initiated allergic reactions and anaphylaxis in mice transgenic for human FcεRIα in vivo.
A disruptive nanobody, called sdab 026 was reported several years later in 2018.212 The authors described an allosteric mechanism for sdab 026, in which both blocking of the IgE:FcεRI interaction as well as disruption of IgE:FcεRI are based on trapping the IgE-Fc Cε3-4 domains in a closed conformation incompatible with FcεRI binding.213 While not yet officially peer-reviewed, various bispecific IgG4 Fc-fusion proteins have been published (patent WO2024003376A1) with sdab 026 (i.e., A1) and an additional less potent or nondisruptive anti-IgE nanobody (i.e., A2-G1), in an analogous approach as previously described for the DARPin Fc-fusions. Several of the bispecific nanobody Fc-fusion molecules, such as NIgG4B1A1(G4S), were shown to efficiently inhibit allergen-mediated reactions in passive cutaneous or systemic anaphylaxis models in mice transgenic for human FcεRIα in vivo.
Compared to classical antibodies, the described alternative scaffolds or fusion molecules feature smaller binding sites, which may be advantageous when targeting of specific epitopes important in disruption of high-affinity protein:protein interactions is essential. As such they represent promising approaches for the generation of next-generation anti-IgE biologicals. However, clearly more work needs to be done in order to assess their developability, manufacturability and in vivo efficacy as well as safety.
4.1.3 Unifying different modes-of-action in one molecule
There is a growing body of evidence suggesting that the therapeutic efficacy of anti-IgE biologicals could be significantly increased, if the treatment simultaneously interfered at multiple levels in the allergy cascade.214 This could potentially be achieved by developing next-generation anti-IgE molecules exerting multiple modes-of-action (Figure 3). Theoretically, such a novel drug candidate should ideally combine the following molecular characteristics: (i) bind free serum IgE with high affinity to neutralize its biological activity, (ii) efficiently and rapidly disrupt IgE from its high-affinity receptor FcεRI on the surface of allergic effector cells in the blood (i.e., basophils, dendritic cells) and in tissues (i.e., mast-cells), (iii) inhibit IgE:CD23 interactions to prevent IgE facilitated antigen presentation on dendritic cells or B cells, (iv) suppress IgE production in B cells or eliminate IgE switched B cells.
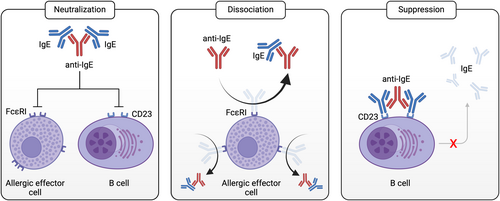
While a lot of effort has focused on maximizing free IgE neutralization in previous anti-IgE development programs, more recent approaches are starting to optimize additional modes-of-action (Table 3). At this stage, however, more research is needed to evaluate how these functional characteristics will translate into improved therapeutic potential and clinical benefit in vivo in humans.
| Anti-IgE biological | Blocking of IgE:FcεRIα | Blocking of IgE:CD23 | Disruption of IgE:FcεRIα | Suppression of IgE production |
|---|---|---|---|---|
| Omalizumab | ++ | ++ | + | − |
| Ligelizumab | +++ | + | − | ++ |
| HAE1 | +++ | ++* | ++ | n.a. |
| Quilizumab | − | − | − | +++ |
| Xmab7195 | +++ | ++ | + | ++ |
| UB221 | +++ | − | n.a. | +++ |
| IgETrap-Fc | +++ | − | + | n.a. |
| C02-H2L2 | +++ | ++* | ++ | n.a. |
| AB1904Am15 | ++ | ++* | n.a. | n.a. |
| KIH_E07_79 | ++ | ++ | +++ | ++ |
| NIgG4B1A1(G4S) | ++ | n.a. | +++ | n.a. |
- Note: * inferred from shared epitope with omalizumab.
- Abbreviations: efficacy scale: − = absent; + = moderate; ++ = strong; +++ = very strong; n.a., not available.
4.2 Combination and repurposing of existing strategies
4.2.1 Omalizumab and allergen-specific immunotherapy
Allergen-specific immunotherapy (AIT) is a clinical procedure aimed at re-establishing immunological tolerance against culprit allergens. The treatment involves administration of incrementally increasing doses of allergen extract, which can be delivered via different routes (i.e., subcutaneous, sublingual, oral or intralymphatic). Starting out with minute concentrations of allergen, decreases the risk of a systemic reaction. Once a certain maintenance dose is achieved repetitive administration is required to achieve clinical benefit. The overarching goal of the treatment is to shift the allergy prone Th2-dominated immune response towards protective Th1 immunity including the induction of regulatory T cells and allergen-specific IgG antibodies. As suggested in the guidelines, the treatment duration typically spans 3–5 years.215 This long-term therapy, results in sustained unresponsiveness and prolonged amelioration of allergic symptoms in a fraction of patients.216, 217 To increase the safety of AIT and to investigate whether there might be synergistic effects between anti-IgE therapy and AIT, Kuehr et al.218 conducted the first clinical trial in which omalizumab was combined with AIT. This randomized, double-blind, placebo-controlled, multi-site study 221 patients suffering from birch and grass pollen-induced seasonal allergic rhinitis. Their results demonstrated that omalizumab conferred a protective effect with different types of allergens and significantly increased the clinical benefit when compared to AIT alone. Many follow-up studies used omalizumab as pretreatment and/or adjunct therapy for AIT mainly in aero, hymenoptera and food allergy,219 with the rational of reducing IgE levels before introducing the allergen to reduce the risk of adverse reactions. Increased safety of AIT upon omalizumab combination has been reported for several of these investigations,220-222 however, omalizumab is not yet officially approved for such combination therapy. The OUtMATCH study, a large multicenter clinical trial in food allergy, is currently assessing the benefits of omalizumab adjunct therapy in AIT in a systematic manner.223
The efficacy of combined anti-IL-4 therapy with a suboptimal dose of grass pollen subcutaneous immunotherapy to induce sustained tolerance to allergen in seasonal allergic rhinitis patients was assessed in a randomized, double-blind clinical trial. Using allergen-induced skin late-phase response as surrogate marker of therapeutic response, no additional benefit over SCIT alone could be found.224 Compared to combination therapy using anti-IgE and AIT, current data indicate that IL-4 targeting in conjunction with AIT appears less promising.
4.2.2 BCMAxCD3 bispecific antibody
B cell maturation antigen (BCMA) is a tumor necrosis factor (TNF) family receptor specifically expressed on plasma B cells (PCs) playing a major role in their survival. The bispecific anti-BCMA/anti-CD3 antibody linvoseltamab (BCMAxCD3) developed at Regeneron to treat multiple myeloma simultaneously engages PCs and T cells inducing T cell mediated killing of the PCs.225 Recently a novel concept using this antibody to broadly target and remove long-lived PCs in an isotype independent manner combined with blockade of the IL4Rα to durably reverse IgE-mediated allergy has been suggested.226 In a long-term house dust mite sensitization model (>15 weeks) in mice expressing human BCMA and human CD3, application of linvoseltamab efficiently depleted bone marrow plasma cells (BMPCs). Importantly, all immunoglobulin isotypes (i.e., IgA, IgM, IgE and IgG1) were significantly reduced 1 week following the treatment. Antibody levels recovered to pre-treatment baseline values within 3–5 weeks under continuation of HDM-sensitization. To specifically prevent this rebound effect for IgE, BCMAxCD3 treatment was combined with anti-IL4Rα antibody injections. Indeed, sustained suppression of IgE levels was observed, while the other antibody levels reached baseline levels within 9 weeks post BCMAxCD3 application. Systemic challenge of mice undergoing a 14-week HDM sensitization and combined treatment of BCMAxCD3 with anti-IL4Rα antibodies did not show any signs of anaphylaxis. Further, the combination of BCMAxCD3 and anti-IL4Rα antibody treatment on resting IgE levels in cynomolgus monkeys was tested. In line with results in the mice, an initial drop in IgE and IgA levels with the BCMAxCD3 single treatment was observed. The levels normalized to baseline between 50 and 100 days post injection. Prolonged suppression of IgE was only achieved in the combination treatment. The bispecific BCMAxCD3 antibody was assessed for its ability to eliminate PCs from human PBMCs and BM samples. The antibody specifically ablated PCs but not other B cell subsets in these samples, which is in line with specific BCMA expression on PCs. When culturing BM samples from allergic and nonallergic donors in vitro IgE only accumulated in the culture supernatants of allergic donors. The production of IgE in these samples was fully suppressed upon bispecific BCMAxCD3 antibody treatment. Additionally, initial human data from clinical trials in multiple myeloma patients who received weekly administration of bispecific BCMAxCD3 antibody indicated that IgE levels are completely reduced as early as 4 weeks posttreatment. These data suggest that transient PC ablation using a BCMAxCD3 bispecific antibody combined with persistent inhibition of IgE class switching using an anti- IL4Rα antibody might be a promising strategy to achieve IgE depletion. However, there are many questions remaining about the potential risks associated with transient depletion of all immunoglobulins.
4.2.3 Combination of approved biologicals
When a single biologic is ineffective or insufficient for the management of a given allergic condition within 4 months of treatment, guidelines generally recommended switching to an alternative option197 instead of adding another therapy. However, interfering with multiple pathophysiological pathways could be beneficial in some patients with mixed allergic phenotypes and/or endotypes. While systematic studies about efficacy and safety of combination therapies are still missing, there are currently around 38 case reports and case series227-234 providing insight into the use of dual biologics in co-occurring asthma and atopic dermatitis. These mostly include combinations of (i) omalizumab with add-on dupilumab/mepolizumab or (ii) benralizumab with add-on dupilumab. Improved efficacy of dual biologic therapy has been reported in 21 cases and no major adverse events or safety signals were observed so far.235 However, further systematic trials and cost-effectiveness evaluations236 are required to make a conclusive statement about the usefulness of these approaches.
5 CONCLUSIONS
The increasingly detailed understanding of the molecular and cellular mechanisms underlying allergic disorders has significantly shaped and advanced the development of therapeutic intervention strategies. Monoclonal antibodies are particularly interesting in this context due to their favorable properties, including long serum half-life, high specificity, customizable affinity and efficacy as well as generally favorable safety profile.237 Compared to more traditional treatment approaches, such as the use of corticosteroids, monoclonal antibodies can target key components involved in the allergic cascade with higher precision, resulting in fewer side effects and potentially more favorable clinical outcomes. Moreover, molecular engineering has enabled the structural fine-tuning of monoclonal antibodies for specific therapeutic actions and at the same time helped to minimize immunogenicity.238, 239 Additionally, the production and manufacturing of monoclonal antibodies have significantly evolved over the years.240 Today, there are established, standardized procedures and protocols that ensure consistency and quality in antibody production. The increasing expertise and advancements in biotechnology have streamlined manufacturing pipelines, allowing for the efficient production of monoclonal antibodies used to treat a wide range of diseases.241 This standardization and accumulated know-how have enabled the reliable and scalable production of these therapeutic agents, ensuring their availability and effectiveness in clinical settings.
Different monoclonal antibody-based therapies have demonstrated efficacy across a spectrum of allergic conditions, including asthma, atopic dermatitis, chronic spontaneous urticaria, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis and food allergies, underscoring the central role of IgE, type 2 cytokine pathways and alarmins in allergy pathophysiology. Having the choice between multiple approved treatment options enables tailoring therapeutic strategies to individual disease endotypes in a personalized medicine approach. Whether combination therapies will be approved and implemented on a routinely basis in this context is rather unlikely due to associated costs and uncertainties about potential risks of such co-treatments. However, there might be an opportunity for newly developed multispecific drugs that simultaneously target different pathways in the allergic cascade. In addition, the combination of anti-IgE therapy with AIT presents a promising therapeutic avenue. Clinical studies have shown that adjunct use of omalizumab can enhance the safety and efficacy of AIT by reducing the risk of adverse reactions during allergen exposure. This combination strategy holds potential for achieving more durable tolerance to allergens and improving clinical outcomes.
Targeting type 2 cytokine pathways, specifically functions of IL-4, IL-5, and IL-13, holds considerable promise for the treatment of allergic disorders. This approach has already demonstrated substantial potential in effectively controlling allergic inflammation with dupilumab, benralizumab mepolizumab and reslizumab. By modulating the activity of these cytokines, therapies can significantly reduce symptoms and enhance the quality of life for patients. However, our understanding of type 2 cytokine signaling is still evolving, and there are gaps in knowledge regarding the full range of functions and interactions of these cytokines. Therefore, the long-term safety profile of cytokine-targeted therapies should remain under investigation. While short-term benefits are evident, potential risks associated with chronic use need to be thoroughly evaluated.
By blocking the activity of alarmins, it is possible to disrupt early stages of allergic inflammation. While targeting TSLP signaling pathways has demonstrated successful treatment outcomes, alarmins are also involved in various physiological processes beyond allergic inflammation and their inhibition could potentially disrupt homeostatic immune functions. While generally providing a promising approach, potential risks, such as impaired tissue repair mechanisms should be further evaluated in this context, especially upon long-term treatment.
The identification of allergen-specific IgE as a crucial factor in the pathogenesis of most allergic conditions led to the conceptualization of therapeutic anti-IgE antibodies.68, 84 Subsequent development efforts mainly focused on generating potently neutralizing, non-anaphylactogenic monoclonal antibodies, specifically targeting free serum IgE rather than FcεRIα-bound cell surface IgE. The primary goal was to block IgE:FcεRIα interactions, with less emphasis on preventing IgE binding to CD23. The development and approval of omalizumab marked a significant breakthrough in the management of allergic diseases. However, the numerous unsuccessful attempts to develop a next-generation monoclonal anti-IgE antibody suggest that our understanding of the essential functional characteristics for an optimized anti-IgE antibody remains incomplete. For instance, omalizumab's potential to actively disrupt pre-existing IgE:FcεRIα complexes on the surface of allergic effector cells has only recently been described. This raises the question: does the ability to dissociate IgE from its high-affinity receptor contribute to omalizumab's therapeutic efficacy? Remarkably, over 20 years later, omalizumab remains the only approved therapeutic anti-IgE antibody, prompting further questions: Does the weaker inhibitory activity of ligelizumab in blocking IgE:CD23 interactions limit its therapeutic benefit? What role does in vivo biodistribution play in targeting locally produced IgE in different tissues? Are the size and half-life of IgE:anti-IgE complexes important in acting as additional allergen sinks? Future research and development efforts should address these unknowns and pave the way for new therapeutic strategies. At this point, results from basic research strongly suggest that next-generation anti-IgE biologicals will have to optimize more than one mode-of-action to leverage synergistic effects and achieve more complete suppression of the allergic response. It is conceivable that combining those different molecular features into a single antibody or antibody-like protein could bring the next revolution in allergy disease management.
In conclusion, the continued exploration of the intricate cellular and molecular network involved in allergic responses is crucial for developing next-generation treatments. These advancements hold the potential to provide more effective, safe, and tailored therapeutic options, ultimately improving the quality of life for patients suffering from various allergic diseases. As research progresses, the integration of personalized medicine approaches, leveraging biomarkers to guide treatment selection, will be essential in optimizing clinical outcomes and addressing the diverse needs of allergy patients.
ACKNOWLEDGMENTS
We thank members of the Eggel and Jardetzky labs for providing valuable feedback on the manuscript. This work was supported by a grant from the Swiss National Science Foundation 310030_219361 (A.E.) and NIH grant R01AI115469 (T.S.J.). Figures were created with BioRender.com. Open access funding provided by Universitat Bern.
CONFLICT OF INTEREST STATEMENT
LFP, TSJ and AE are co-founders and shareholders of Excellergy, Inc.—a company developing novel therapeutic molecules to treat allergic disorders. AE is a co-founder and shareholder of ATANIS Biotech AG—a company developing allergy diagnostic tests.
Open Research
Data available on request from the authors.




