Decoding immunogenic cell death from a dendritic cell perspective
This article is part of a series of reviews covering Mechanisms of programmed cell death appearing in Volume 321 of Immunological Reviews.
Summary
Dendritic cells (DCs) are myeloid cells bridging the innate and adaptive immune system. By cross-presenting tumor-associated antigens (TAAs) liberated upon spontaneous or therapy-induced tumor cell death to T cells, DCs occupy a pivotal position in the cancer immunity cycle. Over the last decades, the mechanisms linking cancer cell death to DC maturation, have been the focus of intense research. Growing evidence supports the concept that the mere transfer of TAAs during the process of cell death is insufficient to drive immunogenic DC maturation unless this process is coupled with the release of immunomodulatory signals by dying cancer cells. Malignant cells succumbing to a regulated cell death variant called immunogenic cell death (ICD), foster a proficient interface with DCs, enabling their immunogenic maturation and engagement of adaptive immunity against cancer. This property relies on the ability of ICD to exhibit pathogen-mimicry hallmarks and orchestrate the emission of a spectrum of constitutively present or de novo-induced danger signals, collectively known as damage-associated molecular patterns (DAMPs). In this review, we discuss how DCs perceive and decode danger signals emanating from malignant cells undergoing ICD and provide an outlook of the major signaling and functional consequences of this interaction for DCs and antitumor immunity.
1 INTRODUCTION
The term immunogenicity was coined for the very first time in 19691, 2 to describe the capacity of an antigenic molecule to provoke a cell-mediated immune response. The search for the “cellular intermediate” collecting antigenic entities and coordinating the clonal expansion of antigen-specific T cells, led to the discovery of dendritic cells (DCs) by the immunologist Ralph Steinman, the founding father of the DCs.3, 4 While the immunogenic role of DCs was originally noted in a transplantation setting, Ralph Steinman predicted DCs to be a critical accessory cell required for the generation of many immune responses,5 a discovery for which he was awarded the Nobel Prize in 2011. DCs were soon recognized to perform a critical function in antitumor immunity6-8 by capturing tumor-derived antigens within the dying cancer cells and presenting them on major histocompatibility (MHC) molecules to naïve T cells in the draining lymph node (dLN).8-10
DCs are short-lived immune cells that develop in the bone marrow from so-called bone marrow-derived precursors (pre-DCs).11 Upon bone marrow egress, DCs reach peripheral tissues via the circulation and undergo further differentiation into either XCR1-expressing Type 1 conventional DCs (cDC1s) or SIRPα-expressing Type 2 cDCs (cDC2s)12 (see Box 1). cDC1s are specialized in cross-presentation (i.e., presenting exogenously acquired dead-cell derived antigens on MHCI to CD8 T cells), and hence they represent the most critical subset driving antitumor immunity.13-15 DCs enter peripheral tissues or the tumor bed as so-called resting or immature DCs with a large phagocytosing capacity.12 Engulfment of dying tumor cells will initiate a coordinated maturation process, including the upregulation of the chemokine receptor CCR7 and the antigen presentation machinery8 (see Figure 1).
BOX 1. DC subsets in vivo and in vitro
The rapid evolution in the field of single-cell transcriptomics combined with the design of novel approaches to delineate the ontogeny of a specific cell type, led to a unified DC nomenclature based on ontogeny rather than on surface marker expression.32, 33 cDCs derive from a common dendritic cell precursor (CDP) and strictly depend on the cytokine FMS-like tyrosine kinase 3 ligand (FLT3L) for their development.90 They comprise two major subsets cDC1s and cDC2s that develop from pre-DCs in response to a distinct set of transcription factors (Batf3, IRF8, ID2, and Bcl6 for cDC1s versus IRF4, KLF4, Notch2, and RBPJ for cDC2s).91 Especially in vivo, each subset exerts unique functions. cDC1s excel in cross-presentation of dead-cell derived antigens to CD8+ T cells via their MHCI complexes, while cDC2s predominantly present phagocytosed material via MHCII molecules to CD4+ T cells.34, 35, 92, 93 This division in labor is not absolute and especially in human DCs, there are several indications that also cDC2s can cross-present.94 While cDC1s are easy to discriminate based on their conserved gene program and the expression of highly unique markers such as XCR1 or DNGR1, cDC2s are more heterogeneous and have been subdivided into cDC2A and cDC2B.95 Especially in inflammatory conditions, additional DC types can be observed. Recently, a new DC subset has been identified in humans and mice in inflammatory conditions that appears to develop from a Ly6C+ monocyte-DC precursor rather than from CDPs, distinguishing it from true cDC2s.88, 96 It has been annotated as cDC3, but its role in anticancer immunity remains to be investigated.
In vitro, the confusion is even greater due to the lack of proper tools to recapitulate bona fide DC differentiation in tissue cultures. Traditionally, DCs have been differentiated in vitro from bone marrow-derived cells in the presence of granulocyte macrophage colony stimulating factor GM-CSF (so-called GM-CSF DCs).97 Although this method has been widely adopted in the DC field, it leads to a very heterogeneous population of cells that mostly represent monocyte-derived DCs (mo-DCs), resembling macrophages or DC-like cells that do not correspond to cDC1 or cDC2.98 A recent review therefore suggested to rename GM-CSF DCs as monocyte-derived cells (MCs) rather than mo-DCs.12 As MCs are easy to cultivate and provide a large yield (in the mouse from bone marrow cells and in humans from peripheral blood mononuclear cells99), MCs are often used for DC vaccination studies in the context of tumor therapy, also in humans.86 While MCs are very potent in taking up tumor antigen, there is still debate whether MCs have the capacity to migrate to the dLN and possess T-cell stimulatory capacity.82, 89 More recently, additional protocols have been developed to differentiate bona fide cDC1s and cDC2s in vitro from human CD34+ stem cells or mouse bone marrow-derived cells by coculturing them on a feeder layer of fibroblasts expressing Notch ligands.100 While these DCs truly recapitulate features and functions of in vivo cDCs, the yield remains low, limiting their widespread use for vaccination purposes. With new technologies emerging to target cDCs in vivo using antibody-based strategies7 or lipid nanoparticles (LNPs) as carriers to deliver tumor antigens to DCs,101, 102 in vivo targeting might become the preferred strategy for DC vaccination purposes.
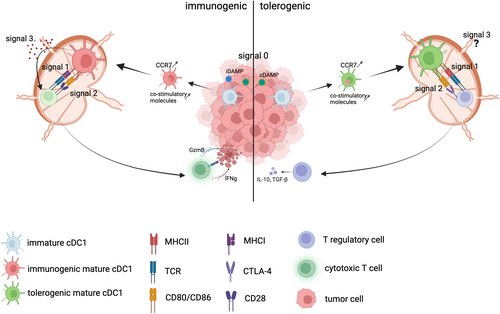
DCs can mature in two different ways, either in an immunogenic or in a homeostatic (also called tolerogenic) manner.16, 17 This depends on how the DC perceives the antigen during its uptake (either as dangerous, pathogenic, or as self entity), which will have a major impact on how the DCs will instruct the adaptive immune system. It is generally accepted that the generation of effector T cells depends on (at least) three signals that are delivered by immunogenic mature DCs: Signal 0 described as the sensing of the environment by the cDC1s, Signal 1 being the presentation of the cognate antigen on MHC molecules, Signal 2 the upregulation of co-stimulatory molecules (e.g., CD80, CD86, and CD40), and signal 3 the release of proinflammatory chemokines and cytokines18, 19 (see Figure 1). These signals will ultimately instruct the T cell to react to the antigen and kill the tumor. In immunological terms, antigen presentation leads in this case to cross-priming, or the induction of an effective immune response. Alternatively, homeostatic mature DCs will instruct the T cell to tolerize the antigen, thereby converting naïve T cells to regulatory T cells and dampen the immune response. In this case, antigen presentation leads to cross-tolerance, or the induction of tolerance against the antigen-expressing cell.18, 19
On a daily basis, millions of cells in our body die by apoptosis, get engulfed by DCs and do not trigger immunity. So, what defines immunogenicity?
Broadly speaking, immunogenicity is determined by a combination of two properties: antigenicity and adjuvanticity. Antigenicity refers to the ability of an antigen to be seen by lymphocytes. For T cells this implies whether an antigen is being engulfed by DCs, protected from lysosomal degradation and processed, carried to the dLN by migratory DCs and presented on MHC molecules to naïve T cells with a cognate T-cell receptor. Adjuvanticity refers to the ability of a substance to induce priming of lymphocytes, which is dependent on the activation of DCs and associated with the induction of an immunogenic DC maturation process. In the presence of a pathogen, this is accomplished by activating receptors for so-called pathogen-associated molecular patterns (PAMPs) on DCs, which drive inflammatory signaling cascades culminating in the activation of interferon responsive factors (IRFs), mitogen-activated protein kinases (MAPK) and nuclear factor κB (NF-κB). In sterile inflammatory conditions, the upstream signals driving immunogenic DC activation, have long remained enigmatic.20
In the original “self versus foreign” model of Polly Matzinger, the innate immune system was postulated to react only to foreign entities (e.g., microbial, nonself antigens) while tolerating the organism's self-antigens/cells.21, 22 Accordingly, sterile cell demise could only be immunologically silent, or tolerogenic as it is in the case of physiological apoptosis, or elicit immune stimulation, if it provides a source of PAMPs. In the early 1990s, several studies established the role of tumor-associated antigens (TAAs) in T cells for mediating antitumor immunity and suggested a role for adjuvants in supporting adaptive immune responses, which together challenged the self versus foreign model.21-26 Studies conducted by Walter Land, examining immunity triggered by tissue injury in patients undergoing allograft rejection,27 surmised that endogenous or “self” entities released or exposed by injured or dying cells could also alert the immune system. He then formulated the “injury hypothesis” and later on coined these self-constituent damage-associated molecular patterns (DAMPs), in analogy with PAMPs.28 After various conceptual changes spurred by several ground-breaking discoveries, Polly Matzinger elaborated the “danger model” (conceptually equivalent to the injury theory), which proposes that the immune system responds to conditions of “danger” rather than “foreignness,” which are driven by both self and nonself entities, irrespective of their origin.29 Notably, the nature of the danger signals exposed by damaged cells or tissues and how these endogenous entities are perceived as dangerous by the innate immune system are still conceptually debated (for a review see Ref. 20). Nowadays cell death-associated DAMPs are defined as endogenous molecules that are exposed to the extracellular milieu during the process of cell death and communicate the status of danger to the organism, thereby initiating immune responses.
Given the key role of danger signals in the context of antitumor immunity, how the innate immune system senses and decodes dying cancer cells has become a central aspect of cancer research.
In this review, we first introduce different dendritic cell (DC) subsets and their potential roles in eliciting an antitumor immune response. We then address the immunomodulatory signals—or DAMPs—delivered by cancer cells undergoing immunogenic cell death (ICD) and how they are sensed and deciphered by DCs. Finally, we discuss the key immunological consequences of this interface on DC biology and antitumor immunity. Other key cancer cell-autonomous (e.g., antigen loss, impairment in antigen presentation machinery, and expression of MHCI molecules) and tumor microenvironmental factors (e.g., tumor-supporting stroma, poor accessibility of T cells to the tumor parenchyma, immunosuppressive tumor vasculature, tumor heterogeneity, coexistence of clones with differential antigenicity, and cell death susceptibility), influence both the ability of cancer cells to undergo ICD and the elicitation of antitumor immunity in response to regulated cell death (RCD). These aspects have been the topic of recent reviews30, 31 and will not be further discussed here.
2 INTRODUCING THE PLAYERS IN THE IMMUNOGENIC CELL DEATH FIELD: DENDRITIC CELL TYPES AND SUBSETS IN ANTITUMOR IMMUNITY
The wealth of flow cytometry markers that have been used to annotate distinct DC types across different tissues or organisms has created much confusion in the field. By annotating DCs purely based on the expression of surface markers, DC subsets and DC states often become intermingled, which complicates the interpretation of findings coming from different labs. The term “DC subset” refers to cell types that ontogenetically derive from different precursors (summarized in Ref. 12). Based on this definition conventional DCs (cDCs) have been classified into two major subsets, cDC1s and cDC2s,32, 33 which develop from pre-DCs in response to a distinct set of transcription factors (see Box 1) and have their own specific function.34, 35 On the contrary, the term “DC state” refers to a specific phenotypic state of a DC, associated with a change in function or location.12 While this will also manifest with changes in surface marker expression, it does not affect the ontogeny of the antigen-presenting cell that still arises from the same precursor. Therefore, these DCs should not be regarded as distinct subsets but rather as distinct phenotypic states. As an example, mature cDCs have been annotated in the past as mRegDCs,36 LAMP3+ DCs,37, 38 or even DC3s39 (not to be confused with the recently identified cDC3 subset, see Box 1), giving the impression that mature DCs would represent a distinct subset, while they are a different cell state within the cDC1 or cDC2 subset.
But what defines DC maturation? The term DC maturation was originally conceived as “the acquisition of T-cell stimulatory capacity”, relying on the expression of CCR7 (i.e., the chemokine receptor driving DC migration to the T cell zones within the lymph node parenchyma along a CCL19/CCL21 gradient) and of co-stimulatory molecules such as CD80, CD86, or CD40 (required for the activation and induction of effector T cells).40 DC maturation was thought to be functionally coupled to the priming of adaptive immune responses. In this regard, the term DC maturation exclusively referred to immunogenic maturation, provoked by the triggering of pattern recognition receptors (PRRs) present on the DC's surface or endosomes.40 At that time, the additional role of DCs in establishing peripheral tolerance had already become apparent,41-43 but it was believed that tolerance was induced by immature DCs, to avoid the induction of autoimmunity.44, 45 This thought was inspired by the generally held belief that the uptake of apoptotic cells would not induce DC maturation, in contrast to the uptake of necrotic cells.46-49 How immature DCs would present self-antigens to T cells in the lymph node in the absence of CCR7 or co-stimulatory molecules remained a conundrum though, and several groups started suggesting that cross-tolerance might require at least some type of DC maturation.50-53
About 10 years later, transcriptional studies by the Malissen and Lawrence labs revealed that two types of DC maturation exist: immunogenic maturation induced by the triggering of PRRs and homeostatic maturation, observed in steady-state conditions.16, 54 Both maturation programs share a common set of maturation genes, and in addition possess a unique maturation signature that directs the DCs in a homeostatic or immunogenic program.16 Both maturation programs lead to the induction of CCR7 and hence allow DC migration, in line with earlier studies showing that steady-state DC migration depends on CCR7.52 In addition, both processes promote the induction of co-stimulatory molecules,50, 55 although in homeostatic mature cDCs the expression levels of co-stimulatory molecules become downregulated upon interaction with CTLA-4 expressing Tregs.56, 57 At the transcriptional level, immunogenic mature cDC1s uniquely express a Type I IFN signature, while homeostatic mature cDC1s are distinctively marked by the expression of a cholesterol biosynthesis program.16 The upstream signal driving the homeostatic maturation program remained enigmatic for a long time, but recent studies revealed that the continuous uptake of endogenous apoptotic cells is an essential step in the process of homeostatic cDC1 maturation.36, 57, 58 At least in steady state, efferocytosis-driven DC maturation appears unique to cDC1s,57, 58 in line with their specific capacity to engulf apoptotic cells.42, 59-66 In tumors, cDC2s do engulf as well67 and the engulfment of dying tumor cells has been proposed to drive both cDC1 and cDC2 maturation into a regulatory phenotype (mReg DC).36
So, what are the DC subsets and states that are most relevant for antitumor immunity?
For tumors to be properly rejected by the adaptive immune system, TAAs need to be cross-presented by DCs to naïve T cells (see Figure 1). Being equipped with a dedicated molecular machinery to engulf and stabilize tumor-derived antigens, such as the engulfment receptors CLEC9A/DNGR-168 or the small GTPase Rac2,69 cDC1s are perfectly suited for cross-presentation,70, 71 explaining their pivotal role in antitumor immune responses.13-15 Tumors with a greater cDC1 infiltrate are better controlled72, 73 and the presence of a cDC1 signature in patient tumors is associated with response to immunotherapy.74, 75 Even though several immune cell types can capture (fluorescently labeled) antigens in the tumor, the rare CD103+ cDC1 subset has been demonstrated to carry the TAAs to the tumor-draining lymph node (tdLN) and prime CD8+ T cells, possibly because of their enhanced capacity to stabilize the antigen.13, 15 Genetic deficiency of cDC1s leads to a failure in tumor rejection, both in full Batf3-deficient mice,14 as in more refined models where Xcr1-dependent cDC1s are targeted specifically.76
In contrast to the well-established role of cDC1s, the role of cDC2s in antitumor immunity remains less explored, in part due to the lack of appropriate markers that would allow the specific deletion of the cDC2 population in genetic models. cDC2s are specialized in presenting antigens to CD4 T cells (see Box 1), hence important for the induction of CD4 T-cell help and the humoral immune response. As such, they are thought to play an essential role in optimizing the activation of cytotoxic T cells and the establishment of central memory responses by providing the “license to kill” signal.19, 77-79 This has important consequences for antitumor immunity in the context of immunotherapies, as it urges the need for combining antigenic epitopes presented on MHCI and MHCII for optimal tumor vaccine design.80, 81 Alternatively, cDC2s have been suggested to reduce tumor growth by reprogramming pro-tumoral tumor-associated macrophages and reduction of myeloid-derived suppressor cells.82 In specific conditions, such as in the absence of cDC1s, cDC2s might even rescue antitumor immunity.83, 84
Finally, the role of monocyte-derived DCs within the tumor remains debated.85, 86 A role for monocyte-derived inflammatory DCs in anthracycline-induced therapy has been surmised,87 but the strategies used to deplete this population are limited due to the lack of specific markers. With the recent identification of a Ly6C+ DC population, termed cDC3,88 and the growing appreciation of mixed DC subsets in conditions of inflammation,89 the unclarities about the role of inflammatory DCs will hopefully be resolved in the near future.
3 DEFINING THE IMMUNOGENIC CELL DEATH CODE: CONSTITUTIVE AND INDUCIBLE DAMPS IN IMMUNOGENIC CELL DEATH
In multicellular organisms, cell demise is a genetically regulated mechanism driven by a specific molecular machinery. At the organism level, apoptosis represents the main form of RCD that shapes (post-)embryonic development, maintains tissue homeostasis, and removes dispensable, damaged, or infected cells (Figure 2 and Box 2). Clearance of apoptotic corpses by the engulfing phagocytes, through the process of efferocytosis, occurs well before the dying cell completely disintegrates and ensures that the intracellular content of the dying cells is not exposed to the environment thus actively preventing inflammation.103 Conversely, lytic forms of RCD, such as necroptosis and pyroptosis of ferroptosis (schematically illustrated in Figure 2 and introduced in Box 2), are hallmarked by the rapid burst of the dying cell with the consequent release of a plethora of intracellular molecules, including nucleotides, proteins including chemokines/cytokines, (oxidized) lipids, all acting as mediators of robust inflammation. In line with this, necroptosis and pyroptosis (Figure 2 and Box 2) have been regarded as manifestations of pathogen-infected cells driving persuasive inflammatory responses.
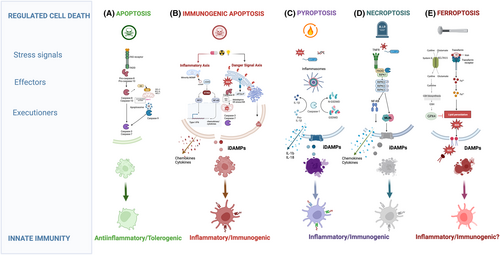
BOX 2. Main types of regulated cell death
Apoptosis
Apoptosis is a highly ordered RCD,189 which is coordinated and executed by a class of highly specific aspartate-specific cysteinyl proteases called caspases. Caspases are produced as inactive zymogens (pro-caspases) and are activated following a variety of developmental cues, stress or death signals upon the assembly of intracellular supramolecular complexes recruiting the so-called initiator caspases via interaction with adaptor proteins.132 Two main pathways lead to the activation of initiator caspases. The intrinsic apoptotic pathway is initiated by MOMP leading to the release of mitochondrial proapoptotic factors, such as cytochrome c and the second mitochondrial activator of caspases (SMAC, or DIABLO) into the cytosol.133 This causes the recruitment of pro-caspase-9 via homotypic interaction with the adaptor protein apoptotic peptidase-activating factor-1 (Apaf-1) to a cytosolic platform called the apoptosome, which results in the activation of the initiator caspase-9 and the consequent proteolytic activation of downstream effector caspase-3 and 7. This mechanism is under the control of BCL2 family members. Cellular stress-induced transcriptional and/or posttranslational modification of BH3 only proapoptotic proteins can directly bind to antiapoptotic BCL2 proteins and neutralize their function.134, 135 This event unleashes the proapoptotic pore-forming activity of BAX and BAK, resulting in MOMP. Inhibitor of apoptosis proteins (IAPs), including XIAP, counteract caspase activation following MOMP.190 The extrinsic pathway is initiated by the recruitment and activation of the prototypical initiator pro-caspase-8 at the death-inducing signaling complex (DISC) upon ligation of death ligands to death receptors belonging to the tumor necrosis factor receptor (TNFR) superfamily, including TNFR and FAS. Following homotypic interaction with FADD and DISC recruitment, pro-caspase 8 is activated by induced dimerization191 and subsequently leads to the downstream activation of the effector caspases-3/7. This mechanism is under the control of FLICE-like inhibitory protein (cFLIP). In cells where the extrinsic pathway is insufficient to drive effector caspase activation, caspase-8 cleaves the BH3-only protein BID generating tBID,192, 193 which further amplifies caspase activation by triggering BAK/BAX-mediated MOMP and the intrinsic apoptosis pathway.
Necroptosis
Necroptosis is a lytic form of cell death, induced upon engagement of different innate immune signaling pathways, in response to stimulation of TLRs, death receptors, RIG-like receptors, or Type I and II interferons (IFNs).194, 195 Necroptosis is therefore thought to be a dominant RCD in pathogen-infected cells or in injured tissues undergoing pronounced degenerative and inflammatory processes. Typically in response to TNF the serine/threonine protein kinases receptor interacting kinase 1 (RIPK1) forms a complex with the TNFR1-associated death domain (TRADD) adaptor protein and other accessory proteins. RIPK1 can regulate cell death and inflammation via kinase-dependent and kinase-independent/scaffolding functions.196 Within this complex RIPK1 can be ubiquitinated by the cellular inhibitor of apoptosis proteins (cIAPs) or linear ubiquitin chain assembly complex (LUBAC),197, 198 resulting in the I-κB kinase (IKK)-mediated activation of NF-κB and the transcriptional activation of pro-inflammatory cytokines, and pro-survival genes. Under conditions of cIAP deficiency or caspase-8 inhibition TNFR1 promotes the formation of the necrosome an amyloid-like complex driven by the interaction of RIPK1 with RIPK3 through their RIP homotypic interaction motif (RHIM).199 The necrosome then results in the RIPK3-mediated phosphorylation and activation of its substrate, the pseudokinase MLKL,200-202 which upon oligomerization and translocation to the plasma membrane leads to cell lysis accompanied by the release of DAMPs which can propagate secondary inflammation. The lytic activity of MLKL is under the regulation of the ESCRT-III complex, which can reverse membrane damage.162 The formation of the necrosome is antagonized by the recruitment of the caspase-8/cFLIP complex through the adapter FADD and RIPK1. Caspase-8 mediated cleavage of RIPK1 then curtails cell death.203, 204 In line with this model, inhibition of caspase-8 is observed in cell infected by pathogens, which cause an increase in the expression of inhibitors of this protease (i.e., vFLIPs). Induction of necroptosis by Type I or Type II IFNs, can involve a RIPK1-independent activation of RIPK3 by the IFN-inducible Z-DNA binding protein (ZBP1).205
Pyroptosis
Pyroptosis is a proinflammatory lytic form of RCD playing a critical role in the host defense against pathogens.206 This RCD is triggered by the activation of inflammatory caspase-1 and caspase-11 (or caspase 4/5 in humans) within the inflammasomes,207 large cytosolic platforms triggered by members of the nucleotide-binding domain and leucine-rich repeat (NLR) containing family in response to diverse types of intracellular insults and products of pathogens.208 In the canonical inflammasome-driven pyroptosis, recruitment of the prototype inflammatory caspase-1 to the NLRP3, a member of the NLR family, via activating adaptors, such as ASC leads to its activation. Upon activation, caspase 1 cleaves GSDMD a member of the gasdermin family of proteases, into an N-terminal and a C-terminal fragment. The cytotoxic N-terminal GSDMD fragment is recruited to the plasma membrane where it assembles in oligomers with pore-inducing function, resulting in loss of osmolarity, consequent cell lysis, and release of DAMPs.209, 210 Active caspase-1 also leads to the proteolytic conversion of pro-IL-1β and pro-IL-18, the precursors of the potent pro-inflammatory cytokines IL-1β and IL-18, into their bioactive forms. These cytokines are released in the extracellular space through GSDMD-mediated pores and further evoke inflammatory responses. In analogy to the MLKL-inducing pore formation, ESCRT-mediated membrane repair mechanisms can subvert the release of DAMPs and pro-inflammatory molecules and support cell survival.211-213 The relevance of pyroptosis as a RCD implicated in host defense against pathogens is supported by the ability of certain pathogens to prevent inflammasome activation. In humans the GSDM family comprises 6 members GSDMA, GSDMB, GSDMC, GSDMD, GSDME (also known as DFNA5), and PJVK (also known as DFNB59). With the exception of PJVK, all human GSDMs are synthesized as precursors that are kept inactive by an autoinhibitory mechanism involving the intramolecular interactions between their N-terminal and the C-terminal domains.214 Beyond the canonical inflammasome-driven pyroptosis, GSDMD and other members of the GSDM family, can be cleaved by apoptotic caspases173, 174, 215 (reviewed in Ref. 176) leading to an amplification of the apoptotic pathway by, for example, the permeabilization of the mitochondria and the release of cytochrome c.175 In cancer cell exposed to chemotherapy the activation of caspases-3 and -7 can also lead to the proteolytic activation of GSDME, resulting in pyroptosis without apoptotic morphology.171
Secondary necrosis
When cells undergoing apoptosis are not efficiently cleared, for example when the capacity of phagocytes to engulf apoptotic cargoes is overruled or in the absence of efferocytosis, they can progress into a so-called secondary necrosis. This process was thought to be an uncontrolled event occurring as a result of osmotic pressure, with a morphological trait of passive necrosis. However, over the past decades it became clear that secondary necrosis is both morphological and immunological distinct from passive or accidental necrosis, which occurs in response to sudden cellular damage that causes the immediate release of the unmodified intracellular content, and from the orderly cell lysis induced by necroptosis (reviewed in Ref. 126). There are indications that the molecular composition of the surface and secretome of secondary necrotic cells, and thus their associated DAMPs, is distinct from accidental necrosis and necroptosis (reviewed in Ref. 126). Recently, apoptosis-driven secondary necrosis has been shown to be regulated by the caspase-3-mediated cleavage of GSDME, driving the permeabilization of the plasma membrane through its pore-forming ability.216 However, apoptosis-driven secondary necrosis may not always involve GSDME as in certain settings loss of GSDME does not affect the progression to secondary cell death (reviewed in Ref. 217) suggesting that other cell death mediators can be involved.
Ferroptosis
Ferroptosis is a caspase-independent, iron-dependent form of RCD driven by the unchecked accumulation of phospholipid-peroxides in cellular membranes.218 Direct or indirect inhibition of the activity of GPX4, a GSH-dependent selenoprotein that counters the oxidation of lipids in membranes, in the presence of labile iron (Fe2+) leads to the peroxidation of polyunsaturated fatty acids (PUFAs) in phospholipid bilayers.217 PUFAs are oxidized either enzymatically, through the activity of Fe2+-dependent lipoxygenases, or via Fenton chemistry.219 Lipophilic antioxidants and iron chelators, but not caspase or RIPK1 inhibitors, block ferroptotic cell death. Recently, other GPX4-independent antioxidant mechanisms of ferroptosis control, including those dependent on the ferroptosis suppressor protein 1 (FSP1)/CoQ10220, 221 and dihydroorotate dehydrogenase (DHODH)222 among others, have been shown to suppress phospholipid peroxidation and ferroptosis.
Cross talk between regulated cell death pathways
Growing evidence indicates that the main RCD pathways described above are not part of distinct linear signaling cascades but interface extensively. Such a functional cross talk between different forms of RCD is thought to ensure that, once a cell is committed to dying, the inhibition of one mechanism cannot prevent cell death to occur, but rather triggers the switch toward a different cell death modality. This is exemplified by the caspase-8 control of the apoptosis-necroptosis-pyroptosis switch.223 However, the recently disclosed interconnections between various RCD, for example, the apoptosis-pyroptosis or necroptosis-pyroptosis cross talks, indicate that cell death pathways do not operate as binary programs but can function in synergy (for recent reviews on this topic see Ref. 164, 165).
In early 2000, several landmark studies challenged the dogmatic view that only lytic forms of cancer cell death could elicit inflammatory responses and endorse immunogenicity104, 105 (reviewed in Refs. 22, 106). In 2005 the concept of ICD was introduced to define a form of RCD induced by an assorted class of anticancer therapies, with the ability to elicit tumor antigen-specific immune responses in an immunocompetent syngeneic host, thus enabling the establishment of immunological memory.107, 108
Originally described as an immunostimulatory variant of chemotherapy-induced apoptosis of cancer cells, the effective antitumor propensity of ICD has since then been extended to lytic forms of RCD (Figure 2 and Box 2) with necroptosis and pyroptosis as most important paradigms. Nowadays, irrespective of the specific cell death program inducing it, according to the Nomenclature Committee on Cell Death, ICD is defined as a “form of RCD that is sufficient to activate an adaptive immune response in immunocompetent mice.”109 In line with this, the gold standard approach to establish whether cancer cells dying in response to a potential ICD inducer are capable of initiating adaptive immunity, requires vaccination assays in immunocompetent syngeneic hosts (see Ref. 109).
Inducers of ICD comprise an assorted compendium of conventional and targeted anticancer therapies that include but is not limited to certain chemotherapeutics such as anthracyclines (e.g., doxorubicin and mitoxantrone), DNA-damaging agents (i.e., cyclophosphamide and oxaliplatin), proteasomal inhibitors (i.e., bortezomib and carfilzomib), mitotic poisons (e.g., docetaxel), the tyrosine kinase inhibitor crizotinib, the epidermal growth factor receptor-specific monoclonal antibody cetuximab, cyclin-dependent kinase inhibitor dinaciclib, the antibiotic bleomycin, oncolytic viruses, and various physical/chemical interverventions, such as irradiation, hypericin-based photodynamic therapy (PDT), and high hydrostatic pressure.109-112
The antitumor immunity properties of ICD depend on its ability to combine the delivery of TAAs (antigenicity) with the robust emission of DAMPs (adjuvanticity). Antigenicity is conferred in malignant cells by the expression of TAAs, tumor neo-antigens, or in rare cases viral antigens, which are not covered (or not strongly covered in the case of TAAs) by central tolerance.26, 113 Adjuvanticity on the other hand has been proposed to rely on the proficient and spatiotemporally coordinated extracellular exposure or release of DAMPs.
ICD associated DAMPs include several constitutively expressed molecules fulfilling housekeeping functions, which acquire de novo immunomodulatory functions once exposed or released extracellularly upon loss of homeostasis triggered by cellular stress or death signals. These are also termed constitutive DAMPs (cDAMPs) and comprise the endoplasmic reticulum (ER) chaperones calreticulin (CRT) and ERp57, heat-shock proteins like HSP70 and HSP90, mitochondria-derived N-formylated peptides and DNA, the non-histone protein high-mobility group Box 1 (HMGB1), annexin A1 (ANXA1), ATP and other nucleotides, nucleic acids including dsRNA and dsDNA, and F-actin (reviewed in Refs. 22, 110, 111). In analogy with PAMPs, cDAMPs act by binding to a similar set of PRRs, like various members of the toll-like receptor family expressed on innate immune cells. In addition, cDAMPs can stimulate innate immune cells by binding to purinergic P2 receptors (e.g., ATP), low-density lipoprotein receptor-related protein 1 (LRP1) (i.e., CRT and HSPs), formyl peptide receptor 1 (FPR1) (i.e., ANXA1), the receptor for advanced glycation end products (RAGE) (i.e., HMGB1), and CLEC9A/DNGR1 (i.e., F-actin).22, 109 These danger signals can exert a plethora of effects, from facilitating the phagocytosis of dying cells to stimulating their activation and release of immunomodulatory cytokines. In the context of DC vaccination strategies, whereby malignant cells undergoing ICD are co-incubated in vitro with monocyte-derived DCs (see Box 1), genetic interventions, or manipulations to block the release of cDAMPs or interfere with their binding to PRRs abrogate their immunogenicity and the protection against a subsequent challenge with living cells of the same type.114-120
More recently, the term “inducible DAMPs” or iDAMPs was introduced to describe the ability of sterile dying cells to actively promote the transcription and translation of an array of inflammatory chemokines and cytokines, in a manner reminiscent of pathogen-infected cells.121 These inflammatory mediators include but are not limited to CCL2, CXCL1, CXCL10, and Type I IFNs. One of the prototypical pathways driving the expression of immunostimulatory iDAMPs is the transcription factor NF-κB, and its upstream regulator receptor interacting protein kinase (RIPK) 1122 (see Figure 2 and Box 2). However, as iDAMPs are generated by a process activated during cell death, their molecular nature and composition are strictly dependent on the stress pathways being engaged.123, 124
Together with cDAMPs, the secretion of iDAMPs by the stressed/dying cancer cells undergoing ICD attracts myeloid cells, including neutrophils, and T cells to the tumor parenchyma, and plays an essential role in sustaining immunogenicity and enabling tumor rejection in several settings.119, 123, 125 The inability of accidental necrosis (see also Box 2) to induce pronounced immunogenicity is likely explained by the absence of iDAMPs, thereby distinguishing uncontrolled cell disintegration from the transcriptionally active and regulated variants of ICD. In contrast to sudden or accidental cell lysis, secondary necrosis (see Box 2), which occurs under conditions of impaired efferocytosis downstream of the induction of apoptosis, might contribute to immunogenicity in different settings.126 However, the molecular composition of immunomodulatory signals released by cancer cell undergoing secondary necrosis involving (or not) caspase-3 mediated cleavage of gasdermin (GSDM) E (see Box 2) and the overall effects on DCs, needs to be scrutinized more systematically.
Additionally, it should be noted as well that certain DAMPs can also elicit immunosuppression. The prototype of these immunosuppressive molecules is the apoptotic “eat-me” signal phosphatidyl serine (PS), but it has become clear that apoptotic cells actively release assorted metabolites, which include signaling factors like polyamines and nucleotides127 with strong anti-inflammatory and tissue repair functions. In addition, during immunosilent apoptosis, several mechanisms actively prevent the immunostimulatory functions of DAMPs, including but not limited to the nuclear sequestration of HMGB1 due to hypoacetylation128 and the caspase-mediated inactivation of IL-33129 or of the cGAS-STING pathway.130, 131
Thus while jointly DAMPs provide a constellation of “find-me,” “eat-me,” “present-me” and maturation signals to DCs, whether all DAMPs are equally capable of inducing immunogenic DC maturation has been debated and will be discussed in more detail later.
3.1 Breaking down the dogma: Immunogenic apoptosis
Cell death by apoptosis is executed by the activation of caspases, a class of highly conserved aspartate specific cysteinyl proteases. Apoptotic caspases are activated via two molecularly well-defined and converging signaling pathways, known as the intrinsic (or mitochondrial) and extrinsic (or death receptor) pathways (schematically shown in Figure 2 and further detailed in Box 2).132-135
Challenging the dogma that cancer cell death by apoptosis is necessarily and uniquely immunosuppressive/non-immunogenic, work over the past two decades demonstrated that an assorted class of anticancer treatments or drugs inducing apoptosis, drive efficient adaptive antitumor immunity.110-112 These widely different classes of anticancer modalities, which as mentioned above, encompass among others anthracyclines, oxaliplatin, irradiation and PDT, share the ability to activate danger signaling pathways resulting in the highly ordered spatiotemporal release of DAMPs110, 111 (Figure 2). The secretion of a rich compendium of adjuvants by the stressed/dying malignant cells relies on the capacity of immunogenic stressors to avert ER homeostasis through the generation of reactive oxygen species (ROS).110 The signaling mechanisms driving the externalization or release of cDAMPs following immunogenic stressors have been molecularly characterized to a great extent and found to require the concomitant loss of ER-Ca2+ homeostasis and the surge of ROS signals functioning as apical danger signals. Congruently, in malignant cells interventions that elicit the activation of ER stress in the absence of pervasive oxidative stress, fail to drive ICD.110, 111
Cancer cells sensing an immunogenic stressor, actively (i.e., during the pre-apoptotic phase) traffic the ER luminal protein CRT, HSP70 and HSP90, to the outer leaf of the plasma membrane (PM), while concomitantly downregulating the “do not eat-me” signal CD47.22, 105 Surface-exposed CRT functions as a potent “eat-me” signal, driving the engulfment of apoptotic corpses by DCs or other myeloid cells, through the interaction with LRP1 (also known as CD91) on their surface.136-139
In cancer cells undergoing immunogenic chemotherapy, ATP efflux is carried out by a lysosomal, LAMP1-mediated translocation to the PM and requires the opening of pannexin 1 channels,140 whereas after ER-photodamage, ATP is trafficked along with CRT via the secretory pathway.136 Once exported extracellularly, ATP is thought to stimulate the chemotaxis of myeloid cells by binding to ionotropic P2RY2 purinergic receptors and to promote NLRP3/ASC/caspase-1 inflammasome-mediated IL-1β release by binding to P2RX7 receptors on monocyte-derived DCs.115 More recently, oxidized lipids were added to the list of DAMPs that can trigger inflammasome activity in DCs, and shown to be required for the induction of memory T cells and long-term antitumor immunity.125 ICD-associated DAMPs further encompass the emission of the cytosolic calcium- and phospholipid-binding protein ANXA1116 and HMGB1116, 141 from dying cancer cells. While the mechanisms underlying their release in response to an ICD inducer remain elusive, both cDAMPs exert robust adjuvant effects once interacting with cognate receptors on DC-like cells. The binding of ANXA1 to FPR1 facilitates the interaction between dying cancer cells and monocyte-derived DCs116 whereas the interaction of HMGB1 with RAGE or with TLR4 favors maturation and antigen processing in mo-DCs.117 In addition, HMGB1 can bind extracellular DNA released from tumor cells thereby facilitating its endocytosis by DCs.142, 143 Bona fide inducers of immunogenic apoptosis such as mitoxantrone and hypericin-PDT (but not the poorly immunogenic cisplatin) elicit the transcription and de novo translation of a common subset of chemokines targets of the NF-κB and AP1 pathways.123 Collectively, these iDAMPs drive the antitumor vaccine potential of chemotherapy.123 Moreover, during ICD induced by chemotherapy, cancer cell-derived dsRNA and the triggering of endosomal TLR3, stimulate the production of Type I IFNs by malignant cells further sustaining immunogenicity.119
It is interesting to note that danger signals induced by immunogenic stressors involve common pathways activated by loss of proteostasis110, 144 that are also evoked by pathogens in infected cells. The ER is naturally designed to swiftly communicate with the extracellular environment and thus the key role of ER stress in conveying the status of danger to the innate immune system is particularly relevant. Consistent with this, infection of cancer cells with Salmonella results in oxidative stress-induced unfolded protein response (UPR), the major pathway elicited following the loss of ER homeostasis, and in the release of immunogenic peptides generated by the tumor proteasome with antitumor immunity promoting effects.145 The mechanism underpinning the surface relocation of CRT during ICD has been shown to require the UPR sensor eukaryotic translation initiation factor 2 alpha kinase 3 (EIF2AK3, best known as PERK) and its direct downstream target, the eukaryotic translation initiation factor 2 subunit alpha (eIF2α).105, 136, 146, 147 PERK coordinates the ER-to-Golgi transport and SNARE-mediated exocytosis of CRT to the PM, through a pathway incited by loss of ER-Ca2+ homeostasis and ROS.136, 147 Why PERK among the members of the UPR provides the dominant signal for the relocation of CRT to the PM, is still unclear. In response to the depletion of the ER-Ca2+ store, PERK operates as an apical Ca2+ sensor that orchestrates the remodeling of the actin-cytoskeleton, to facilitate the formation of ER-PM contact sites and Ca2+ entry,148 which may support ER-to-Golgi trafficking and vesicle exocytosis. Of note, an inhibitor often used in the field to block PERK, GSK2606414 and GSK2656157, potently inhibits RIPK1 activity as well, warranting against the use of this inhibitor as sole tool to make firm statements on the role of PERK in ICD.149
Emission of CRT on the surface of cells undergoing ER stress triggered by ICD-inducing chemotherapy or by viruses, also provides a ligand for NKp46 on natural killer (NK) cells resulting in the stimulation of NK-mediated immunosurveillance,150 which highlights the “viral mimicry” ability of this sterile variant of RCD. In line with this, viruses and other pathogens have evolved mechanisms to subvert ER stress and the trafficking process underpinning the release of DAMPs.106
A central and still open question in ICD is the role of caspases. Chemotherapy-induced ICD relies on sublethal activation of caspase-8, which is dispensable for cell death while being required to mobilize CRT to the plasma membrane.151 On the other hand, caspases have long been known for their ability to dampen inflammation in the context of both intrinsic and extrinsic apoptosis, and it is, therefore, difficult to reconcile these data in one paradigm.152 For example, caspase-induced inhibition of the STING-Type I IFN and NF-κB pathways is mediated by the cleavage of various components of the cGAS-STING pathway, including cGAS itself.130, 131 Consistently, blocking caspase activity following mitochondria outer membrane permeabilization (MOMP), the central mechanism of intrinsic apoptosis (see Box 2), stimulates Type I IFN response and NF-κB activation thus promoting immune responses, without ultimately preventing killing. Notably, inflammation can also ensue under conditions of so-called minority MOMP, which is insufficient to fully activate caspase signaling.153-155 Whether the strength of (MOMP-induced) caspase signaling ultimately determines its contextual role in regulating inflammation during ICD remains speculative. Furthermore, caspase-8 has a scaffolding function driving the assembly of a complex together with Fas-associated death domain (FADD) and RIPK1 ripoptosome, which promotes NF-κB activation and chemokine production after ER stress-mediated TRAIL stimulation.151 Thus it is possible yet still speculative, that at least in certain settings, caspase-8 regulates ICD by supporting inflammation through its scaffolding functions. Notwithstanding, it seems that ICD-mediated inflammation can be uncoupled from caspase-mediated cell death.123
3.2 Immunogenic necroptosis and pyroptosis driven by inflammation
Necroptosis and pyroptosis are lytic forms of RCDs (schematically shown in Figure 2 and introduced in Box 2) sharing similarities in their mechanisms of execution and in their ability to release a wide range of danger signals. Both RCDs are also prominently inflammatory, suggesting that the immunogenicity of these lytic cell death modalities is supported by the release of chemokines and cytokines.
Necroptosis is typically induced by innate immune signaling pathways triggered by death receptors, and primarily TNFR1, TLRs, and various nucleic acid sensors (Figure 2 and Box 2) (reviewed in Refs. 156, 157). Necroptosis is induced by the RIPK3-mediated phosphorylation of the mixed lineage kinase domain-like pseudokinase (MLKL), upon the formation of the necroptosis initiating the RIPK1-RIPK3 complex called the necrosome. MLKL permeabilization of the cell membrane results in the leakage of the cellular contents and the release of a plethora of danger signals, inflammatory cytokines, and chemokines (Figure 2 and Box 2). Necroptotic DAMPs comprise polymerized actin, self-nucleic acids, the proinflammatory cytokine IL-33, which upon passive release binds to ST2 (IL1-R4158), HMGB1, ATP, and in certain immunogenic chemotherapy settings also surface exposed CRT.159, 160 Of note, recent data using a FRET-based biosensor for necroptosis, suggest that the release of HMGB1 and IL-33 follows different kinetics,161 possibly implicating distinct MLKL-mediated mechanisms for their plasma membrane release. Furthermore, MLKL-mediated cell lysis is under the control of the endosomal sorting complexes required for transport (ESCRT) and repair mechanism,162 thus exerting an additional regulatory checkpoint in the immunogenic and inflammatory output of necroptosis.
Necroptosis (but not accidental necrosis) procured by the genetic induction of FADD dimerization combined with inducible expression of RIPK3, promoted antitumor immunity in vaccination settings. This genetically induced necroptotic pathway elicited the release of HMGB1, ATP and CXCL1, in the absence of ER stress and NF-κB-induced inflammation.159 Using a similar dimerizable system to induce necroptosis, the release of cDAMPs was found per se insufficient to drive cross-priming of CD8+ T cells and antitumor immunity.122 Activation of NF-κB-mediated inflammation downstream of RIPK1 was required to unleash the immunogenic potential of necroptotic cancer cells,122 while uncoupling of NF-κB driven inflammatory iDAMPs from cell death compromised antitumor immunity. In line with this, RIPK1-mediated ICD and concomitant NF-κB activation promoted tumor rejection and abscopal antitumor immunity following locoregional therapy for soft tissue sarcoma with melphalan in combination with TNF and SMAC mimetics.163
In certain settings, RIPK1 and RIPK3 can also promote inflammasome activation,164, 165 and the subsequent release of the potent mediators of inflammation IL-1β and IL-18. In fact, both types of lytic RCD, necroptosis and pyroptosis (see Box 2) harbor the potential to evoke massive cytokine release (cytokine release syndrome), typical of infection diseases. This exacerbated cytokine release can be detrimental to tumor growth control as it can favor pro-tumorigenic chronic inflammation166, 167 and dampen the therapeutic effects of cancer (immune)therapies.
Pyroptosis is implicated in the removal of pathogen infected cells and is driven by the activation of a class of pore-forming molecules called GSDMs (further described in Box 2). The main molecular driver of pyroptosis is the pore-forming fragment of GSDMD following inflammasome-driven activation of caspase-1, in what is known as the canonical pyroptosis.168 In a manner reminiscent of necroptosis, the NTD of GSDMD causes plasma membrane permeabilization with consequent loss of osmolarity and cellular rupture.169 This results in the emission of DAMPs, and in the release of the mature cytokines IL-1β and IL-18 upon processing of their pro-form by the active caspase-1 (see further Box 2). The pyroptotic cytokine IL-18 can directly support both innate and adaptive lymphoid immunity by favoring NK priming and stimulating IFN-γ production by CD4+ type 1 T helper (Th1) cells, while IL-1β sustains T-cell expansion and Th17 differentiation among other effects.158, 165, 166 However, both cytokines have a broad spectrum of activities158, 166 and cellular targets nurturing chronic inflammation. This likely explains why pyroptosis can in certain contexts supports tumorigenesis.170
Some chemotherapy drugs and targeted therapies can induce pyroptosis in cancer cells without the activation of the inflammasome, through the caspase-3-mediated cleavage of GSDME.171, 172 The caspase-GSDMs axis is however not restricted to caspase-3 as depending on the initiating cellular stress, caspase-8 activation can process GSDMD and GSDME, resulting in pyroptosis.173, 174 GSDME is also activated in cells undergoing secondary necrosis (see Box 2) suggesting that when the phagocytic clearance of apoptotic cells is impaired, GSDME-mediated cell lysis could ensue. Of note, beyond their pyroptotic activity, both GSDME and inflammasome-induced GSDMD can permeabilize mitochondria, leading to the release of cytochrome c and caspase-mediated apoptosis.175 Other GSDM family members, including GSDMA/B/C, can crosstalk with caspase signaling and the apoptotic machinery.176, 177 In hypoxic cancer cells the nuclear pool of the immune checkpoint programmed cell death ligand (PD-L1) switches TNF-induced apoptosis to pyroptosis, by eliciting the expression of GSDMC and its further processing by caspase-8.178 Interestingly, this caspase-8-GSDMC-pyroptosis axis driven by the nonimmune function of PD-L1 in hypoxic cancer cells is also stimulated by chemotherapies such as doxorubicin, a known ICD inducer.178
Together these findings illustrate how crosstalk between different RCD, which is potentially induced by anticancer treatments in vivo, can convert noninflammatory caspases and apoptosis into proinflammatory pyroptosis and vice versa. Further studies are required to fully understand the molecular mediators and the inflammatory and immunological impact of the crosstalk among RCD pathways in anticancer treatment.
3.3 The many unknowns of ferroptosis in immunogenic cell death
Ferroptosis is a non-apoptotic, iron-mediated cell death driven by lipid-peroxidation following impairment of main antioxidant systems179 (schematically shown in Figure 2 and Box 2). Over the past decade, research in ferroptosis has gained momentum in anticancer therapy because of its ability to overcome drug resistance and favor killing of persistent cancer cells, which are ultimately responsible of tumor relapse.180, 181
Ferroptotic cells are capable of emitting key DAMPs, linked to cell lysis such as ATP and HMGB1, and to secrete inflammatory and immunomodulatory cytokines and chemokines.182, 183 However, whether this lytic form of RCD is immunogenic remains controversial and is still under scrutiny. In one study ferroptotic cancer cells were shown to induce maturation of bone marrow-derived DCs and when used in a prophylactic tumor vaccination model protected mice against a rechallenge.184 In another study, using an inducible glutathione peroxidase 4 (GPX4) knockdown model and comparing early versus terminal stages of ferroptosis, only late ferroptotic cancer cells demonstrated the ability to induce maturation of bone marrow-derived DCs.185 Under these settings, cancer cells initiating the ferroptosis process and exhibiting pronounced intracellular lipid peroxidation, failed to be engulfed and recognized by DCs. Furthermore, cancer cells undergoing ferroptosis were unable to mount antitumor responses in settings of therapeutic DC vaccination and even repressed the immunogenicity of bona fide chemotherapy-induced ICD.185 While the reasons for this discrepancy are still elusive, immunosuppressive factors released by cells undergoing ferroptosis, including the COX-2 product prostaglandin E2 and various oxidized lipid species, could impair a proficient interface between dying cells and antigen presenting cells (APCs). However, given that recent reports indicate that induction of ferroptosis can potentiate immunotherapy regimens,186-188 the immunogenic potential of ferroptosis strategies in cancer therapy warrants further studies.
4 CONNECTING THE DOTS: HOW DENDRITIC CELLS SENSE AND DECODE IMMUNOGENIC CELL DEATH
While DAMPs are currently defined as adjuvants stimulating the establishment of immunological memory, an increasing number of molecules are assigned as DAMPs based on their ability to activate innate immune receptors and to trigger pro-inflammatory signaling cascades in innate immune cells, irrespective of their subsequent effects on the adaptive immune response.224, 225 This confusion largely stems from the fact that in many studies, immunogenicity is judged by the induction of co-stimulatory molecule expression on DCs, often performed by coincubation of dying tumor cells in vitro with monocyte-derived GM-CSF DCs. However, as mentioned before, the induction of co-stimulatory molecule expression on DCs does not necessarily reflect their immunogenic maturation, and can as well lead to cross-tolerance.50 Furthermore, as MCs represent a heterogenous population consisting of mostly monocytes/macrophages98 (see Box 1) expressing a different set of PRRs, these APCs might react in a different way to the exposure of DAMPs than cDCs do in in vivo settings. Some of the proposed DAMP receptors such as TLR4 for HMGB1, or P2RX7 for ATP are lowly expressed in cDC1s, both in steady state and in tumor context (see ImmGen Databrowser226 and the publicly available database227). Furthermore, in two recent studies genetic deficiency of TLR4, MyD88, TRIF, MAVS, or P2RX7 in cDCs had no impact on the antitumor immune response in spontaneous or radiation-induced tumor control,228-230 which is inconsistent with earlier observations in DC vaccination studies.114, 117 While this discrepancy might be explained by differences in treatment, tumor models and/or in the DC subtype involved, it urges for studies reconciling the discrepancies in DC vaccination models versus in vivo tumor models relying on endogenous DC populations.
Other studies also questioned the adjuvanticity of cDAMPs. As mentioned before, depending on the type of RCD mediating ICD, the mere release of well-established cDAMPs is often not sufficient to induce an adaptive immune response, in the absence of the concomitant expression of NF-κB-driven iDAMPs by the dying cell.122, 123 Along these lines, despite the initial enthusiasm for DNGR1 (CLEC9A), as a cDC1-specific DAMP receptor for F-actin exposed by dying cells,68, 231, 232 more recent studies revealed that DNGR1 was dispensable for the dead cell-induced expression of co-stimulatory molecules and cytokines in DCs.233 On the contrary, DNGR1 was needed to divert the dead cell cargo into a non-degradative recycling endosome and to assist in phagosomal rupture enabling cross-presentation,233, 234 indicating a role for DNGR1 in antigenicity rather than adjuvanticity. In addition to the well-known find-me and eat-me signals, these studies proposed the term “present-me” signal to describe the role of DNGR1 in this context.225 Similarly, both the de novo surface translocated CRT and HSPs136, 137 can assist TAAs transfer to DCs, again suggesting that certain cell death associated cDAMPs can also contribute to the antigenicity.
So, what is needed from a DC perspective to trigger an adaptive immune response?
Emerging data point toward a critical role for Type I IFN and the activation of cGAS-STING pathways in DCs as a bridge between innate immune sensing of stressed/dying cancer cells and the activation of TAAs-specific T-cell-mediated antitumor immunity230 (Figure 3). Early studies already demonstrated the essential role for the production of Type I IFN in host DCs in response to tumor cells for cross-presentation and the generation of TAAs-specific T cells.9, 10 This was confirmed unequivocally in more recent studies demonstrating the critical contribution of the cGAS-STING pathway in DCs for tumor control in both spontaneous model of tumorigenesis and in response to irradiation.228, 229 In humans, the importance of a Type I IFN response in DCs is further supported by several patient studies that highlighted the predictive power of a Type I IFN transcriptional signature present in tumors or myeloid cells toward positive clinical responses.119, 235, 236 The upstream trigger of the cGAS-STING pathway in DCs remains elusive at this point, but several studies hint toward the role of cancer cell dead-derived DNA, either from genomic or mitochondrial origin.143, 237 Different danger signaling might be important for the release of tumor cell-derived DNA and/or enable its uptake. The adjuvant effect of alum, a compound of nonmicrobial origin, has been explained by its ability to kill cells at the site, leading to the release of DNA.238 Bernard and collaborators, showed that oxidized self-DNA released from malignant cells during UV exposure is sufficient to trigger the activation of cGAS-STING,239 potentially linking the activation of ROS to the generation of iDAMPs. Interestingly, two studies indicated an essential role for HMGB1 in the binding and subsequent endocytosis of extracellular DNA released from dying tumor cells by DCs.117, 240 TIM3, which is highly expressed in tumor infiltrating CD103+ cDC1s, interferes with this process providing the rationale for anti-TIM3 therapy in combination with paclitaxel.143, 240 Activation of the cGAS-STING pathway in DCs is linked to the induction of chemokines such as CXCL9 and CXCL10, together with other Type I IFN response genes, which mediate recruitment of effector T cells to the tumor bed.73, 241, 242
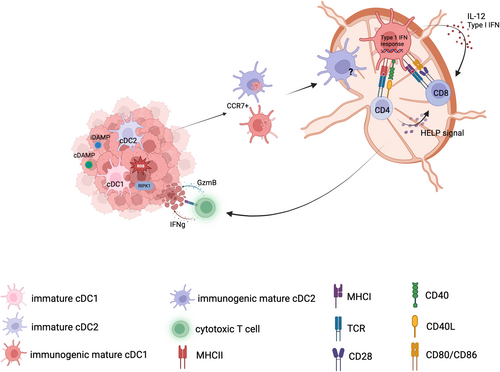
Of note, transcriptional profiling revealed that the presence of a Type I IFN signature in mature DCs is a hallmark of their immunogenic maturation program.16, 57 In homeostatic conditions, a transient type I IFN signature can be observed in late immature cDC1s, immediately after uptake of apoptotic cells, which is STING-dependent as well, but it is turned off in an LXR-dependent manner before the cDC1s start to gain CCR7 expression.57
So, the question circles back as to why tumor-derived DNA, released from cells dying from chemotherapy or irradiation, would be recognized as dangerous and stimulate an immunogenic response in DCs, while the DNA released from spontaneously dying cells does not. Likely, the combination of DNA together with released cDAMPs such as HMGB1 or de novo-generated iDAMPs, might create an immunostimulatory complex.142, 143 Alternatively, immunogenic anticancer procedures generating oxidative stress, including but not limited to radiotherapy or photodynamic therapy might modify the DNA in such a way that it becomes immunogenic.239 Chemotherapeutics such as doxorubicin are known to induce DNA and chromatin damage, which could release certain danger signals. Along these lines, recent data suggest that doxorubicin could cause histone eviction,243 a signal that might be recognized by the engulfing cell.244 Finally, a role for endogenous retroviral elements cannot be excluded.245
Most likely, no single agent will on itself be sufficient to break tolerance and several key-lock systems will contribute to ensure proper recognition of the dying cell by DCs and coordinate essential immune cell interactions to promote an optimal immune answer81, 246, 247 (Figure 3).
5 CONCLUSIONS AND OUTSTANDING QUESTIONS
Despite the remarkable advances in DC vaccination strategies based on the concept of ICD in preclinical mouse models, this success has not been translated to the clinic yet, and most vaccines developed so far demonstrated only limited efficacy in late-stage clinical trials.248, 249 This might indicate that we still do not understand all components involved in the process of ICD. Discrepancies observed in models based on DC vaccination strategies versus models relying on spontaneous antitumor immunity might be explained by different types of DCs that are engaged and/or a different spectrum of danger receptors that they express. Future research needs to address these discrepancies to understand the mode of action of ICD-based therapies and how we can harness them to improve current anticancer regimens. Emerging data highlight the essential role of cGAS-STING and Type I IFN signaling pathways in DCs for proper tumor control, hinting toward a potential role for tumor-derived DNA to be recognized as danger signal. How DAMPs mediate adjuvanticity in this context is still speculative, but might be linked to their ability to facilitate endocytosis and/or endosomal release of tumor-derived DNA in DCs. Considering the immense power of ICD to signal danger to our immune system, understanding how various variants of ICD are being decoded by DCs remains of utmost importance for the future of tumor therapy.
Outstanding questions
- Are all types of stress-induced RCD equally suitable to promote DC maturation?
- How are immunogenic dying cells being recognized by DCs?
- Why is tumor-derived DNA different from apoptotic cell-derived DNA? And how does tumor DNA reach the cytosol of engulfing DCs?
- What is the role of inflammatory monocyte-derived DCs and cDC2s in the antitumor immune response?
- How do the inflammatory and immunogenic output of cells dying through connected programs, which is likely to reflect the in vivo situation, affect adjuvanticity and the interface with DCs?
- Why do DC vaccination studies have only limited success in the clinic?
- Which ICD program can be harnessed to design more efficacious anticancer DC-vaccines?
ACKNOWLEDGMENTS
P.A. and S.J are supported by the EOS DECODE consortium No. 30837538. P.A. is supported by grants from the Flemish Research Foundation (FWO- Vlaanderen; G0A3320N), the Stichting tegen Kanker (F/2022/2037), the KU Leuven C14/21/095 InterAction consortium, the EOS MetaNiche consortium No. 40007532, the iBOF/21/053 ATLANTIS network. SR is supported by a BOF grant (University of Ghent, 01D02419). S.J. is supported by grants from the Flemish Research Foundation (FWO- Vlaanderen; G017521N, G050622N) and ERC Co-G DC-RIDDLE (CoG 819314). The authors would like to apologize for not citing the work of other colleagues due to space limitations.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.