Ferroptosis in immunostimulation and immunosuppression
This article is part of a series of reviews covering Mechanisms of programmed cell death appearing in Volume 321 of Immunological Reviews.
Summary
Ferroptosis is a form of iron-dependent regulated cell death characterized by the accumulation of toxic lipid peroxides, particularly in the plasma membrane, leading to lytic cell death. While it plays a crucial role in maintaining the overall health and proper functioning of multicellular organisms, it can also contribute to tissue damage and pathological conditions. Although ferroptotic damage is generally recognized as an immunostimulatory process associated with the release of damage-associated molecular patterns (DAMPs), the occurrence of ferroptosis in immune cells or the release of immunosuppressive molecules can result in immune tolerance. Consequently, there is ongoing exploration of targeting the upstream signals or the machinery of ferroptosis to therapeutically enhance or inhibit the immune response. In addition to introducing the core molecular mechanisms of ferroptosis, we will focus on the immune characteristics of ferroptosis in pathological conditions, particularly in the context of infection, sterile inflammation, and tumor immunity.
1 INTRODUCTION
Cell death is a natural and essential part of the cellular life cycle, playing a fundamental role in the development, maintenance, and overall health of multicellular organisms. Cells can die through different molecular mechanisms that are classified into accidental cell death (ACD) and regulated cell death (RCD).1 Unlike ACD, RCD is a controlled process with multiple subroutines that are time-dependent and ultimately halt cell functions.2 In 1972, pathologists observed a specific type of cell death in human or rat tissues characterized by condensed and fragmented cells and nuclei.3 They named this cell death process “apoptosis.” As research in cell death progresses, the focus has expanded from the very well-characterized process of apoptosis to include an increasing number of non-apoptotic cell death modalities.
Among these modalities, iron-dependent and non-apoptotic ferroptosis have garnered significant attention in translational medicine.4 The concept of ferroptosis was coined in 2012 during the screening of small molecules that selectively induced cell death in cancer cell lines (HT1080 and Calu1) harboring oncogenic RAS mutations.5 We now know that ferroptosis can also occur in normal cells or tissues, independent of mutant RAS activation, and its induction is involved in the disruption of oxidative and antioxidant balance.6 It is noteworthy that the core steps of ferroptosis, including glutathione depletion, glutathione peroxidase 4 (GPX4) inactivation, and lipid peroxidation, closely resemble those of oxytosis.7 Oxytosis, initially described in a neuronal cell line (N18-RE-105) during the late 1980s, represents a non-excitotoxic pathway for cell death induced by glutamate.8
In the immune system, cell death plays a pivotal role in the elimination of damaged, infected, or abnormal cells. RCD allows the removal of cells that pose a threat to the overall health and function of the organism. Proper regulation of cell death is crucial for maintaining immune tolerance and homeostasis.9 Dysregulation of RCD pathways, including ferroptosis, can lead to immune dysfunctions, such as excessive inflammation, autoimmune diseases, or immunodeficiency.10 Understanding the delicate balance between cell death and immune tolerance is vital for preserving immune homeostasis and preventing immune-related disorders.11
In this narrative review, we aim to provide an overview of the basic principles of ferroptosis while delving into the dual role of ferroptosis in both immunostimulation and immunosuppression. By exploring the intricate pathways associated with ferroptosis,12 we provide new insights into disease pathogenesis and identify potential therapeutic targets. The interplay between ferroptosis and immune responses adds another layer of complexity to our understanding of the immune system and its implication in various diseases.
2 MOLECULAR MACHINERY: KEY HALLMARKS OF FERROPTOSIS
Ferroptosis can occur through two major pathways: the extrinsic or transporter-mediated pathway that can be activated by the inhibition solute carrier family 7 member 11 (SLC7A11), and the intrinsic or enzyme-related pathway that results from the inhibition of GPX4 (Figure 1).13 The core molecular machinery and signaling regulation have primarily been identified in cancer cells using small molecular compounds like erastin and RSL3, which inhibit SLC7A11 and GPX4, respectively.5, 14 SLC7A11 is the main functional component of the amino acid transporter system xc− located on the cell membrane. This transporter plays a crucial role in the transport of cystine (a sulfur-containing amino acid) and glutamate (an important neurotransmitter in the central nervous system). The transporter exchanges intracellular glutamate with extracellular cystine in a one-to-one stoichiometry. This exchange is vital for maintaining cellular redox balance and providing cysteine, a precursor for the synthesis of glutathione, an essential antioxidant that acts as a cofactor for GPX4.15 GPX4 stands out among glutathione peroxidases due to its distinctive ability to directly reduce and detoxify lipid hydroperoxides.16 These lipid hydroperoxides are generated through the oxidation of polyunsaturated fatty acids (PUFA), which are fatty acids containing two or more double bonds between carbon atoms, primarily found in cell membranes.17 This property renders GPX4 particularly important in protecting cells from oxidative stress-induced lipid peroxidation, which can lead to cell damage, inflammation, and various diseases.15
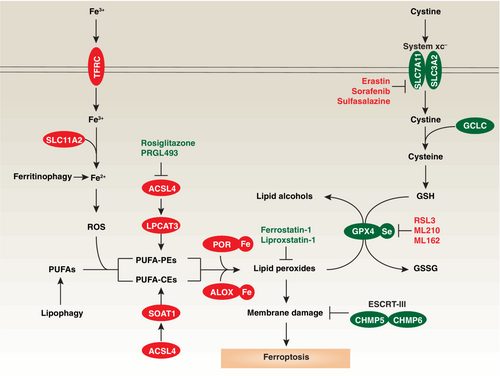
Unlike other classical cell death inducers that typically activate executors of cell death, the initiation of ferroptosis primarily occurs through blocking a cell death-inhibitory system involving antioxidant mechanisms. In addition to erastin and RSL3, several drugs (e.g., sorafenib and sulfasalazine) or yet-to-be developed compounds (e.g., ML210 and ML162) have been found to inhibit SLC7A11 or GPX4 activity, thus inducing lipid peroxidation and triggering ferroptosis (Figure 1). GPX4 is ubiquitously expressed in various tissues. It can be found in the cytosol, mitochondria, and nucleus.18 However, current evidence indicates that its primary role is to inhibit ferroptosis specifically within the cytosol.14 Other antioxidant enzymes, such as apoptosis-inducing factor mitochondria-associated 2 (AIFM2; also known as FSP1),19 dihydroorotate dehydrogenase (DHODH),20 GTP cyclohydrolase 1 (GCH1),21 and microsomal glutathione S-transferase 1 (MGST1),22 play GPX4-dependent or -independent roles in inhibiting ferroptosis in cancer or non-cancer cells. These observations highlight the importance of understanding how different antioxidant systems collaborate to form an integrated defense against ferroptotic damage. Notably, NFE2 like BZIP transcription factor 2 (NFE2L2; also known as NRF2), a nuclear transcription factor activated in response to oxidative stress, plays a central role in transactivating anti-ferroptotic genes,23 indicating the existence of a transcriptional machinery that shapes the response to ferroptosis.
Another distinguishing characteristic of ferroptosis, compared to other forms of cell death, is that the effector molecules of ferroptosis appear to be toxic lipids rather than proteins.24, 25 In apoptosis, necroptosis, and pyroptosis, caspases, mixed-lineage kinase domain-like pseudokinase (MLKL), and gasdermin D (GSDMD), respectively, serve as lethal effector molecules due to their ability to cleave proteins or to form pores in the cell membrane.2 However, these traditional cell death mediators are not required for ferroptosis.5 Instead, the accumulation of lipid peroxidation products, such as 4-hydroxynonenal (4HNE) and malondialdehyde (MDA), can subvert cell membrane structure and function, ultimately leading to membrane rupture and cell death.26, 27 Ferrostatin-1 and liproxstatin-1 are widely used ferroptosis inhibitors due to their ability to act as radical-trapping antioxidants, thereby slowing down the accumulation of lipid hydroperoxides.28
The activation of lipid peroxidation in ferroptosis involves specific enzymes, such as those from the lipoxygenase (ALOX) family and cytochrome P450 oxidoreductase (POR). Interestingly, ALOX and POR play independent roles in mediating lipid peroxidation for ferroptosis.29, 30 Although the threshold to distinguish physiological and pathological concentrations of 4HNE or MDA remains unclear, the loss of membrane repair machinery, such as endosomal sorting complexes required for transport (ESCRT)-III, can impair membrane fluidity, accelerate cell edema, and ultimately result in ferroptotic cell death.31, 32 Thus, genetic depletion of the core components of the membrane repair machinery, such as charged multivesicular body protein 5 (CHMP5) and charged multivesicular body protein 6 (CHMP6), can enhance ferroptosis-mediated tumor suppression in human cancer cells (PANC1 and HepG2).31
The sensitivity of cells to ferroptosis induction can be influenced at multiple levels, with various steps of iron or lipid metabolism controlling the levels of ferroptosis. Excessive iron not only produces reactive oxygen species (ROS) through the Fenton reaction but also activates iron-dependent lipid peroxidation enzymes, such as ALOXs or POR.33 These processes collectively enhance the sensitivity to ferroptosis. Similarly, the accumulation of lipids, especially PUFAs primarily localized in cell membranes, provides targets for lipid peroxidation under oxidative stress. This process is driven by the acyl-CoA synthetase long chain family member 4 (ACSL4)-lysophosphatidylcholine acyltransferase 3 (LPCAT3) pathway, where free PUFAs are ligated with coenzyme A (CoA) to produce PUFA-CoAs for subsequent incorporation into phosphatidylethanolamines (PEs).34, 35 In contrast, activation of the ACSL4-sterol O-acyltransferase 1 (SOAT1) pathway produces PUFA-cholesteryl esters (CEs) instead of PUFA-PEs, contributing to lipid peroxidation in solute carrier family 47 member 1 (SLC47A1)-deficient human pancreatic cancer cells.36 Thus, the lipid flippase SLC47A1 on the cell membrane may serve as a metabolic checkpoint influencing the induction of various ferroptosis pathways. It is worth noting that ACSL4-independent ferroptosis has been reported in certain cases, such as photodynamic therapy in human HeLa cancer cells.37 In addition, ACSL3-dependent monounsaturated fatty acid (MUFA) production completely inhibits PUFA-dependent ferroptosis in HT1080 cells,38 suggesting that different lipid species may have antagonistic effects.
In addition to direct enzymatic processes, iron or lipid metabolism can be regulated through degradation pathways. Specifically, the activation of selective autophagy, such as ferritinophagy39 or lipophagy,40 promotes the degradation of ferritin (an iron storage protein) in pancreatic cancer cells and mouse embryonic fibroblasts, as well as the catabolism of lipid droplets in liver cancer cells. Lipid droplets are cellular organelles that store lipids, primarily neutral lipids like triglycerides and cholesterol esters. During this degradation process, free iron and lipids are released, making them available for subsequent lipid peroxidation. These findings support the notion that ferroptosis is an autophagy-dependent cell death mechanism.41 However, autophagy can also inhibit ferroptosis in specific contexts, such as asthma.42 Gaining a deeper understanding of the heterogeneity and plasticity of the ferroptosis machinery is crucial for identifying pathways that may be targeted for disease modulation in specific circumstances.
3 IMMUNE CONSEQUENCE: DUAL ROLE OF FERROPTOSIS
In many cases, ferroptosis exerts immunostimulatory effects, triggering immune responses and promoting inflammation (Figure 2). However, it is important to acknowledge that the occurrence of ferroptosis in immune cells can also lead to immunosuppression (Figure 3). The immunostimulatory or immunosuppression effects of ferroptosis may have both beneficial and detrimental consequences depending on context. In certain situations, ferroptosis can contribute to immune defense against pathogens or tumor cells, while in other instances, it may promote chronic inflammation, tissue damage, or autoimmune responses. In this section, our focus is to explore how ferroptotic stress influences sterile inflammation, infection by pathogens, and tumor immunosurveillance.
3.1 Sterile inflammation
Sterile inflammation is triggered by endogenous molecules released from damaged or stressed cells rather than by invading pathogens.43 This type of inflammation can arise from various factors, including tissue injury, trauma, autoimmune reactions, metabolic dysregulation, or exposure to certain environmental agents.
Studies using conditional Gpx4−/− mice, which lack the antioxidant enzyme GPX4, have provided valuable insights into the involvement of ferroptosis in sterile inflammatory diseases. For instance, the specific loss of Gpx4 in the pancreas,44 kidney,45 liver,46 or intestine47 from mice has been demonstrated to induce or increase susceptibility to experimental pancreatitis, acute renal failure, liver necrosis, and Crohn's disease, respectively. Pancreatitis is an inflammatory disease characterized by the premature activation of digestive enzymes, such as trypsin. Loss of Gpx4 leads to increased sensitivity of pancreatic acinar cells to ferroptosis due to proteasome 26S subunit ubiquitin receptor, non-ATPase 4 (PSMD4)-dependent GPX4 protein degradation.44 Oxi-lipidomics analysis of Gpx4−/− kidneys reveals complex lipid oxidation patterns in phosphatidylcholine, PE, as well as in cardiolipin, a mitochondria-specific phospholipid.45 In Gpx4−/− livers, there is an upregulation of NFE2L2-targeted antioxidant genes as a defense mechanism.46 Additionally, Gpx4-deficient small intestinal epithelial cells exhibit an inflammatory response upon exposure to PUFAs, resulting in the production of interleukin 6 (IL6) and C-X-C motif chemokine ligand 1 (CXCL1), thereby mediating Crohn's disease.47 Furthermore, mice with Gpx4 haploinsufficiency in neutrophils develop spontaneous lupus-like disease characterized by the presence of autoantibodies, neutropenia, skin lesions, and proteinuria.48 These pathological processes observed in Gpx4-deficient mice can be successfully inhibited by ferroptosis inhibitors, such as ferrostatin-1, liproxstatin-1, or vitamin E.
Additionally, several studies have demonstrated that ferrostatin-1 or liproxstatin-1 can protect against tissue ischemia–reperfusion injury, which occurs when blood supply to an organ or tissue is initially restricted and subsequently restored.49-53 Pharmacological inhibition of ferroptosis confers protection against experimental diabetes54 and non-alcoholic fatty liver disease,55 the most common manifestation of metabolic liver disease. These findings further underscore the broad role of ferroptotic damage in sterile inflammatory diseases.
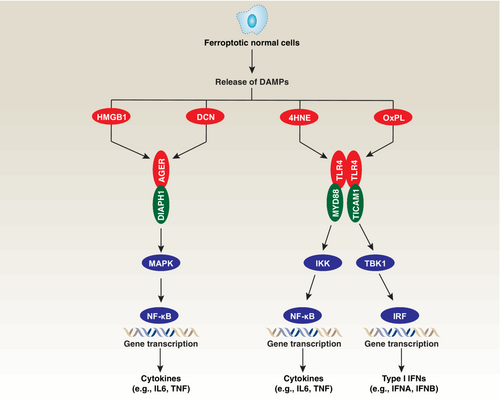
Mechanistically, ferroptosis can induce an inflammatory response through the release of intracellular contents, including damage-associated molecular patterns (DAMPs) (Figure 2).43 The release of DAMPs during ferroptosis has the potential to trigger immune activation and inflammation. These DAMPs usually activate pattern recognition receptors (PRRs) on immune cells, leading to the production of pro-inflammatory cytokines and recruitment of immune cells. For example, one well-known DAMP released during ferroptosis is high mobility group box 1 (HMGB1), which can activate immune cells and promote inflammation.56 Moreover, another specific DAMP released during ferroptosis is decorin (DCN), which is recognized as a unique marker of ferroptotic death.57 Both extracellular HMGB1 and DCN are agonists of advanced glycosylation end-product specific receptor (AGER; also known as RAGE) in macrophages.56, 57 AGER activation subsequently triggers the production and release of pro-inflammatory cytokines, including IL6 and tumor necrosis factor (TNF), through the positive regulation of NF-κB pathways.58
In the context of myocardial ischemia–reperfusion injury, a mouse model of coronary artery ligation-induced myocardial damage, ferroptotic cell death initiates neutrophil recruitment and subsequent production of type I interferon (IFN) through the toll like receptor 4 (TLR4) and TIR domain containing adaptor molecule 1 (TICAM1; also known as TRIF) signaling pathway.59 This implies that ferroptosis-induced inflammation likely plays a role in the pathogenesis of myocardial ischemia–reperfusion injury. Additionally, 15-hydroperoxyeicosatetraenoic acid, an intermediate metabolite derived from arachidonic acid by ALOX15, acts as a trigger for myocardial ischemia–reperfusion injury by inducing cardiomyocyte ferroptosis.60 Both 4HNE and oxidized phospholipids (OxPL) function as ligands for TLR4, leading to the activation of proinflammatory transcription factors, such as NF-κB or the interferon-regulatory factor (IRF) family, via an action on different adaptor proteins.61
Overall, blocking the signaling pathways activated by DAMPs and PRRs can potentially ameliorate inflammation and tissue damage caused by ferroptotic cell death. Inhibiting the release or recognition of DAMPs, including HMGB1, DCN, 4HNE, and OxPL, may provide therapeutic benefits by mitigating ferroptosis-induced inflammation. Further investigation is warranted to elucidate the precise mechanisms by which DAMPs released during ferroptosis modulate immune responses and the potential for targeting these pathways as therapeutic strategies in the context of ferroptotic cell death and associated inflammatory diseases.
3.2 Infection by pathogens
Iron plays a crucial role in the proper functioning of immune cells, including macrophages, neutrophils, and lymphocytes.62 During infection, the host can regulate iron distribution and availability as a means to modulate immune cell activity. In response to an infection, the body can limit iron availability to pathogens while providing this metal to immune cells, thereby promoting their antimicrobial functions.63 Interestingly, pathogens can exploit iron metabolism to induce ferroptosis in host cells, thereby promoting their propagation or evading host immune surveillance.
For example, Mycobacterium tuberculosis can trigger macrophage necrosis associated with iron overload, lipid peroxidation, and downregulation of GPX4 in both in vivo and in vitro models.64 In contrast, treatment with a ferroptosis inhibitor, such as ferrostatin-1, increases the survival of macrophages, as well as their ability to clear the bacteria.64 Similarly, in Pseudomonas aeruginosa infection, the ALOX15 enzyme catalyzes lipid peroxidation and induces ferroptosis in human bronchial epithelial cells, contributing to lung damage in the context of cystic fibrosis.65, 66 Accordingly, the use of a ferroptosis inhibitor protects against P. aeruginosa-induced lung damage in vivo.65, 66 Furthermore, the induction of tumor protein p53 (TP53)-dependent ferroptosis in macrophages during Listeria infection leads to massive intracellular iron accumulation, promoting the progression of the infection.67 These independent studies suggest that increased ferroptosis in macrophages contributes to the pathogenesis of certain infections. However, in specific circumstances, iron present in macrophages may trigger the oxidative death of intracellular bacteria such as Staphylococcus aureus and Escherichia coli, hence improving the microbiocidal function of the immune system.68
In addition to bacterial infection, dysfunctional ferroptosis has been implicated in viral and parasitic infections. For example, the promotion of certain enteroviruses and coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the strain responsible for causing coronavirus disease 2019 (COVID-19), is facilitated by ACSL4-mediated ferroptosis.66 This highlights the potential of developing inhibitors that target ACSL4 as a promising approach for treating viral diseases. The mechanistic target of rapamycin kinase (MTOR) encompasses two protein complexes, namely MTORC1 and MTORC2, which serve as crucial regulators within the cell's nutrient sensing pathways. MTORC2 deficiency in memory CD4+ T cells can induce ferroptosis by suppressing GPX4 activity.69 This condition results in heightened vulnerability to acute infection with lymphocytic choriomeningitis virus in mice.69 Given that blocking MTOR activation can result in the autophagic degradation of GPX4 protein,70 it is essential to further investigate the impact of autophagy on viral infection.
GPX4 is also a selenoenzyme that relies on selenium for its function in preventing lipid ROS-induced ferroptosis. Activation of the selenium-GPX4 axis plays a role in preventing viral infections and enhancing the immune effects of vaccines by inhibiting ferroptosis in follicular helper T cells (TFH).71 Regarding parasitic diseases, infection of Gpx4-deficient mice with Leishmania leads to a reduction in the number of CD4+ T cells, which contributes to the persistence of Leishmania in vivo.72 On the other hand, the induction of TP53-dependent ferroptosis in liver cells limits infection by the Plasmodium parasite.73
The consequences of pathogen infection can lead to the development of sepsis, a clinical syndrome associated with multiple organ dysfunction or failure.74 Pharmacological inhibition of ferroptosis reduces inflammatory responses and alleviates organ dysfunction.75, 76 Nevertheless, the conditional knockout of Gpx4 in myeloid cells expedites experimental sepsis triggered by cecal ligation and puncture or bacterial lipopolysaccharides.77 This acceleration predominantly occurs through pyroptosis-dependent mechanisms rather than ferroptosis,77 emphasizing the fact that lipid peroxidation is also implicated in non-ferroptotic death.78 Interestingly, pro-inflammatory M1 macrophages are more resistant to ferroptosis, while anti-inflammatory M2 macrophages are more susceptible to ferroptotic cell death.79 Although the underlying mechanisms remain poorly understood, one possibility is that nitric oxide synthase 2 (NOS2; also known as iNOS) is upregulated in M1 macrophages, which may play an antioxidant role to limit ferroptosis.79
Understanding the intricate interplay between pathogens and host cells, as well as the activation of antioxidant pathways in response to ferroptotic stress, is of utmost importance in designing approaches to limit infection. By deciphering the mechanisms by which specific immune cell subsets modulate ferroptosis and by exploring the antioxidant pathways that regulate ferroptosis resistance or sensitivity, we can gain insights into potential therapeutic strategies to combat sepsis and other infectious diseases.
3.3 Tumor immunity
The immune system plays a critical role in recognizing and eliminating cancerous cells, contributing to the surveillance and control of tumor growth. Tumor immunity involves a complex interplay of various components and processes that work together to recognize and target tumor cells. Emerging evidence suggests that ferroptotic responses have a multifaceted role in tumor immunity, with implications that vary depending on the tumor stage, model, and treatment context.80
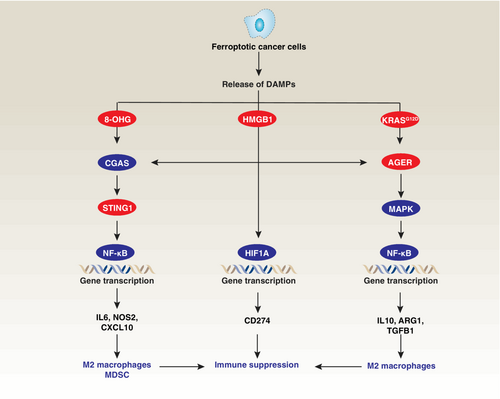
While inflammation is a vital part of the immune response against pathogens and tissue injury, chronic or persistent inflammation within the tumor microenvironment can create a favorable environment for tumor development and progression.81 Particularly, the effects of ferroptotic damage on macrophage polarization, shifting them from an antitumor to a protumor phenotype, have been observed to promote Kras-driven pancreatic tumorigenesis in mice (Figure 3).82 Induction of ferroptotic damage, either through high-iron diets or depletion of Gpx4 in pancreatic acinar cells, triggers the release of nuclear DNA containing 8-hydroxy-2′-deoxyguanosine (8-OHG) into the cytosol, subsequently activating the stimulator of interferon response cGAMP interactor 1 (STING1)-dependent DNA sensor pathway.82 This, in turn, leads to macrophage infiltration and protumor M2 polarization during Kras-driven pancreatic ductal adenocarcinoma development in mice.82 Additionally, the release of HMGB1 and oncogenic KRAS protein from ferroptotic cells can promote M2 macrophage polarization in pancreatic cancer or the recruitment and activation of myeloid-derived suppressor cells (MDSCs) in mice with Gpx4-deficient liver tumors.83, 84 Furthermore, HMGB1 can potentiate the cyclic GMP-AMP synthase (CGAS)-STING1 pathway85 and induce the expression of immune checkpoint CD274 (also known as PD-L1) through the activation of hypoxia inducible factor 1 subunit alpha (HIF1A) transcription factor in pancreatic tumor.86 These mechanisms contribute to the establishment of an inflammation-associated immune-suppressive tumor microenvironment.
In established tumors, robust induction of ferroptosis has emerged as a promising approach to target tumors,87 particularly drug-resistant cells that overexpress GPX4.88 One of the advantageous aspects of ferroptosis induction is its potential to activate antitumor immunity by triggering immunogenic cell death (ICD), which is a form of regulated cell death that elicits an immune response and promotes immune system activation.89, 90 Ferroptosis-related ICD is characterized by the release of specific molecular signals, such as adenosine triphosphate (ATP) and HMGB1, as well as the presentation of antigens that alert the immune system to the presence of dying cells (Figure 4).91 This immune activation can lead to the recognition and elimination of dying cells, as well as associated pathogens or tumor cells.
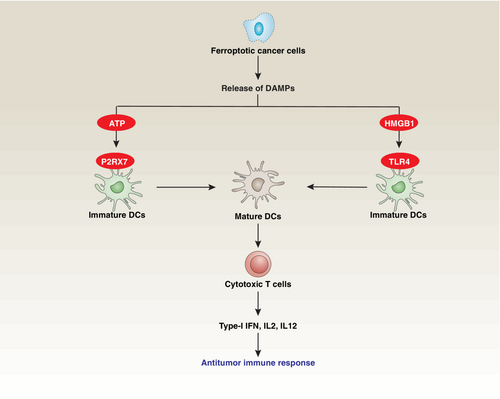
However, recent studies suggest that ferroptosis can have a negative impact on antigen-presenting cells, such as dendritic cells, impairing the activation of CD8+ T cells-mediated adaptive immunity.92 The release of immune-suppressive DAMPs during ferroptotic cell death may contribute to this immune suppression.93 While previous studies suggest that oxidized HMGB1 can mediate immune tolerance during apoptosis,94 further investigation is needed to determine the redox status of extracellular HMGB1 in the context of ferroptosis. Additionally, the induction of ferroptosis in dendritic cells can directly impair the immunogenicity of ferroptotic cells, potentially dampening the immune response.95 Furthermore, the clearance of ferroptotic cells by macrophages may dampen the immunogenicity of these cells. A recent study suggests that toll-like receptor 2 (TLR2) on macrophages can recognize an oxidized phospholipid, specifically 1-stearoyl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine (SAPE-OOH), on the surface of ferroptotic cells, thereby promoting their phagocytosis.96 Gaining deeper insights into the mechanisms underlying the “eat me” and “don't eat me” signals is crucial for elucidating how immune cells engage in the phagocytosis of ferroptotic cells.
Most current ferroptosis inducers primarily target GPX4 or SLC7A11 in cancer cells, both of which are expressed in immune cells as well. However, the non-selective induction of ferroptosis can have contrasting effects on immune cells within the tumor microenvironment. While ferroptosis can eliminate immunosuppressive cells, such as regulatory T cells (Treg)97 and MDSCs,98 to enhance antitumor immunity, it can also kill immune effectors including CD8+ T cells,99 natural killer cells,100 neutrophils,101 and macrophages,79 thus dampening antitumor immune responses. Therefore, when evaluating the long-term effects of ferroptosis inducers on tumor immunity in vivo, careful assessment of the diverse immune cell components within the tumor microenvironment is essential. Overcoming this challenge requires the development of strategies that simultaneously inhibit ferroptosis in anti-tumor immune effectors while promoting ferroptosis in immunosuppressive cells to maximize the benefits of tumor immunotherapy. At the present stage of research, directly targeting the GPX4 protein is not considered an effective approach for enhancing ferroptosis-related antitumor immunity in vivo. This is primarily due to its potential side effects, including the elimination of immune effectors.
Of note, immune cells can release cytokines that trigger cancer cell death through a ferroptotic mechanism. For example, interferon gamma (IFNG; also known as IFNγ) released from CD8+ T cells downregulates the expression of SLC7A11.102 This downregulation impairs the uptake of cystine by tumor cells, leading to increased lipid peroxidation and subsequent ferroptosis.102 The combination of locally produced arachidonic acid with IFNG can further enhance IFNG-induced immunogenic ferroptosis of cancer cells, partly through the involvement of ACSL4.103 However, in infection models, IFNG can restrict iron uptake by upregulating the expression of the iron exporter solute carrier family 40 member 1 (SLC40A1; also known as FPN1).104 Thus, IFNG plays a dual role in the regulation of ferroptosis, exerting both restrictive effects on iron intake and potential influences on other aspects of ferroptotic pathways. In contrast, the proinflammatory cytokine interleukin 1 beta (IL1B, also known as IL1β) triggers the translocation of lysine acetyltransferase 2B (KAT2B, also known as PCAF) to mitochondria, where it catalyzes the acetylation of nicotinamide nucleotide transhydrogenase (NNT) at the K1042 site.105 This acetylation event inhibits ferroptosis and promotes tumor immune evasion by ensuring sufficient maintenance of iron–sulfur clusters.105 Thus, different cytokines play opposing roles in the regulation of ferroptosis in cancer cells.
Combining ferroptosis inducers with immune checkpoint inhibitors represents another promising approach for treating various cancers. Although preclinical studies are encouraging,102, 106, 107 clinical studies investigating this combination are currently lacking. The identification of reliable biomarkers to assess ferroptosis and its correlation with immune response parameters is important for patient stratification and predicting treatment responses in the future. Validated biomarkers could guide personalized therapeutic approaches and help monitor the efficacy of ferroptosis-targeted interventions.108 For example, a bioinformatics analysis of osteosarcoma patients utilizing the Cancer Genome Atlas database unveiled a 4-gene panel (WASP actin nucleation promoting factor [WAS], cortistatin [CORT], Wnt family member 16 [WNT16], and galactosidase beta 1 like 2 [GLB1L2]) as biomarkers associated with both ferroptosis and immune response in the tumor microenvironment.109 Furthermore, the redox status of HMGB1 released during cell death, such as ferroptosis, has the potential to shift the antitumor response from immunostimulation to immunosuppression.110
4 CONCLUSION AND PERSPECTIVE
Over the last 10 years, the field of ferroptosis research has witnessed a remarkable surge in advancements and discoveries.4, 111 It has become evident that ferroptosis influences, and intersects with, various pathological processes traditionally associated with apoptosis or other forms of cell death. This observation suggests the existence of intricate cross-talk and feedback loops among different cell death pathways. Ferroptosis is a distinctive form of oxidative cell death that arises from dysfunctions in multiple antioxidant systems.112 However, the interplay, competition, and complementation between these antioxidant systems in regulating ferroptosis remain poorly understood.
The advancements in our understanding of ferroptosis mechanisms and its immune consequences have had a profound impact on the field of cell biology and hold promise for therapeutic applications in medicine. Modulating ferroptosis and its impact on immune responses may offer opportunities for therapeutic interventions. Combining strategies targeting ferroptosis regulators, such as lipid or iron metabolism pathways, with immunotherapies could enhance anti-tumor immune responses or suppress pathological immune reactions. Given the complex interactions between ferroptosis and immune responses, exploring combination therapies that integrate ferroptosis modulators with immune checkpoint inhibitors, targeted therapies, or other immunomodulatory agents holds potential to improve treatment outcomes.80
- Immune cell-specific functions: Explore the unique functions of diverse immune cell subsets in the regulation of ferroptosis and their implications for overall immune responses. By comprehending the precise mechanisms through which specific immune cells modulate ferroptosis within specific contexts, we can gain valuable insights into their roles in the development of disease and unveil potential therapeutic approaches.
- Immune memory and ferroptosis: Explore the influence of ferroptosis on immune memory responses, such as the generation of memory T cells and long-term immune protection. Elucidating the relationship between ferroptosis and immune memory will have implications for vaccine development and the establishment of long-lasting immune control against pathogens or tumors.
- Translational applications: Utilize the insights obtained from preclinical investigations to advance clinical implementations through the development of therapies aimed at targeting ferroptosis, thereby influencing immune responses. It is essential to carry out clinical trials to assess the effectiveness and safety of combined strategies that involve the modulation of ferroptosis and immunotherapies across various disease contexts.
By pursuing these research directions, we will ultimately succeed in unraveling the intricate interplay between ferroptosis and immune responses. This carries the promise of novel therapeutic strategies that harness the potential of ferroptosis modulation in the context of immune-mediated diseases.
ACKNOWLEDGMENTS
Research by D.T. and R.K. was supported by grants from the National Institutes of Health (R01CA160417, R01CA229275, and R01CA211070). GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR)—Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); European Research Council Advanced Investigator Award (ERC-2021-ADG, ICD-Cancer, Grant No. 101052444), European Union Horizon 2020 Projects Oncobiome, Prevalung (grant No. 101095604) and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
CONFLICT OF INTEREST STATEMENT
GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys, and Vascage. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. GK is in the scientific advisory boards of Hevolution, Institut Servier and Longevity Vision Funds. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. GK'wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk and Roche, was on the on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. GK's brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. The other authors declare no conflicts of interest or financial interests. The funders had no role in the design of the study; in the writing of the manuscript, or in the decision to publish the results.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.